Abstract
Cancer stem cells (CSCs) are known as the major reason for therapy resistance. Recently, natural herbal compounds are suggested to have a significant role in inhibiting the breast cancer stem cells (BCSCs). The aim of this study was to explore the effective natural herbal compounds against BCSCs.This review article was designed based on the BCSCs, mechanisms of therapy resistance and natural herbal compounds effective to inhibit their activity. Therefore, Science direct, PubMed and Scopus databases were explored and related original articles were investigated from 2010 to 2019. BCSCs use different mechanisms including special membrane transporters, anti-apoptotic, pro-survival, and self-renewal- related signaling pathways. Natural herbal compounds could disturb these mechanisms, therefore may inhibit or eradicate the BCSCs. Studies show that a broad range of plants, either as a food or medicine, contain anti-cancer agents that phenolic components and their different derivatives share a large quantity. Natural herbal compounds play a pivotal role in the eradication of BCSCs, through the inhibition of biological activities and induction of apoptosis. Although it is necessary to conduct more clinical investigation.
Key Words: Breast neoplasm, Neoplasm, Phenolic compounds, Stem cells, Therapy resistance
Introduction
Breast cancer (BC) is one of the most challenging forms of cancer because of its high prevalence rate (about 1.7 million new cases diagnosed) and also a high rate of death reports among women (approximately 0.5 million death cases) (1). International Agency for Research on Cancer has reported that approximately 2.1 million cases diagnosed in 2018 (2). Although in the recent decade, different therapeutic techniques including surgery, radio/chemo/ hormonal therapy or targeted therapies have been presented (3), and in spite of the development of innovative anti-cancer drugs acting effectively, patients suffer from consequences of relapse and metastasis (4).
Through all of the possible reasons that have been suggested to explain the occurrence of metastasis, the theory of the presence of breast cancer stem cells (BCSCs) has been faced with more success. BCSCs are defined with unique properties including high proliferation, the ability to self-renewal and the generation of heterogeneous lineages (5). This great achievement, along with studies that have been made in the field of natural compounds found in diet plants and herbal medicine, has tended scientists to explore the role of these compounds in cancer therapy with special attention to target CSCs.
Nowadays, it is well known that natural compounds, generally obtained from plants are useful to treat some of the human diseases (6) including cancer. However, scientists have explored the role of marine natural compounds as anti-cancer agents (7), but most of the studies have been established based on herbal compounds (8). Recently, in addition to different types of normal stem cells and their characteristics and applications in medicine that have been investigated (9), different aspects of CSCs especially BCSCs characteristics have been reviewed by scientists (10). In this article, in addition to the elucidation of the role of BCSCs to therapy resistance, natural herbal compounds against BCSCs are also reviewed.
Therapy resistance, facing issue to breast cancer treatment
Although most chemotherapeutic drugs decline the size of tumors significantly (11), they fail to vanish it completely. As a result, the tumor resists to treatment and relapses. Proving the existence of CSCs in different cancers such as breast cancer (12), it has been demonstrated that chemo/radiotherapy interventions are unable to kill CSCs efficiently (13). Several studies state the inefficiency of chemotherapy (14) and radiotherapy (15) to eradicate CSCs and increased CSCs content of tumors following chemotherapy (16). Possible reasons for therapy resistance are listed below and are shown in Figure 1.
Figure 1.
Mechanisms of therapy resistance in breast cancer stem cells
Active anti-apoptotic pathways
The apoptotic response of CSCs to treatment strongly depends on the equilibrium between pro-apoptotic and anti-apoptotic molecules that are expressed by CSCs. Pro-apoptotic molecules that are expressed including Bcl-2 homologous antagonist killer (BAK), Bcl-2 associated X protein (BAX) and Bcl-2 associated death promoter (BAD). In contrast, anti-apoptotic molecules belonging to the Bcl-2 family (BCL2, MCL1, BCL- XL) regulate the permeability of the outer membrane of the mitochondrion (17). Overexpression of anti-apoptotic molecules within the CSCs leads to therapy resistance. Suppressing the activity of these molecules targets CSCs effectively (18, 19).
Cancer stem cell niche
Within the tumor, CSCs reside in special environmental conditions, where they are not only affected by local physico/chemical factors (oxygen, PH, nutrients) but also they interact with other immune cells or fibroblasts existing on the same site. This special environmental condition is known as “niche”. External stimuli could influence niche severely. For example, radio/chemotherapy leads to the activation of interleukin6 (IL6) by niche elements. IL6, in turn, activates the nuclear factor kappa-light-chain-enhancer of activated B cells (STAT3/NF-Kβ) signaling pathway, which is related to increased BCSC population as well as resistance to Trastuzumab (20). Hypoxic niche has a key role in therapy resistance because of less production of reactive oxygen species (ROS) (21), induction of quiescence state of CSC (22), and eventually releasing the hypoxia-inducible factors 1 and 2 (HIF1, HIF2) (23).
ATP- dependent drug efflux
CSCs possess a thrifty and useful mechanism to elude from applied stress due to the cytotoxic drugs administered to the cells during chemotherapy. Chemotherapeutic drugs have high toxicity on CSCs, but the best answer to how CSCs resist against these agents is that CSCs have an advanced transport system in which ATP is consumed and cytotoxic drugs are pumped outside of the cell actively. The fundamental components of this membrane port are ATP- binding cassette (ABC) proteins, a family of membrane proteins with three well-known members involved in CSCs drug resistance, p- glycoprotein (P- gp), breast cancer resistant protein (BCRP), and multidrug-resistant protein 1 (MDR1). Therefore, ABC proteins lead to resistance to different types of chemotherapeutic agents such as antimetabolites, topoisomerase, and tyrosine kinase inhibitors and taxanes (24).
Pro- survival signaling pathways
Different signaling pathways are contributed to maintaining self-renewal and differentiation of CSCs. These pathways seem to be somewhat responsible for resistance to therapy, therefore could be regarded as new therapeutic targets. The most important pathways are Notch and Hedgehog (25), as well as the Wnt pathway that play an important role to maintain the undifferentiated state and self-renewal conditions of BCSCs (26).
DNA repair and ROS scavenging systems
Radio/chemotherapy treatments lead to DNA damage and following tumor cell death. Instead, CSCs’ response to DNA damage appears as activation of ataxia-telangiectasia-mutated-and-Rad3-related kinase- checkpoint kinase 1 and 2 (ATR-ChK1 and ATM-ChK2) kinase signaling pathways. At the next step, cell cycle progress is suppressed and conditions are provided to DNA repair (27). Also, radiotherapy leads to the production of potentially lethal oxygen radicals known as ROS within the cell. BCSC’s strategy to evade cell death is an efficient ROS removal system in which following irradiation, little quantities of ROS are produced. Also, enzymes involving in ROS scavengings such as catalase, superoxide dismutase (SOD), and glutathione peroxidase are up-regulated in BCSCs (28).
Natural herbal compounds, future anti-cancer drugs with a different prospect
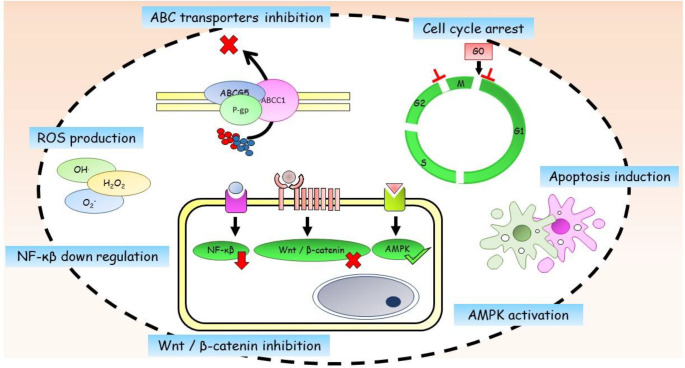
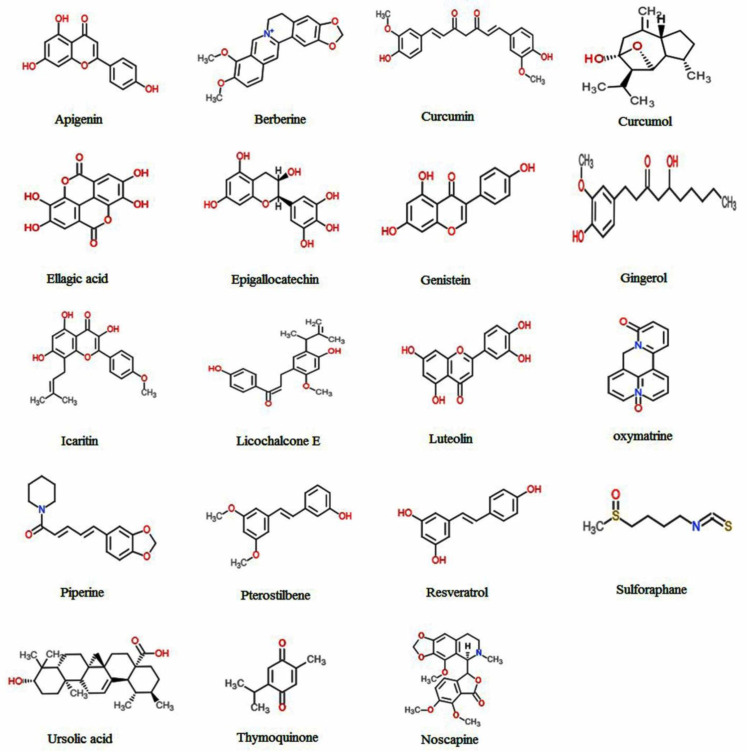
Recently, extensive studies about breast cancer, verifying the presence of BCSCs (10) and therapy resistance issues altogether, have forced scientists to recognize more effective anti-cancer drugs. Isolation, identification and inspecting the therapeutic effects of natural herbal compounds against cancer is a fascinating field of research. The advantage of these compounds is that they are tolerable and also could be added to the dietary regimen. As well, phytochemical compounds are used to inhibit tumor growth or to diminish the possibility of tumor relapse (29, 30). Some of the herbal compounds that have been investigated so far to suppress the BCSCs are listed below. The chemical structure and mechanism of action of these compounds are shown in Figure 2 and Table 1, respectively.
Figure 2.
Chemical structures of natural herbal compounds with anti-cancer and anti-cancer stem cell effects
Table 1.
The mechanism of action of natural herbal compounds with anti-cancer and anti-cancer stem cell effects
| Compound name | Kind of compound | Mechanism of action | Reference |
|---|---|---|---|
| Apigenin | Flavonoid | ABC transporter inhibition, TNFα blockage, MMP2,9 down-regulation Apoptosis induction, cell cycle arrest STAT3 pathway inhibition, Phospho-JAK1,2 inhibition |
41-47 |
| Berberine | Alkaloid | ABC transporter inhibition, apoptosis induction, MMP2,9 down-regulation, Decreased CXCR4, CCR6, CCR9 expression |
52-56 |
| Curcumin | Polyphenol | NF-κB, Wnt/β-catenin, Shh inhibition | 59-62 |
| Curcumol | Sesquiterpenoid | Cell cycle arrest, NF-κB, Akt/JNK1/2 pathways inhibition | 63 (review) |
| Ellagic acid | Polyphenol | Cell cycle arrest, apoptosis induction, TGFβ/Smad3 inhibition | 69-71 |
| Ursolic acid | Polyphenol | Cell cycle arrest, apoptosis induction | |
| Luteolin | Polyphenol | Cell cycle arrest, apoptosis induction VEGF secretion inhibition, inhibiting MPA induced acquisition state |
69,72 |
| Epigallocatechin | Polyphenol | Cell cycle arrest, apoptosis induction, HIF1α degradation, AMPK activation, decrease the expression of estrogen receptor-α36, MMP9, mTOR, FASN inhibition | 73-78 |
| Genistein | Isoflavone | Increase the expression of PTEN, PI3K/Akt, MEK/ERK, Hedgehog–Gli1 inhibition, cell cycle arrest | 81,82.84,85 |
| Gingerol | Phenol | Hedgehog/ Akt/ GSK3β pathway inhibition, MMP2,9 suppression | 86,87 |
| Icaritin | Flavonoid | Cell cycle arrest, apoptosis induction, decrease EGFR and estrogen receptor- α36 expression | 94,95 |
| Licochalcone E | Phenol | Reducing Sp1 expression, apoptosis induction, MMP9 inhibition, angiogenesis inhibition | 96-98 |
| Noscapine | Alkaloid | Cell cycle arrest and prolonged S-phase, apoptosis induction, disrupting tubulin dynamics | 101,102,104,105 |
| Oxymatrine | Alkaloid | Wnt/β-catenin, αⅤβ3 integrin/FAK/ PI3K/Akt inhibition, EMT suppressing Apoptosis induction |
110-113 |
| Piperine | polyphenol | Wnt/β-catenin down-regulation, Apoptosis induction, cell cycle arrest, MMP9,13 inhibition, ROS production, survivin suppression, p65 phosphorylation | 61,116-120 |
| Pterostilbene | Phenol | Inducing Argonaute2 expression Hedgehog/Akt/GSK3β suppression |
86,121 |
| Resveratrol | Phenol | Cell cycle arrest, Apoptosis induction Wnt inhibition, |
125-128 |
| Sulphorafane | Isothiocyanate | Wnt/β-catenin down-regulation Apoptosis induction Cripto/Alk4 suppression |
133-135,137,138 |
| Thymoquinone | Phenol | ROS production, cell cycle arrest Apoptosis induction, NF-κB inhibition |
142-146 |
Phenolic compounds and their derivatives
Phenolic compounds are natural herbal compounds derived from phenylalanine and tyrosine, which are found in food sources extensively [reviewed by Weng and Yen (31)]. Astragalin as a phenolic compound exists in some medicinal plants (32). Their chemical structures differ, but typically pose an aromatic ring with one or two hydroxyl groups. According to the number of aromatic rings and functional groups, phenolic compounds are divided into phenolic acids, monophenols, and polyphenols. Phenolic derivatives are found in a broad range that among of them simple phenols, flavonoids, stilbene, phenylpropanoids, and benzoic acid derivatives are found in various types of plants and nutrients.
Apigenin
Apigenin is a natural flavonoid found in a great number of vegetables and fruits such as French peas, garlic, cabbage, bell pepper and many others (33, 34). Also, Apigenin has been found as an effective element of medical herbs such as Salvia officinalis (35), Turnera aphrodisiaca (36), Lawsonia inermis (37), Ocimum basilicum and Tamarindus indica (38). Apigenin has acquired more attention because of its preventive effects on cancer (39) and diverse effects including antioxidant, ROS removal, antiviral, anti-inflammatory and anti-mutagenic roles that have been evaluated in different systems of mammalian (40).
The role of Apigenin in the inhibition of ABC transporters (p- glycoprotein and ABCB5 transporters) shows that it attaches to the ATP- binding domain of transporters, preventing the ATP attachment; therefore, the required energy to efflux the cytotoxic drugs is not provided. In addition, the activity of Apigenin does not interfere with classical strategies of multidrug resistance, so it seems that it could be conquered to multidrug resistance of different tumors (41). Also, it was shown that Apigenin is able to block tumor necrosis factor-alpha (TNFα) pathway in MDA- MB231 cancer cells; therefore, the following release of chemokines such as CCL2, granulocyte-macrophage colony-stimulating factor (GMCSF), IL-1α and IL-6 is inhibited. These pro-inflammatory chemokines have an important role in tumor growth and metastatic invasion (42).
A study showed that Apigenin suppressed the migration of prostate CSCs via down-regulating matrix metalloproteinase 2, 9 (MMP2,9), slug and snail. Also, Apigenin induces apoptosis through an extrinsic pathway, which is associated with the expression of caspases 3 and 8 (43). Synergic anti-cancer effects of Apigenin and chrysin, a flavonoid with structural similarity to Apigenin, were evaluated. It was shown that both Apigenin and chrysin decreased MDA-MB-231 cell viability and motility, and induced apoptosis via down-regulating the expression of low-density lipoprotein receptor related-protein 6 (LRP6) and S- phase kinase-associated protein 2 (Skp2) (44).
Apigenin could reverse drug resistance of adriamycin-resistant MCF7 cancer cells, which is associated with the arrest of cells in the sub-G0/G1 phase and increased apoptosis. It seems that the suppression of drug resistance by Apigenin depends on the blockade of the signal transducer and activator of transcription 3 (STAT3) signaling pathway (45). A similar data was previously reported in which Apigenin decreased the expression of phospho Janus Kinase 1/2 (phospho-JAK1/ JAK2), phospho-STAT3, and STAT3-dependent luciferase reporter gene activity in human epidermal growth factor receptor 2 (HER2)- expressing BT- 474 cancer cells (46). Li et al. demonstrated that Apigenin significantly suppressed the proliferation and migration of triple-negative breast cancer (TNBC) cells and inhibited stemness features of TNBC cells in both in vitro and in vivo assays. It seemed that Apigenin decreased yes-associated protein/ transcriptional coactivator with PDZ-binding motif (YAP/TAZ) activity and the expression of target genes, such as connective tissue growth factor (CTGF) and Cystein rich 61 (CYR61). They also showed that Apigenin disrupted the YAP/TAZ-TEADs (TEA domain transcription factors) protein-protein interaction and decreased expression of TAZ sensitized TNBC cells to Apigenin treatment (47).
Berberine
Berberine is an alkaloid derivative and a member of the Berberidaceae family, which is found in medicinal herbs Coptis chinensis and Hydrastis canadensis with anti-bacterial (48), anti-inflammatory (49) and verified anti-cancer properties (50, 51). It has been reported that berberine-containing liposomes pass through the membrane of CSCs, inhibit ABC transporters and finally assemble within the mitochondria. Also, berberine leads to activation of pro-apoptotic protein BAX, inhibition of anti-apoptotic protein Bcl-2, increased the permeability of the mitochondrial membrane, the release of cytochrome C and the activation of caspases 3, and 9 (52, 53).
Berberine suppresses MCF7 and ZR- 75- 30 cell growth and migration through reducing the expression of ephrin-B2 and inhibition of MMP2 and 9, respectively (54). One study suggests that migration- inhibitory effect of berberine in MCF7 cancer cells is due to the down-regulation of chemokine receptors 4 (CXCR4), 6 (CCR6), and 9 (CCR9) (55). A combination of berberine with cisplatin suppressed MCF7 cell growth and induced apoptosis through up-regulation of caspases 3, and 9 and down-regulation of Bcl-2 (56).
Curcumin and curcumol
Curcumin is a polyphenolic element of turmeric, an Indian spice used to produce mustard and curry (57). Curcumin bears antioxidant and anti-inflammatory properties (57, 58). It has been shown that curcumin inhibits tumor growth and angiogenesis in the mouse model of human breast cancer through modulating NF- Kβ pathway (59). Also, it has a potential inhibitory effect on Wnt/β- catenin and Sonic hedgehog (shh) pathways relating to BCSCs self-renewal (60). Curcumin leads to the diminished ability of mammosphere formation and decreased the number of ALDH+ cells during consecutive passages. In contrast, curcumin poorly influences differentiated cancer cells (61). Curcumin and epigallocatechin (EPGC) could suppress the phosphorylation of STAT3, followed by decreased levels of STAT3/ NF-Kβ expression in CD44+ CSCs of MCF7- HER2 and MDA- MB231 cell lines (62). Curcumol is a sesquiterpenoid entity of species that belongs to the Zingiberaceae family including turmeric. Curcumol seems to induce cell cycle arrest at G2/M and G0/G1 phases of breast cancer cells and inhibits NF- Kβ pathway through the eliminating the protein kinase B/c-Jun N-Terminal kinases 1/2 (Akt/JNK1/2) pathway and disallowance of MMP9 (63).
A study investigated the role of curcumin to sensitize CSCs from MCF7 and MDA- MB231 cell lines to chemotherapy agents. Data showed that curcumin was able to reduce mammosphere formation. Also, curcumin sensitized BCSCs to mitomycin C (MMC) and the combination of mitomycin C and curcumin decreased CD44+/ CD24- BCSCs population more than 75%. The mechanism in which curcumin sensitizes BCSCs is attributed to the decrease of ABC transporters ABCC1 and ABCG2 (64). The other mechanism of curcumin to chemosensitization of cancer cells is the up-regulation of microRNAs (miRNAs) involving in epithelial-mesenchymal transition and down-regulation of transcription factors B lymphoma Mo-MLV insertion region 1 homolog (BMI1), polycomb protein SUZ12, and enhancer of zeste homolog 2 (EZH2) (65). Curcumin could inhibit the migration and invasion activities of MDA-MB-231 cells by inhibiting the epithelial-mesenchymal transition (EMT) process. For example, curcumin was able to down-regulate the mRNA expression of β-catenin, Vimentin, and Fibronectin, while it up-regulated mRNA expression of E-cadherin. It was proved that curcumin diminishes the protein expression of Oct4, Nanog and Sox2 (66). Recently, novel strategies using curcumin have been developed. Curcumin-loaded nanoparticles in combination with 5-fluorouracil (5- FU) could inhibit cell growth and tumor progression via disturbing the E-cadherin. Also, this combination decreased lipid-peroxidation, while increased malondialdehyde (MDA) and SOD levels (67). It was shown that curcumin- doxorubicin encapsulated nanoparticles could inhibit mammosphere formation in vitro and tumor progression in vivo and decrease the number of CSCs to one fifth (68).
Ellagic acid, ursolic acid, and luteolin
Ellagic acid, ursolic acid, and luteolin are several polyphenolic elements of pomegranate that seem to have anti-cancer properties. Effect of standardized pomegranate extract (PE) on the CSCs isolated from the WA4 cell line shows that pomegranate extract shuts off cell cycle progression at the phase G0/G1 (69) and apoptosis induction through activating the caspase 3. Pomegranate extract components reduce the proliferation rate and viability of the WA4 cell line. It must be mentioned that all of the extracted components do not have such efficiency; for example, caffeic acid has no considerable inhibitory effect on CSCs. Another study showed that the cell cycle arrest of MCF7 cancer cells occurs due to the regulatory role of ellagic acid on the transforming growth factor-beta/Smad3 (TGFβ/ Smad3) signaling pathway (70). The combination of ellagic acid and radiation creates synergistic tumor cytotoxicity in which colony-forming potential of MCF7 cells is reduced significantly. In addition, an increased level of BAX vs decreased level of Bcl-2 pushed cancer cells into apoptosis (71). Luteolin was shown to inhibit the angiogenesis via blocking progestin-dependent vascular endothelial growth factor (VEGF) secretion by breast cancer cells. Also, it prevented medroxyprogesterone acetate (MPA)-induced acquisition state in BCSCs (72).
Epigallocatechin
Epigallocatechin (EPGC) is a natural compound found in several herbal extracts such as the Spatholobus suberectus (SS). This compound causes cell cycle arrest, progression of apoptosis and inhibition of lactate dehydrogenase A (LDH A) of MCF7 and MDA-MB 231 cell lines. Also, it inhibits LDH A expression and in vivo tumor growth. Inhibited expression of LDH A seems to be due to the dispersion of heat shock protein 90 (Hsp90) from hypoxia-inducible factor1α (HIF-1α) and finally acceleration of proteasome degradation of HIF-1α (73).
In addition, Epigallocatechin gallate (EPGCG) of green tea and especially some of its analogs (analogs 4, 6) potentially activate the AMP-activated protein kinase (AMPK) in breast cancer cells. AMPK controls activities such as cell cycle progress, energy situation, protein synthesis, and cell growth and vitality. AMPK inhibits proliferation and up-regulation of an inhibitor of cyclin-dependent kinase (CDK) p21cip1. Also, it inhibits the down-regulation of the mammalian target of rapamycin (mTOR). Eventually, all of these events give rise to the suppression of the CSC population (74).
Epigallocatechin inhibited the growth of CSCs in MDA- MB231 and MDA- MB436 cell lines and decreased the expression of estrogen receptor-α36 (75). Combination of epigallocatechin and a synthetic agonist of retinoid X receptor-γ (RXRγ), known as 6-OH-11-O-hydroxyphenanthrene (IIF), was shown to reduce expression of epidermal growth factor receptor (EGFR) and invasion- associated markers of CD44, MMP2, and 9 in breast cancer cell lines MCF7, MCF7- TAM, and MDA- MB231 (76). An in vivo study showed that epigallocatechin-3-gallate applies its anti- CSC activity via several mechanisms including inducing apoptosis through activation of caspase 3, inhibiting the expression of CD44 and MMP2, suppressing the angiogenesis through inhibition of VEGF and enhanced oxidative stress (77). Recently, researchers have suggested that a new derivative of EPGC, called G28, is able to inhibit the mammosphere forming potential of MDA-MB-231 cell line and its doxorubicin-resistant type. G28 inhibits the fatty acid synthase (FASN) enzyme of cancer cells, which seems to be responsible for cancer cells drug resistance (78).
Genistein
Soybean is a rich source of isoflavonoids among which the anti-cancer properties of genistein and formononetin (79) are well-studied. Animal studies show that soy and its genistein content reduce the quantity of mammary adipose tissue, and increase the expression of E-cadherin, mammary tumor suppressor phosphatase and tensin homolog (PTEN). Especially, it seems that genistein inhibits the differentiation of adipose tissue and in contrast, it enhances the expression of estrogen receptor β (ERβ) of the fibroblast-like cells of mammary stroma in vivo. In addition, genistein with similar effects decreased the amounts of CSCs and generated mammospheres in vitro and in vivo. Analyzing mechanisms of action of genistein suggest that it inhibits Akt (protein kinase B, PKB) pathway and increases PTEN expression (80). Also, MCF7 cells cultured under the genistein-treated adipocytes medium tend to form fewer mammospheres (81).
Genistein could suppress the proliferation potential of mammospheres from the MDA- MB231 cell line through paracrine signaling pathways. The differentiation-inducing effect of genistein seems to arise from the activation of phosphatidylinositol 3-kinase/ Protein Kinase B (PI3K/Akt) and mitogen-activated protein kinase/extracellular signal-regulated kinase (MEK/ERK) signaling pathways through paracrine factors that have been released from ER+ cancer cells (82). As a contradictory result, one study has shown that genistein induces the expression of ABCC1 and ABCG2 transporters in MCF7 and MDA- MB231 cell lines and inhibits miR-181a, an inhibitor of ABCG2 translation (83). Genistein could suppress MCF-7 CSCs proliferation in vitro and in vivo through inducing inhibitory effects on Hedgehog–Gli1 signaling pathway (84). The synergic effects of genistein and Sulforaphane (SFN) were assessed and it was found that these compounds successfully inhibited the cancer cell cycle at G2 or G1 phases. This combination could act as histone methyltransferase (HMT) and histone deacetylase (HDAC) inhibitor via down-regulating HDAC2 and HDAC3 (85).
Gingerol
Gingerol is a phenolic compound existing in the oil extracted from ginger root, generating its special taste and flavor. The fresh root contains a high concentration of gingerol, while dried root bears abundant amounts of shogaol, a dehydrated form of gingerol. It has been shown that 6- shogaol could kill BCSCs effectively, inhibit mammosphere formation and increase the chemosensitivity of CSCs. Mechanism of action of 6- shogaol is down-regulation of CD44 expression, and targeting the hedgehog/ Akt/ GSK3β (Glycogen synthase kinase 3 beta) signaling pathway, leading to phosphorylation of β- catenin and decrease in expression of C- myc and cyclin D, which target stemness of BCSCs (86). It has been demonstrated that gingerol inhibits the motility, attachment, and invasion of cancer cells of the MDA-MB231cell line via an inhibitory effect on MMP2/9 activity (87).
Icaritin
Icaritin is a prenyl flavonoid compound isolated from Epimedium genus that has been used as Chinese herbal medicine for a long time. As a medication, icaritin has various properties such as protective effects on nervous system (88), stimulation of cardiac and neural differentiation (89), steroid-related osteonecrosis prevention (90), osteoblast hyperactivity and suppression of action and differentiation of osteoclasts (91) and inhibitory effect on growth of human prostate carcinoma cell line (92).
Anti-cancer effect of icaritin in the COLO- 205 colon cancer cell line is attributed to the production of ROS, down-regulation of Bcl-2 and signaling of D1/ E cyclin, and activation of caspases 3, and 9 (93). The effect of icaritin on BCSCs is arresting the cell cycle in the G2/M phase, induction of apoptosis, and down-regulation of Bcl-2 (94). Icaritin reduces the expression of EGFR and estrogen receptor- α36 in MDA- MB231 and MDA- MB453 cells (95).
Licochalcone E
Another natural herbal compound that is known as a potent anti-cancer is licochalcone E (LicE), a licorice phenol derivative, that inhibits breast tumor growth and metastasis in vitro and in vivo. In vitro experiment showed that LicE reduced the expression of specificity protein 1 (Sp1) in MCF7 and MDA- MB231 cell lines (96). Sp1 is a protein that involves in regulating cell- cycle controlling protein as well as promoting carcinogenesis and tumor metastasis (97).
LicE decreases the expression of cyclins and CDK and stimulates the apoptosis associated with higher expression of BAX and cloven caspases 3 and lower expression of Bcl-2. Also, LicE has other key roles including inhibition of migration and invasion of cultured cells, preventing the release of MMP9, VEGF-A, and plasminogen activator of urokinase-type and increasing the release of metalloproteinase-2 tissue inhibitor. In addition, LicE drastically limits vessel formation by endothelial cells (98).
Noscapine (Nos)
Noscapine is a natural alkaloid derived from Papaver somniferum with anti-cancer, anti-tussive, and anti-metastasis properties with low toxicity and without a sedative, addictive and analgesic properties (99). Noscapine inhibits cellular proliferation of breast, ovarian and prostate cancers (100). It was demonstrated that noscapine arrests CSCs derived from MCF-7 and MDA-MB-231 at the G2/M phase (101). It was also demonstrated that treating MDA-MB-231 cells with sub-therapeutic doses of noscapine sensitizes the cells to docetaxel through down-regulation of Bcl- 2, survivin, and pAKT, which illustrates apoptosis induction (102). Another study showed that noscapine increased BAX expression, while decreases the expression of Bcl- xl and Bcl-2. Also, it was found that noscapine initiates both extrinsic and intrinsic apoptotic pathways. Decreased level of anti-apoptotic factors may be due to decreased and increased expression of NF- Kβ and IκBα (nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha), respectively (103). Noscapine analog seems to disrupt tubulin stability, and changes the surface configuration of tubulin fibers, preventing microtubule formation. In fact, the noscapine analog prolongs the S- phase of the cell cycle (104). Noscapine and its derivatives can interfere with the effluxion activity of P- glycoprotein (P-gp). Moreover, the combination of noscapine and vinblastine inhibited MCF7 cancer cell proliferation via tubulin dynamic disruption (105).
Oxymatrine
Oxymatrine is a plant alkaloid with anti-microbial (106) and anti-inflammatory (107) effects. Also, its anti-tumor effect on cancer cells is characterized by apoptosis induction and cell cycle inhibitory mechanisms (108) and an inhibitory effect on cell cycle progress following the DNA damage (109). Oxymatrine leads to a decreased rate of proliferation of MCF-7 cells, as well as a decrease in the side population (SP) cells (cancer cells with stem cell properties). The inhibitory effect of oxymatrine seems to be due to the inhibition of the Wnt/b-catenin pathway (110). Inhibition of the Wnt/b-catenin pathway leads to a suppressed Bevacizumab-induced EMT process of BCSCs (111). Chen et al. showed a different mechanism in which oxymatrine effectively suppressed the EMT process induced by fibronectin. Oxymatrine could hamper the αVβ3 integrin/FAK/PI3K/Akt signaling pathway via inhibiting the phosphorylation of FAK (focal adhesion kinase), PI3K, and Akt (112). An in vitro study on MCF7 and MDA- MB231 cell lines showed that oxymatrine influences the viability and proliferation of cells in a time and dose-dependent manner. In addition, treatment with oxymatrine arrests cell cycle at S- phase, and activation of caspases 3, and 9 takes cells into apoptosis (113).
Piperine
Piperine is a polyphenolic compound isolated from long and black peppers. Studies on the animal model show that piperine reduces the rate of lung cancer. Recently, piperine has been used as a chemosensitizer agent in combination with rapamycin to treat breast cancer (114). Using a combination of thymoquinone and piperine in breast cancer mouse model showed significant shrinkage of tumor and occurrence of apoptosis as well as reduced levels of VEGF, suggesting the suppression of angiogenesis within the tumor (115). Also, piperine makes it possible to inhibit the Wnt/β-catenin pathway; therefore, it could be effective to target BCSCs (61). Lai et al. investigated the anti-cancer effect of piperine on mouse 4T1 breast cancer. They showed that piperine inhibited tumor progression in a time and dose-dependent state and induced apoptosis through activating caspase 3. Piperine made cancer cells cycle arrest at the G2/M phase with decreased expression of cyclin B1. Also, piperine prevented cancer cell migration by reducing MMP9 and MMP13 levels (116). It seems that MMP9 reduction is due to blockage of Akt, ERK1/2, and p38 MAPK signaling pathways that prevented the activation of AP-1 (activator protein 1) and NF- Kβ (117). It has been suggested that piperine could act as a pro-oxidant compound leading to ROS production, MCF7 cancer cells arrest at the G2/M phase and death (118). Moreover, piperine was able to down-regulate the expression of proteins associated with different phases of TNBC cell cycles such as cyclin D3, cyclin B, CDK4, CDK1 and p21 (119). Piperine improves anti-cancer effects of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) on MDA-MB-468 and MDA-MB-231 cell lines. Cancer cell growth suppression and apoptosis, survivin suppression and p65 phosphorylation within cancer cells reported as significant results of piperine- TRAIL combination treatment (120).
Pterostilbene
Pterostilbene is a natural stilbene and dimethylated analog of resveratrol, which is found in blueberries and bears anti-cancer properties. Studies on the effect of pterostilbene on BCSCs have shown that pterostilbene induces the expression of Argonaute2 (Ago2), a kind of interfering RNA, followed by increased levels of tumor-suppressive microRNAs miR-16, miR-141, miR-143, and miR-200c. This mechanism resulted in a reduced number of BCSCs and suppressed mammosphere formation (121). This compound modulates the generation of BCSCs and tumor-associated macrophages. Tumor-associated macrophages are related to metastasis and malignancy so the co-culture of tumor-associated macrophages with cancer cells of MCF7 and MDA- MB231 cell lines is accompanied by a higher percentage of CSC content and higher expression of HIF-1α, Twist1, β- catenin and NF-Κβ transcriptional factors. Treatment with pterostilbene reduces CSC content of culture and expression of NF-Κβ, vimentin, and twist1. In contrast, the expression of E-cadherin increases. Examination of animal models shows that pterostilbene suppresses tumorigenicity and tumor metastasis (122).
It was demonstrated that pterostilbene has anti- BCSCs effects similar to 6- shogaol. In the same way, pterostilbene could reduce BCSCs’ survival and increase the chemosensitivity of BCSCs to paclitaxel. Also, pterostilbene suppresses the mammosphere formation potential and induces membrane damage in BCSCs. Both 6- shogaol and pterostilbene share the same mechanisms to inhibit BCSCs, including decrease in the CD44 expression, and promote the phosphorylation of β- catenin as a result of suppression of hedgehog/ Akt/ GSK3β signaling pathway. Inhibition of this pathway leads to reduced expression of C- myc, and cyclin D that is associated with limited stemness potential of BCSCs (86).
Resveratrol
Resveratrol (Res) is a natural dietary compound found in a vast range of fruits, vegetables and red grape juice with preventive effects on cardiovascular disorders and cancer (123). The effect of resveratrol on normal cells appears as an antioxidant agent. However, in cancer cells, it has pro-oxidant and cytotoxic effects. These different effects depend on the biological characteristics of cancer cells and the unique properties of the microenvironment (124). Resveratrol seems to move intracellular copper ions, in turn contributing to Fenton reactions, leading to the production of ROS. ROS causes to oxidative break of DNA and finally cell death. Several factors, such as low PH of environment, as observed in CSCs niche, increase the number of reactions (124).
Resveratrol could induce G1 arrest, as well as activation of caspases 8/ 9, decrease the cell viability in a dose-dependent manner in both MCF7 and MDA- MB231 cell lines, and modulate miRNAs involving in apoptosis (125). It was demonstrated that resveratrol inhibits RhoA/Lats1/YAP signaling pathway in MDA- MB231 and MDA- MB468 breast cancer cells. Inactivation of RhoA leads to activation of Lats1, which promotes the phosphorylation of YAP and finally inhibits the invasion of cancer cells. Also, a combination of resveratrol and salinomycin was known to induce mitochondria dysfunction, caspase activation followed by apoptosis, and intracellular production of ROS in four BCSC lines (126). Also, another study reported that a combination of resveratrol/ salinomycin targets the Wnt signaling pathway, which results in suppressing the viability of cancer cells, and cell cycle arrest, leading to apoptosis induction (127). Resveratrol may inhibit cell cycle at G1/ S phase in MCF7 and MDA- MB231 cancer cells through targeting Aurora protein kinase (AURKA) and the Polo-like kinase-1 (PLK1) pathways (128). It was proved that resveratrol could alter breast tumor microenvironment through inhibiting the effects of cellular interactions on stem-like breast cancer cells. Resveratrol seemed to hamper the proliferation, migration, and invasion of breast cancer cells as well as the expression of c- myc, cyclin D1, MMP2, MMP9, CD44, SOX2 and Bmi-1 (129).
Isothiocyanates
Isothiocyanates (ITCs), abundantly found in cruciferous vegetables, have an effective role to prevent human tumors. Diets containing adequate quantities of ITCs reduce the incidence risk of several cancers including lung, breast, and colon (130). Mechanisms of action for ITCs to prevent cancer are different and include activating the carcinogen detoxifying agents, and increased apoptosis versus stopped cell cycle. In addition, ITCs make it possible to suppress cell proliferation, angiogenesis, self-renewal and epithelial-mesenchymal transition of CSCs. Also, oncogene signaling pathways up-regulated in several cancers are inhibited by the ITCs. Examples of these pathways are STAT3, NF-Κβ and hormone receptors (131). One of the more known ITCs that has been studied so far is sulforaphane (SFN).
Sulforaphane
SFN is the main glucosinolate component of the broccoli that has been studied because of its anti-cancer effects. SFN could regulate the activity of different signaling pathways including NF-Κβ, shh, and Wnt/β-catenin. SFN has a significant role in modulating epithelial-mesenchymal transition in various types of cancers; therefore, it is regarded to target CSCs. SFN has been proposed as a chemotherapy adjuvant compound during the preclinical studies (132).
Effects of SFN on the regulation of BCSCs have been investigated in vitro and in vivo. SFN lowers the percentage of human BCSCs determined with a decreased level of ALDH+ cells and cultured mammospheres. Administration of SFN into animal models decreased the quantity of ALDH+ cells by more than 50% and inhibited the growth of cancer cells that have been cultured into the secondary hosts. The effects of SFN seemed to be applied to the down-regulation of the Wnt/β-catenin pathway that involves in self-renewal of cells (133).
It was demonstrated that a combination of withaferin A (WA), from the Indian winter cherry, and SFN with low concentration leads to a decrease in viability and an increased apoptosis of MCF7 and MDA- MB231 cell lines. The apoptotic effect of these compounds is observed through an increased level of BAX and decreased level of Bcl- 2 (134). A similar study showed that SFN, alone or in combination with metformin (MTFN), was associated with decreased expression of Bcl-2, Wnt/β-catenin, and HER2 and increased expression of BAX in CSCs. The diminished expression of HER2 was directly correlated with cell apoptosis (135). A study showed that SFN could reduce the number of BCSCs in triple-negative breast cancer cell lines SUM149 and SUM159. SFN seems to inhibit NF- Kβ pathway. A combination of SFNwith docetaxel or paclitaxel not only increases the chemo-toxicity of chemotherapy agents but also reduces the CSC population significantly (136). SFN reduced the expression of CSCs markers including ALDH1A1, CR1, CRIPTO-3/TDGF1P3 (CR3, a homolog of CR1), Nanog, Notch4 and Wnt3. Mechanism of action in which SFN inhibits CSCs is through suppression of the Cripto/Alk4 protein signaling pathway (137). It was demonstrated that docetaxel/ SFN-loaded nanoparticles could target BCSCs and reduce their self- renewal potential. SFN nanoparticles apply their effect via down-regulating β- catenin expression (138).
Thymoquinone
Thymoquinone (TQ) is the main component of Nigella sativa seed oil with anti-inflammatory, antioxidant, and anti-cancer activity (139). The protective effects of N. sativa extract and TQ have been investigated against breast cancer (140). It was shown that TQ has an anti-proliferative, and pro-apoptotic effect via p38 phosphorylation and production of ROS. Also, TQ induced apoptosis in breast cancer cells via inhibiting the expression of anti-apoptotic genes like Bcl- 2, and Bcl- xL (141). It was demonstrated that TQ inhibited breast cancer cell growth through arresting cell cycle at the G1 phase, activating caspases 8, and 9 apoptotic pathways, and enhancing chemosensitivity to cisplatin and docetaxel (142). Rajput et al. showed that co-treatment with TQ and tamoxifen decreases the expression of X-linked inhibitor of apoptotic protein (XIAP), resulting in activation of caspase 9 apoptotic cascade. Also, breast cancer cell survival is disrupted because of inhibition of the PI3K/ Akt pathway followed by Akt phosphorylation (143). TQ augments the anti-cancer effect of gemcitabine (GCB) with an increased population of apoptotic and S phase arrested MCF-7 and T47D cell lines (144). TQ decreased the expression of Eukaryotic elongation factor-2 kinase (eEF-2K), which was associated with decreased proliferation, migration, invasion, and colony formation of triple-negative breast cancer cells both in vitro and in vivo. It seems that TQ inhibits Eef- 2k via miR603/ NF- Kβ axis; miR603 inhibits eEF-2K, while inhibition of the NF- Kβ induces the expression of miR603 (145). TQ could inhibit the metastasis of TNBC cells via down-regulating CXCR4 followed by inhibiting the NF-Κβ signaling pathway in a dose and time-dependent manner. Also, TQ treatment caused decreased migration and invasion induced by CXCL2 (146).
Royal jelly (RJ), a novel anti-cancer agent with different origin
Royal jelly (RJ) is a natural bee product, a white to a pale yellow jelly substance that is secreted from mandibular and hypopharyngeal glands of worker bees (147). RJ is mainly composed of water, carbohydrates, proteins, lipids, vitamins, minerals (148), acetylcholine, adenosine, adenosine monophosphate N1 oxide, polyphenols, and hormones such as progesterone, testosterone, prolactin, and estradiol (149). 10-hydroxydecanoic acid (10 HDA) and 24-methylenecholesterol are the most important bioactive compounds of RJ. 10 HDA is an unsaturated fatty acid that is only found in RJ in nature (150) and has immune-modulatory properties (151). RJ and some other compounds processed by honey bees such as propolis possess desirable therapeutic properties (152). Propolis and phenolic components of RJ have various properties including anti-inflammatory, anti-microbial, antioxidant properties and so on (152, 153). In addition to the therapeutic effects mentioned above, the anti-cancer property of RJ has been investigated in several types of cancers. It was demonstrated that 10 HDA inhibits VEGF- induced angiogenesis through both proliferation and migration of endothelial cells (154). RJ has drastic anti-proliferative potential to inhibit the proliferation of SH- SY5Y neuroblastoma cell lines (155). Recently, a study showed that RJ not only inhibited 4T1 breast cancer cells growth but also augmented the immunity of breast cancer-induced animal models indicated by the increased level of TNF- α and decreased levels of IL- 6 and IL- 10 (156).
Discussion
Breast cancer is a sophisticated disease in which treatment requires more time and cost and imposes economic and psychic pressures to the patient and the healthcare system. In addition to different therapeutic strategies and various range of drugs administered, therapy resistance and disease recurrence are regarded as a point of weakness in the treatment of breast cancer (14, 15). In recent decades, conducted studies in the field of breast cancer and introducing BCSCs theory have been accompanied by the identification of BCSCs (12), altogether have made a new era of research in front of scientists.
On the other side, in the recent years, natural herbal compounds that were thought to be applied as traditional medicine only, have been attractive for researchers who were looking for more efficient drugs to perish the CSCs. Natural herbal compounds found in fruits and vegetables that are obtained easily and in abundance could interfere with biological and metabolic pathways of CSCs, suppress these pathways, leading to inhibition of action or eradication of CSCs. Also, it must be mentioned that obtained results are on the basis of in vivo and in vitro investigations and a few numbers of these compounds have been evaluated in the form of preclinical studies (94, 132). Moreover, the anti-cancer effect of some herbal compounds such as Eupatilin on breast cancer has not been studied (157), or new targeting pathways for cancer prevention have been introduced including Malic enzyme 2 and Phosphoglycerate mutase 1 that are noteworthy (158, 159).
Conclusion
In closing, natural herbal compounds serve as promising therapeutic agents with multifunctional properties in prevention and inhibition of breast cancer but it is substantially suggested that the well-investigated studies be assessed in preclinical experiments with attention to ethical principles, and more experiments be designed to explore the anti-breast cancer effects of those compounds that have not been determined favorably.
Acknowledgment
Authors of the present manuscript gratefully acknowledge Fertility and Infertility Research Center of Kermanshah University of Medical Sciences (KUMS), Kermanshah, Iran for supporting the ideas of the manuscript.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Pineros M, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941–1953. doi: 10.1002/ijc.31937. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R MJ, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 4.Group EBCTC. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. The Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 5.Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, et al. Cancer stem cells—perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66:9339–9344. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- 6.Rasul A, Al-Shawi AA, Malik SA, Anwar H, Rasool B, Razzaq A, et al. Neurada procumbens promotes functions regain in a mouse model of mechanically induced sciatic nerve injury. Pak J Pharm Sci. 2019;32:1761–1766. [PubMed] [Google Scholar]
- 7.Rezakhani L, Rashidi Z, Mirzapur P, Khazaei M. Antiproliferatory effects of crab shell extract on breast cancer cell line MCF7. J Breast Cancer. 2014;17:219–225. doi: 10.4048/jbc.2014.17.3.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu B, Cheng Y, Liu Q, Bao J-k, Yang J-M. Autophagic pathways as new targets for cancer drug development. Acta Pharm Sinic. 2010;31:1154–1164. doi: 10.1038/aps.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khazaei M, Bozorgi A, Khazaei S, Khademi A. Stem cells in dentistry, sources, and applications. Dent Hypotheses. 2016;7:42–52. [Google Scholar]
- 10.Bozorgi A, Khazaei M, Khazaei MR. New findings on breast cancer stem cells: a review. J Breast Cancer. 2015;18:303–312. doi: 10.4048/jbc.2015.18.4.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 12.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proceed Natl Acad Sci. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shafee N, Smith CR, Wei S, Kim Y, Mills GB, Hortobagyi GN, et al. Cancer stem cells contribute to cisplatin resistance in Brca1/p53–mediated mouse mammary tumors. Cancer Res. 2008;68:3243–3250. doi: 10.1158/0008-5472.CAN-07-5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee HE, Kim JH, Kim YJ, Choi S, Kim S, Kang E, et al. An increase in cancer stem cell population after primary systemic therapy is a poor prognostic factor in breast cancer. Br J Cancer. 2011;104:1730–1738. doi: 10.1038/bjc.2011.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zielske SP, Spalding AC, Wicha MS, Lawrence TS. Ablation of breast cancer stem cells with radiation. Transl Oncol. 2011;4:227–233. doi: 10.1593/tlo.10247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li X, Lewis MT, Huang J, Gutierrez C, Osborne CK, Wu M-F, et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer I. 2008;100:672–679. doi: 10.1093/jnci/djn123. [DOI] [PubMed] [Google Scholar]
- 17.Sotiropoulou PA, Christodoulou MS, Silvani A, Herold-Mende C, Passarella D. Chemical approaches to targeting drug resistance in cancer stem cells. Drug Discov today. 2014;19:1547–1562. doi: 10.1016/j.drudis.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Piggott L, Omidvar N, Pérez SM, Eberl M, Clarkson RW. Suppression of apoptosis inhibitor c-FLIP selectively eliminates breast cancer stem cell activity in response to the anti-cancer agent, TRAIL. Breast Cancer Res. 2011;13:1–15. doi: 10.1186/bcr2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yin S, Xu L, Bandyopadhyay S, Sethi S, Reddy KB. Cisplatin and TRAIL enhance breast cancer stem cell death. Int J Oncol. 2011;39:891–898. doi: 10.3892/ijo.2011.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korkaya H, Kim G-i, Davis A, Malik F, Henry NL, Ithimakin S, et al. Activation of an IL6 inflammatory loop mediates trastuzumab resistance in HER2+ breast cancer by expanding the cancer stem cell population. Mol Cell. 2012;47:570–584. doi: 10.1016/j.molcel.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wardman P. Chemical radiosensitizers for use in radiotherapy. Clin Oncol. 2007;19:397–417. doi: 10.1016/j.clon.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 22.Pece S, Tosoni D, Confalonieri S, Mazzarol G, Vecchi M, Ronzoni S, et al. Biological and molecular heterogeneity of breast cancers correlates with their cancer stem cell content. Cell. 2010;140:62–73. doi: 10.1016/j.cell.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 23.Semenza GL. Hypoxia-inducible factors: mediators of cancer progression and targets for cancer therapy. Trends Pharmacol Sci. 2012;33:207–214. doi: 10.1016/j.tips.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muñoz P, Iliou MS, Esteller M. Epigenetic alterations involved in cancer stem cell reprogramming. Mol Oncol. 2012;6:620–636. doi: 10.1016/j.molonc.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vasiliou V, Vasiliou K, Nebert DW. Human ATP-binding cassette (ABC) transporter family. Hum Genomics. 2009;3:281–290. doi: 10.1186/1479-7364-3-3-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farnie G, Clarke RB. Mammary stem cells and breast cancer-role of notch signalling. Stem Cell Rev. 2007;3:169–175. doi: 10.1007/s12015-007-0023-5. [DOI] [PubMed] [Google Scholar]
- 27.Aggarwal BB, Sethi G, Baladandayuthapani V, Krishnan S, Shishodia S. Targeting cell signaling pathways for drug discovery: an old lock needs a new key. J Cell Biochem. 2007;102:580–592. doi: 10.1002/jcb.21500. [DOI] [PubMed] [Google Scholar]
- 28.Niida H, Nakanishi M. DNA damage checkpoints in mammals. Mutagenesis. 2006;21:3–9. doi: 10.1093/mutage/gei063. [DOI] [PubMed] [Google Scholar]
- 29.Selamoglu Z. Biotechnological approaches on anticancer activity of flavonoids-Mini review. Modern Approaches to Drug Design. 2017;1:1. [Google Scholar]
- 30.Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie MJ, Kulp AN, et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458:780–783. doi: 10.1038/nature07733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weng C-J, Yen G-C. Chemopreventive effects of dietary phytochemicals against cancer invasion and metastasis: phenolic acids, monophenol, polyphenol, and their derivatives. Cancer Treat Rev. 2012;38:76–87. doi: 10.1016/j.ctrv.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 32.Riaz A, Rasul A, Hussain G, Zahoor MK, Jabeen F, Subhani Z, et al. Astragalin: A bioactive phytochemical with potential therapeutic activities. Adv Phar Sc. 2018;2018:1–15. doi: 10.1155/2018/9794625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miean KH, Mohamed S. Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. J Agr Food Chem. 2001;49:3106–112. doi: 10.1021/jf000892m. [DOI] [PubMed] [Google Scholar]
- 34.Ninfali P, Angelino D. Nutritional and functional potential of Beta Vulgaris cicla and rubra. Fitoterapia. 2013;89:188–199. doi: 10.1016/j.fitote.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 35.Đorđević S, Cakić M, Amr S. The extraction of apigenin and luteolin from the sage Salvia officinalis L from Jordan Facta universitatis-series. Working and Living Enviromental Protection. 2000;1:87–93. [Google Scholar]
- 36.Afzal M, Aloriquat G, Alhassan J, Muhammad N. Flavone glycosides from lawsonia innermis. Heterocycles. 1980;14:1973–1976. [Google Scholar]
- 37.Khalid H, Abdalla WE, Abdelgadir H, Opatz T, Efferth T. Gems from traditional north-African medicine: Medicinal and aromatic plants from Sudan. Nat Prod Bioprospect. 2012;2:92–103. [Google Scholar]
- 38.Kumar S, Sharma A. Apigenin: The anxiolytic constituent of turnera aphrodisiaca. Pharm. 2006;44:84–90. doi: 10.4103/0250-474X.49143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patel D, Shukla S, Gupta S. Apigenin and cancer chemoprevention: progress, potential and promise. Int J Oncol. 2007;30:233–246. [PubMed] [Google Scholar]
- 40.Middleton E, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol Rev. 2000;52:673–751. [PubMed] [Google Scholar]
- 41.Saeed M, Kadioglu O, Khalid H, Sugimoto Y, Efferth T. Activity of the dietary flavonoid, apigenin, against multidrug-resistant tumor cells as determined by pharmacogenomics and molecular docking. J Nutr Biochem. 2015;26:44–56. doi: 10.1016/j.jnutbio.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 42.Bauer D, Redmon N, Mazzio E, Soliman KF. Apigenin inhibits TNFα/IL-1α-induced CCL2 release through IKBK-epsilon signaling in MDA-MB-231 human breast cancer cells. PloS one. 2017;12:1–17. doi: 10.1371/journal.pone.0175558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Erdogan S, Doganlar O, Doganlar ZB, Serttas R, Turkekul K, Dibirdik I, et al. The flavonoid apigenin reduces prostate cancer CD44+ stem cell survival and migration through PI3K/Akt/NF-κB signaling. Life Sci. 2016;162:77–86. doi: 10.1016/j.lfs.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 44.Huang C, Wei Y-X, Shen M-C, Tu Y-H, Wang C-C, Huang H-C. Chrysin, abundant in morinda citrifolia fruit water–etoac extracts, combined with apigenin synergistically induced apoptosis and inhibited migration in human breast and liver cancer cells. J Agr Food Chem. 2016;64:4235–4245. doi: 10.1021/acs.jafc.6b00766. [DOI] [PubMed] [Google Scholar]
- 45.Seo HS, Ku JM, Choi HS, Woo JK, Lee BH, Kim DS, et al. Apigenin overcomes drug resistance by blocking the signal transducer and activator of transcription 3 signaling in breast cancer cells. Oncol Rep. 2017;38:715–724. doi: 10.3892/or.2017.5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seo H-S, Jo JK, Ku JM, Choi H-S, Choi YK, Woo J-K, et al. Induction of caspase-dependent extrinsic apoptosis by apigenin through inhibition of signal transducer and activator of transcription 3 (STAT3) signalling in HER2-overexpressing BT-474 breast cancer cells. Biosci Rep. 2015;35:1–14. doi: 10.1042/BSR20150165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li YW, Xu J, Zhu GY, Huang ZJ, Lu Y, Li XQ, et al. Apigenin suppresses the stem cell-like properties of triple-negative breast cancer cells by inhibiting YAP/TAZ activity. Cell Death Discov. 2018;4:1–9. doi: 10.1038/s41420-018-0124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun D, Abraham SN, Beachey EH. Influence of berberine sulfate on synthesis and expression of pap fimbrial adhesin in uropathogenic escherichia coli. Antimicrob Agents Chemother. 1988;32:1274–1277. doi: 10.1128/aac.32.8.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hahn FE, Ciak J Berberine. Mechanism of action of antimicrobial and antitumor agents. Springer; 1975. pp. 577–584. [Google Scholar]
- 50.Katiyar SK, Meeran SM, Katiyar N, Akhtar S. p53 cooperates berberine-induced growth inhibition and apoptosis of non-small cell human lung cancer cells in vitro and tumor xenograft growth in vivo. Mol Carcinog. 2009;48:24–37. doi: 10.1002/mc.20453. [DOI] [PubMed] [Google Scholar]
- 51.Liu Z, Liu Q, Xu B, Wu J, Guo C, Zhu F, et al. Berberine induces p53-dependent cell cycle arrest and apoptosis of human osteosarcoma cells by inflicting DNA damage. Mutat Res/Fund and Mol M. 2009;662:75–83. doi: 10.1016/j.mrfmmm.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 52.Ma X, Zhou J, Zhang C-X, Li X-Y, Li N, Ju R-J, et al. Modulation of drug-resistant membrane and apoptosis proteins of breast cancer stem cells by targeting berberine liposomes. Biomaterials. 2013;34:4452–4465. doi: 10.1016/j.biomaterials.2013.02.066. [DOI] [PubMed] [Google Scholar]
- 53.Zhao Y, Jing Z, Lv J, Zhang Z, Lin J, Cao X, et al. Berberine activates caspase-9/cytochrome c-mediated apoptosis to suppress triple-negative breast cancer cells in vitro and in vivo. Biomed Pharmacother. 2017;95:18–24. doi: 10.1016/j.biopha.2017.08.045. [DOI] [PubMed] [Google Scholar]
- 54.Ma W, Zhu M, Zhang D, Yang L, Yang T, Li X, et al. Berberine inhibits the proliferation and migration of breast cancer ZR-75-30 cells by targeting Ephrin-B2. Phytomedicine. 2017;25:45–51. doi: 10.1016/j.phymed.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 55.Ahmadiankia N, Moghaddam HK, Mishan MA, Bahrami AR, Naderi-Meshkin H, Bidkhori HR, et al. Berberine suppresses migration of MCF-7 breast cancer cells through down-regulation of chemokine receptors. Iran J Basic Med Sci. 2016;19:125–131. [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao Y, Jing Z, Li Y, Mao W. Berberine in combination with cisplatin suppresses breast cancer cell growth through induction of DNA breaks and caspase-3-dependent apoptosis. Oncol Rep. 2016;36:567–572. doi: 10.3892/or.2016.4785. [DOI] [PubMed] [Google Scholar]
- 57.Park CH, Hahm ER, Park S, Kim H-K, Yang CH. The inhibitory mechanism of curcumin and its derivative against β-catenin/Tcf signaling. FEBS lett. 2005;579:2965–2971. doi: 10.1016/j.febslet.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 58.Satoskar R, Shah S, Shenoy S. Evaluation of anti-inflammatory property of curcumin (diferuloyl methane) in patients with postoperative inflammation. Int J Clin Pharmacol Ther Toxicol. 1986;24:651. [PubMed] [Google Scholar]
- 59.Bimonte S, Barbieri A, Palma G, Rea D, Luciano A, D’Aiuto M, et al. Dissecting the role of curcumin in tumour growth and angiogenesis in mouse model of human breast cancer. BioMed Res Int. 2015;2015:1–7. doi: 10.1155/2015/878134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li X, Wang X, Xie C, Zhu J, Meng Y, Chen Y, et al. Sonic hedgehog and Wnt/beta-catenin pathways mediate curcumin inhibition of breast cancer stem cells. Anti-cancer drugs. 2018;29:208–215. doi: 10.1097/CAD.0000000000000584. [DOI] [PubMed] [Google Scholar]
- 61.Kakarala M, Brenner DE, Korkaya H, Cheng C, Tazi K, Ginestier C, et al. Targeting breast stem cells with the cancer preventive compounds curcumin and piperine. Breast Cancer Res Treat. 2010;122:777–785. doi: 10.1007/s10549-009-0612-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chung SS, Vadgama JV. Curcumin and epigallocatechin gallate inhibit the cancer stem cell phenotype via down-regulation of STAT3–NFκB signaling. Anticancer Res. 2015;35:39–46. [PMC free article] [PubMed] [Google Scholar]
- 63.Wei W, Rasul A, Sadiqa A, Sarfraz I, Hussain G, Nageen B, et al. Curcumol: from plant roots to cancer roots. Int J Biol Sci. 2019;15:1600–1609. doi: 10.7150/ijbs.34716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou Q, Ye M, Lu Y, Zhang H, Chen Q, Huang S, et al. Curcumin improves the tumoricidal effect of mitomycin C by suppressing ABCG2 expression in stem cell-like breast cancer cells. PloS one. 2015;10:1–12. doi: 10.1371/journal.pone.0136694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Toden S, Okugawa Y, Jascur T, Wodarz D, Komarova NL, Buhrmann C, et al. Curcumin mediates chemosensitization to 5-fluorouracil through miRNA-induced suppression of epithelial-to-mesenchymal transition in chemoresistant colorectal cancer. Carcinogenesis. 2015;36:355–367. doi: 10.1093/carcin/bgv006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hu C, Li M, Guo T, Wang S, Huang W, Yang K, et al. Anti-metastasis activity of curcumin against breast cancer via the inhibition of stem cell-like properties and EMT. Phytomedicine. 2019;58:1–32. doi: 10.1016/j.phymed.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 67.Hashemzehi M, Behnam-Rassouli R, Hassanian SM, Moradi-Binabaj M, Moradi-Marjaneh R, Rahmani F, et al. Phytosomal-curcumin antagonizes cell growth and migration, induced by thrombin through AMP-kinase in breast cancer. J Cell Biochem. 2018;119:5996–6007. doi: 10.1002/jcb.26796. [DOI] [PubMed] [Google Scholar]
- 68.Yuan JD, ZhuGe DL, Tong MQ, Lin MT, Xu XF, Tang X, et al. pH-sensitive polymeric nanoparticles of mPEG-PLGA-PGlu with hybrid core for simultaneous encapsulation of curcumin and doxorubicin to kill the heterogeneous tumour cells in breast cancer. Artif Cells Nanomed Biotechnol. 2018;46:302–313. doi: 10.1080/21691401.2017.1423495. [DOI] [PubMed] [Google Scholar]
- 69.Dai Z, Nair V, Khan M, Ciolino HP. Pomegranate extract inhibits the proliferation and viability of MMTV-Wnt-1 mouse mammary cancer stem cells in vitro. Oncology Rep. 2010;24:1087–1091. [PubMed] [Google Scholar]
- 70.Chen H-S, Bai M-H, Zhang T, Li G-D, Liu M. Ellagic acid induces cell cycle arrest and apoptosis through TGF-β/Smad3 signaling pathway in human breast cancer MCF-7 cells. Int J Oncol. 2015;46:1730–1738. doi: 10.3892/ijo.2015.2870. [DOI] [PubMed] [Google Scholar]
- 71.Ahire V, Kumar A, Mishra KP, Kulkarni G. Ellagic acid enhances apoptotic sensitivity of breast cancer cells to γ-radiation. Nutr Cancer. 2017;69:904–910. doi: 10.1080/01635581.2017.1339811. [DOI] [PubMed] [Google Scholar]
- 72.Cook MT, Liang Y, Besch-Williford C, Goyette S, Mafuvadze B, Hyder SM. Luteolin inhibits progestin-dependent angiogenesis, stem cell-like characteristics, and growth of human breast cancer xenografts. Springerplus. 2015;4:1–16. doi: 10.1186/s40064-015-1242-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang Z, Wang D, Han S, Wang N, Mo F, Loo TY, et al. Bioactivity-guided identification and cell signaling technology to delineate the lactate dehydrogenase A inhibition effects of spatholobus suberectus on breast cancer. PLoS One. 2013;8:1–12. doi: 10.1371/journal.pone.0056631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen D, Pamu S, Cui Q, Chan TH, Dou QP. Novel epigallocatechin gallate (EGCG) analogs activate AMP-activated protein kinase pathway and target cancer stem cells. Bioorg Med Chem. 2012;20:3031–3037. doi: 10.1016/j.bmc.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pan X, Zhao B, Song Z, Han S, Wang M. Estrogen receptor-alpha36 is involved in epigallocatechin-3-gallate induced growth inhibition of ER-negative breast cancer stem/progenitor cells. J Pharmacol Sci. 2016;130:85–93. doi: 10.1016/j.jphs.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 76.Farabegoli F, Govoni M, Spisni E, Papi A. EGFR inhibition by epigallocatechin-3-gallate and IIF treatments reduces breast cancer cell invasion. Biosci Rep. 2017;37:1–14. doi: 10.1042/BSR20170168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.El-Rahman SSA, Shehab G, Nashaat H. Epigallocatechin-3-gallate: the prospective targeting of cscs and preventing metastasis of chemically-induced mammary cancer in rats. Am J Med Sci. 2017;354:54–63. doi: 10.1016/j.amjms.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 78.Giro-Perafita A, Rabionet M, Planas M, Feliu L, Ciurana J, Ruiz-Martinez S, et al. EGCG-derivative G28 Shows high efficacy inhibiting the mammosphere-forming capacity of sensitive and resistant TNBC models. Molecules. 2019:24;1–15. doi: 10.3390/molecules24061027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jiang D, Rasul A, Batool R, Sarfraz I, Hussain G, Mateen Tahir M, et al. Potential anticancer properties and mechanisms of action of formononetin. Biomed Res Int. 2019;2019:1–11. doi: 10.1155/2019/5854315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Montales MTE, Rahal OM, Kang J, Rogers T, Prior RL, Wu X, et al. Repression of mammosphere formation of human breast cancer cells by soy isoflavone genistein and blueberry polyphenolic acids suggests diet-mediated targeting of cancer stem-like/progenitor cells. Carcinogenesis. 2012;33:652–660. doi: 10.1093/carcin/bgr317. [DOI] [PubMed] [Google Scholar]
- 81.Montales MTE, Rahal OM, Nakatani H, Matsuda T, Simmen RC. Repression of mammary adipogenesis by genistein limits mammosphere formation of human MCF-7 cells. J Endocrinol. 2013;218:135–149. doi: 10.1530/JOE-12-0520. [DOI] [PubMed] [Google Scholar]
- 82.Liu Y, Zou T, Wang S, Chen H, Su D, Fu X, et al. Genistein-induced differentiation of breast cancer stem/progenitor cells through a paracrine mechanism. Int J Oncol. 2016;48:1063–1072. doi: 10.3892/ijo.2016.3351. [DOI] [PubMed] [Google Scholar]
- 83.Rigalli J, Tocchetti G, Arana M, Villanueva S, Catania V, Theile D, et al. The phytoestrogen genistein enhances multidrug resistance in breast cancer cell lines by translational regulation of ABC transporters. Cancer lett. 2016;376:165–172. doi: 10.1016/j.canlet.2016.03.040. [DOI] [PubMed] [Google Scholar]
- 84.Fan P, Fan S, Wang H, Mao J, Shi Y, Ibrahim MM, et al. Genistein decreases the breast cancer stem-like cell population through hedgehog pathway. Stem Cell Res Ther. 2013;4:1–10. doi: 10.1186/scrt357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Paul B, Li Y, Tollefsbol TO. The effects of combinatorial genistein and sulforaphane in breast tumor inhibition: role in epigenetic regulation. Int J mole sci. 2018;19:1–22. doi: 10.3390/ijms19061754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wu C-H, Hong B-H, Ho C-T, Yen G-C. Targeting cancer stem cells in breast cancer: potential anticancer properties of 6-shogaol and pterostilbene. J Agr Food Chem. 2015;63:2432–2441. doi: 10.1021/acs.jafc.5b00002. [DOI] [PubMed] [Google Scholar]
- 87.Lee HS, Seo EY, Kang NE, Kim WK. [6]-Gingerol inhibits metastasis of MDA-MB-231 human breast cancer cells. J Nutr Biochem. 2008;19:313–319. doi: 10.1016/j.jnutbio.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 88.Wang Z, Zhang X, Wang H, Qi L, Lou Y. Neuroprotective effects of icaritin against beta amyloid-induced neurotoxicity in primary cultured rat neuronal cells via estrogen-dependent pathway. Neuroscience. 2007;145:911–922. doi: 10.1016/j.neuroscience.2006.12.059. [DOI] [PubMed] [Google Scholar]
- 89.Wo Yb, Zhu Dy, Hu Y, Wang ZQ, Liu J, Lou YJ. Reactive oxygen species involved in prenylflavonoids, icariin and icaritin, initiating cardiac differentiation of mouse embryonic stem cells. J Cell Biochem. 2008;103:1536–1550. doi: 10.1002/jcb.21541. [DOI] [PubMed] [Google Scholar]
- 90.Zhang G, Qin L, Sheng H, Wang X-L, Wang Y-X, Yeung DK-W, et al. A novel semisynthesized small molecule icaritin reduces incidence of steroid-associated osteonecrosis with inhibition of both thrombosis and lipid-deposition in a dose-dependent manner. Bone. 2009;44:345–356. doi: 10.1016/j.bone.2008.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Huang J, Yuan L, Wang X, Zhang T-L, Wang K. Icaritin and its glycosides enhance osteoblastic, but suppress osteoclastic, differentiation and activity in vitro. Life Sci. 2007;81:832–840. doi: 10.1016/j.lfs.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 92.Huang X, Zhu D, Lou Y. A novel anticancer agent, icaritin, induced cell growth inhibition, G 1 arrest and mitochondrial transmembrane potential drop in human prostate carcinoma PC-3 cells. Eur J Pharmacol. 2007;564:26–36. doi: 10.1016/j.ejphar.2007.02.039. [DOI] [PubMed] [Google Scholar]
- 93.Li C, Peng W, Song X, Wang Q, Wang W. Anticancer effect of icaritin inhibits cell growth of colon cancer through reactive oxygen species, Bcl-2 and cyclin D1/E signaling. Oncol Lett. 2016;12:3537–3542. doi: 10.3892/ol.2016.5089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Guo Y, Zhang X, Meng J, Wang Z-Y. An anticancer agent icaritin induces sustained activation of the extracellular signal-regulated kinase (ERK) pathway and inhibits growth of breast cancer cells. Eur J Pharmacol. 2011;658:114–122. doi: 10.1016/j.ejphar.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang X, Zheng N, Dong J, Wang X, Liu L, Huang J. Estrogen receptor-α36 is involved in icaritin induced growth inhibition of triple-negative breast cancer cells. J Steroid Biochem Mol Biol. 2017;171:318–327. doi: 10.1016/j.jsbmb.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 96.Kang T, Seo J, Oh H, Yoon G, Chae J, Shim J. Licochalcone A suppresses specificity protein 1 as a novel target in human breast cancer cells. J Cell Biochem. 2017;118:4652–4663. doi: 10.1002/jcb.26131. [DOI] [PubMed] [Google Scholar]
- 97.Beishline K, Azizkhan-Clifford J. Sp1 and the hallmarks of cancer. FEBS J. 2015;282:224–258. doi: 10.1111/febs.13148. [DOI] [PubMed] [Google Scholar]
- 98.Kwon SJ, Park SY, Kwon GT, Lee KW, Kang Y-H, Choi M-S, et al. Licochalcone E present in licorice suppresses lung metastasis in the 4T1 mammary orthotopic cancer model. Cancer Prev Res. 2013;6:603–613. doi: 10.1158/1940-6207.CAPR-13-0012. [DOI] [PubMed] [Google Scholar]
- 99.Empey D, Laitinen L, Young G, Bye C, Hughes D. Comparison of the antitussive effects of codeine phosphate 20 mg, dextromethorphan 30 mg and noscapine 30 mg using citric acid-induced cough in normal subjects. Eur J Clin Pharmacol. 1979;16:393–397. doi: 10.1007/BF00568199. [DOI] [PubMed] [Google Scholar]
- 100.DeBono A, Capuano B, Scammells PJ. Progress toward the development of noscapine and derivatives as anticancer agents. J Med Chem. 2015;58:5699–5727. doi: 10.1021/jm501180v. [DOI] [PubMed] [Google Scholar]
- 101.Sajadian S, Vatankhah M, Majdzadeh M, Kouhsari SM, Ghahremani MH, Ostad SN. Cell cycle arrest and apoptogenic properties of opium alkaloids noscapine and papaverine on breast cancer stem cells. Toxicol Mech Methods. 2015;25:388–395. doi: 10.3109/15376516.2015.1045656. [DOI] [PubMed] [Google Scholar]
- 102.Doddapaneni R, Patel K, Chowdhury N, Sachdeva M. Noscapine chemosensitization enhances docetaxel anticancer activity and tumor stroma disruption against triple negative breast cancer. AACR. 2016;346:65–73. doi: 10.1016/j.yexcr.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Quisbert-Valenzuela EO, Calaf GM. Apoptotic effect of noscapine in breast cancer cell lines. International J Oncol. 2016;48:2666–2674. doi: 10.3892/ijo.2016.3476. [DOI] [PubMed] [Google Scholar]
- 104.Mahaddalkar T, Manchukonda N, Choudhary S, Cheriyamundath S, Mohanpuria N, Kantevari S, et al. Subtle alterations in microtubule assembly dynamics by Br-TMB-noscapine strongly suppress Triple-negative breast cancer cell viability without mitotic arrest. ChemistrySelect. 2016;1:4313–4319. [Google Scholar]
- 105.Muthiah D, Henshaw GK, DeBono AJ, Capuano B, Scammells PJ, Callaghan R. Overcoming p-glycoprotein-mediated drug resistance with noscapine derivatives. Drug Metab Dispos. 2019;47:164–172. doi: 10.1124/dmd.118.083188. [DOI] [PubMed] [Google Scholar]
- 106.Lu LG, Zeng MD, Mao YM, Fang JY, Song YL, Shen ZH, et al. Inhibitory effect of oxymatrine on serum hepatitis B virus DNA in HBV transgenic mice. World J gastroenterol. 2004;10:1176–1179. doi: 10.3748/wjg.v10.i8.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zheng P, Niu FL, Liu WZ, Shi Y, Lu LG. Anti-inflammatory mechanism of oxymatrine in dextran sulfate sodium-induced colitis of rats. World J Gastroenterol. 2005;11:4912–4915. doi: 10.3748/wjg.v11.i31.4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang M, Huang J. Recent research progress of anti-tumor mechnism matrine. Zhongguo Zhong Yao Za Zhi. 2004;29:115–118. [PubMed] [Google Scholar]
- 109.Jin Y, Hu J, Wang Q, Li Z, Chen Y. Effects of oxymatrine on the apoptosis of human esophageal carcinoma Eca109 cell line and its mechanism. J Huazhong U Sci Med. 2008;28:314–316. doi: 10.1007/s11596-008-0319-y. [DOI] [PubMed] [Google Scholar]
- 110.Zhang Y, Piao B, Zhang Y, Hua B, Hou W, Xu W, et al. Oxymatrine diminishes the side population and inhibits the expression of β-catenin in MCF-7 breast cancer cells. Med Oncol. 2011;28:99–107. doi: 10.1007/s12032-010-9721-y. [DOI] [PubMed] [Google Scholar]
- 111.Xie W, Zhang Y, Zhang S, Wang F, Zhang K, Huang Y, et al. Oxymatrine enhanced anti-tumor effects of bevacizumab against triple-negative breast cancer via abating Wnt/beta-catenin signaling pathway. Am J Cancer Res. 2019;9:1796–1814. [PMC free article] [PubMed] [Google Scholar]
- 112.Chen Y, Chen L, Zhang JY, Chen ZY, Liu TT, Zhang YY, et al. Oxymatrine reverses epithelial-mesenchymal transition in breast cancer cells by depressing alphabeta3 integrin/FAK/PI3K/Akt signaling activation. Onco Targets Ther. 2019;12:6253–6265. doi: 10.2147/OTT.S209056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wu J, Cai Y, Li M, Zhang Y, Li H, Tan Z. Oxymatrine promotes S-phase arrest and inhibits cell proliferation of human breast cancer cells in vitro through mitochondria-mediated apoptosis. Biol Pharm Bull. 2017;40:1232–1239. doi: 10.1248/bpb.b17-00010. [DOI] [PubMed] [Google Scholar]
- 114.Katiyar SS, Muntimadugu E, Rafeeqi TA, Domb AJ, Khan W. Co-delivery of rapamycin-and piperine-loaded polymeric nanoparticles for breast cancer treatment. Drug Deliv. 2016;23:2608–2616. doi: 10.3109/10717544.2015.1039667. [DOI] [PubMed] [Google Scholar]
- 115.Talib WH. Regressions of breast carcinoma syngraft following treatment with piperine in combination with thymoquinone. Sci Pharm. 2017;85:1–11. doi: 10.3390/scipharm85030027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lai LH, Fu QH, Liu Y, Jiang K, Guo QM, Chen QY, et al. Piperine suppresses tumor growth and metastasis in vitro and in vivo in a 4T1 murine breast cancer model. Acta Pharmacol Sin. 2012;33:523–530. doi: 10.1038/aps.2011.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Do MT, Kim HG, Choi JH, Khanal T, Park BH, Tran TP, et al. Antitumor efficacy of piperine in the treatment of human HER2-overexpressing breast cancer cells. Food Chem. 2013;141:2591–2599. doi: 10.1016/j.foodchem.2013.04.125. [DOI] [PubMed] [Google Scholar]
- 118.Jain S, Meka SRK, Chatterjee K. Engineering a piperine eluting nanofibrous patch for cancer treatment. ACS Biomater Sci Eng. 2016;2:1376–1385. doi: 10.1021/acsbiomaterials.6b00297. [DOI] [PubMed] [Google Scholar]
- 119.Greenshields AL, Doucette CD, Sutton KM, Madera L, Annan H, Yaffe PB, et al. Piperine inhibits the growth and motility of triple-negative breast cancer cells. Cancer Lett. 2015;357:129–140. doi: 10.1016/j.canlet.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 120.Abdelhamed S, Yokoyama S, Refaat A, Ogura K, Yagita H, Awale S, et al. Piperine enhances the efficacy of TRAIL-based therapy for triple-negative breast cancer cells. Anticancer Res. 2014;34:1893–1899. [PubMed] [Google Scholar]
- 121.Hagiwara K, Kosaka N, Yoshioka Y, Takahashi RU, Takeshita F, Ochiya T. Stilbene derivatives promote Ago2-dependent tumour-suppressive microRNA activity. Sci Rep. 2012;2:1–9. doi: 10.1038/srep00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mak KK, Wu AT, Lee WH, Chang TC, Chiou JF, Wang LS, et al. Pterostilbene, a bioactive component of blueberries, suppresses the generation of breast cancer stem cells within tumor microenvironment and metastasis via modulating NF-κB/microRNA 448 circuit. Mol Nutr Food Res. 2013;57:1123–1134. doi: 10.1002/mnfr.201200549. [DOI] [PubMed] [Google Scholar]
- 123.Ignatowicz E, Baer-Dubowska W. Resveratrol, a natural chemopreventive agent against degenerative diseases. Pol J Pharmacol. 2001;53:557–569. [PubMed] [Google Scholar]
- 124.Muqbil I, WJ Beck F, Bao B, H Sarkar F, M Mohammad R, M Hadi S, et al. Old wine in a new bottle: the Warburg effect and anticancer mechanisms of resveratrol. Curr Pharm Des. 2012;18:1645–1654. doi: 10.2174/138161212799958567. [DOI] [PubMed] [Google Scholar]
- 125.Venkatadri R, Muni T, Iyer AKV, Yakisich JS, Azad N. Role of apoptosis-related miRNAs in resveratrol-induced breast cancer cell death. Cell Death Dis. 2016;7:1–12. doi: 10.1038/cddis.2016.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kim YN, Choe SR, Cho KH, Cho DY, Kang J, Park CG, et al. Resveratrol suppresses breast cancer cell invasion by inactivating a RhoA/YAP signaling axis. Exp Mol Med. 2017;49:1–9. doi: 10.1038/emm.2016.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Venkatadri R, Iyer AKV, Kaushik V, Azad N. A novel resveratrol–salinomycin combination sensitizes ER-positive breast cancer cells to apoptosis. Pharmacol Rep. 2017;69:788–797. doi: 10.1016/j.pharep.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 128.Medina-Aguilar R, Marchat LA, Arechaga Ocampo E, Gariglio P, García Mena J, Villegas Sepúlveda N, et al. Resveratrol inhibits cell cycle progression by targeting Aurora kinase A and Polo-like kinase 1 in breast cancer cells. Oncol Rep. 2016;35:3696–3704. doi: 10.3892/or.2016.4728. [DOI] [PubMed] [Google Scholar]
- 129.Suh J, Kim DH, Surh YJ. Resveratrol suppresses migration, invasion and stemness of human breast cancer cells by interfering with tumor-stromal cross-talk. Arch Biochem Biophys. 2018;643:62–71. doi: 10.1016/j.abb.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 130.Zhang Y. Cancer-preventive isothiocyanates: measurement of human exposure and mechanism of action. Mutat Res/Fund Mol M. 2004;555:173–190. doi: 10.1016/j.mrfmmm.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 131.Singh SV, Singh K. Cancer chemoprevention with dietary isothiocyanates mature for clinical translational research. Carcinogenesis. 2012;33:1833–1842. doi: 10.1093/carcin/bgs216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li Y, Zhang T. Targeting cancer stem cells with sulforaphane, a dietary component from broccoli and broccoli sprouts. Future Oncol. 2013;9:1097–1103. doi: 10.2217/fon.13.108. [DOI] [PubMed] [Google Scholar]
- 133.Li Y, Zhang T, Korkaya H, Liu S, Lee HF, Newman B, et al. Sulforaphane, a dietary component of broccoli/Broccoli sprouts, inhibits breast cancer stem cells. Clin Cancer Res. 2010;16:2580–2590. doi: 10.1158/1078-0432.CCR-09-2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Royston KJ, Udayakumar N, Lewis K, Tollefsbol TO. A novel combination of withaferin A and sulforaphane inhibits epigenetic machinery, cellular viability and induces apoptosis of breast cancer cells. Int J Mol Sci. 2017;18:1–17. doi: 10.3390/ijms18051092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Keshandehghan A, Nikkhah S, Tahermansouri H, Heidari-Keshel S, Gardaneh M. Co-treatment with sulforaphane and nano-metformin molecules accelerates apoptosis in HER2+ breast cancer cells by inhibiting key molecules. Nutr Cancer. 2019:1–14. doi: 10.1080/01635581.2019.1655073. [DOI] [PubMed] [Google Scholar]
- 136.Burnett JP, Lim G, Li Y, Shah RB, Lim R, Paholak HJ, et al. Sulforaphane enhances the anticancer activity of taxanes against triple negative breast cancer by killing cancer stem cells. Cancer Lett. 2017;394:52–64. doi: 10.1016/j.canlet.2017.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Castro NP, Rangel MC, Merchant AS, MacKinnon G, Cuttitta F, Salomon DS, et al. Sulforaphane suppresses the growth of triple-negative breast cancer stem-like cells in vitro and in vivo. Cancer Prev Res. 2019;12:147–158. doi: 10.1158/1940-6207.CAPR-18-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Huang J, Tao C, Yu Y, Yu F, Zhang H, Gao J, et al. Simultaneous targeting of differentiated breast cancer cells and breast cancer stem cells by combination of docetaxel-and sulforaphane-loaded self-assembled Poly (D, L-lactide-co-glycolide)/Hyaluronic acid block copolymer-based nanoparticles. J Biomed Nanotechnol. 2016;12:1463–1477. doi: 10.1166/jbn.2016.2234. [DOI] [PubMed] [Google Scholar]
- 139.Woo CC, Kumar AP, Sethi G, Tan KHB. Thymoquinone: potential cure for inflammatory disorders and cancer. Biochem Pharmacol. 2012;83:443–451. doi: 10.1016/j.bcp.2011.09.029. [DOI] [PubMed] [Google Scholar]
- 140.Linjawi SA, Khalil WK, Hassanane MM, Ahmed ES. Evaluation of the protective effect of Nigella sativa extract and its primary active component thymoquinone against DMBA-induced breast cancer in female rats. Arch Med Sci: AMS. 2015;11:220–229. doi: 10.5114/aoms.2013.33329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Woo CC, Hsu A, Kumar AP, Sethi G, Tan KHB. Thymoquinone inhibits tumor growth and induces apoptosis in a breast cancer xenograft mouse model: the role of p38 MAPK and ROS. PLoS One. 2013;8:1–14. doi: 10.1371/journal.pone.0075356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sutton KM, Greenshields AL, Hoskin DW. Thymoquinone, a bioactive component of black caraway seeds, causes G1 phase cell cycle arrest and apoptosis in triple-negative breast cancer cells with mutant p53. Nutr Cancer. 2014;66:408–418. doi: 10.1080/01635581.2013.878739. [DOI] [PubMed] [Google Scholar]
- 143.Rajput S, Kumar BP, Sarkar S, Das S, Azab B, Santhekadur PK, et al. Targeted apoptotic effects of thymoquinone and tamoxifen on XIAP mediated Akt regulation in breast cancer. PLoS One. 2013;8:1–13. doi: 10.1371/journal.pone.0061342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bashmail HA, Alamoudi AA, Noorwali A, Hegazy GA, G AJ, Choudhry H, et al. Thymoquinone synergizes gemcitabine anti-breast cancer activity via modulating its apoptotic and autophagic activities. Sci Rep. 2018;8:1–11. doi: 10.1038/s41598-018-30046-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kabil NN, Bayraktar R, Kahraman N, Ozpolat B. Thymoquinone inhibits elongation factor 2 kinase signaling axis by inducing tumor suppressor miR-603 in triple negative breast cancer cells. AACR . 2017;2017:1–5. [Google Scholar]
- 146.Shanmugam MK, Ahn KS, Hsu A, Woo CC, Yuan Y, Tan KHB, et al. Thymoquinone inhibits bone metastasis of breast cancer cells through abrogation of the CXCR4 signaling axis. Front Pharmacol. 2018;9:1–15. doi: 10.3389/fphar.2018.01294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pasupuleti VR, Sammugam L, Ramesh N, Gan SH. Honey, propolis, and royal jelly: a comprehensive review of their biological actions and health benefits. Oxid Med Cell Longev. 2017;2017:1–21. doi: 10.1155/2017/1259510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Nagai T, Inoue R. Preparation and the functional properties of water extract and alkaline extract of royal jelly. Food Chem. 2004;84:181–186. [Google Scholar]
- 149.Ramadan MF, Al-Ghamdi A. Bioactive compounds and health-promoting properties of royal jelly: a review. J Funct Foods. 2012;4:39–52. [Google Scholar]
- 150.Khazaei M, Ansarian A, Ghanbari E. New findings on biological actions and clinical applications of royal jelly: a review. J Diet Suppl. 2018;15:757–775. doi: 10.1080/19390211.2017.1363843. [DOI] [PubMed] [Google Scholar]
- 151.Sugiyama T, Takahashi K, Mori H. Royal jelly acid, 10-hydroxy-trans-2-decenoic acid, as a modulator of the innate immune responses. Endocr Metab Immune Dis Drug Targets. 2012;12:368–376. doi: 10.2174/187153012803832530. [DOI] [PubMed] [Google Scholar]
- 152.Badr G, Sayed EA, Waly H, Hassan KA, Mahmoud MH, Selamoglu Z. The therapeutic mechanisms of propolis against ccl4 -mediated liver injury by mediating apoptosis of activated hepatic stellate cells and improving the hepatic architecture through PI3K/AKT/mTOR, TGF-beta/Smad2, Bcl2/BAX/P53 and iNOS Signaling Pathways. Cell Physiol Biochem. 2019;53:301–322. doi: 10.33594/000000140. [DOI] [PubMed] [Google Scholar]
- 153.Biesalski HK, Dragsted LO, Elmadfa I, Grossklaus R, Muller M, Schrenk D, et al. Bioactive compounds: definition and assessment of activity. Nutrition. 2009;25:1202–1205. doi: 10.1016/j.nut.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 154.Izuta H, Chikaraishi Y, Shimazawa M, Mishima S, Hara H. 10-Hydroxy-2-decenoic acid, a major fatty acid from royal jelly, inhibits VEGF-induced angiogenesis in human umbilical vein endothelial cells. Evid-Based Complement Alternat Med. 2009;6:489–494. doi: 10.1093/ecam/nem152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gismondi A, Trionfera E, Canuti L, Di Marco G, Canini A. Royal jelly lipophilic fraction induces antiproliferative effects on SH-SY5Y human neuroblastoma cells. Oncol Rep. 2017;38:1833–1844. doi: 10.3892/or.2017.5851. [DOI] [PubMed] [Google Scholar]
- 156.Zhang S, Shao Q, Shen Z, Su S. Immunomodulatory response of 4T1 murine breast cancer model to camellia royal jelly. Biomed Res. 2017;28:1223–1230. [Google Scholar]
- 157.Nageen B, Sarfraz I, Rasul A, Hussain G, Rukhsar F, Irshad S, et al. Eupatilin: a natural pharmacologically active flavone compound with its wide range applications. J Asian Nat Prod Res. 2020;22:1–16. doi: 10.1080/10286020.2018.1492565. [DOI] [PubMed] [Google Scholar]
- 158.Sarfraz I, Rasul A, Hussain G, Hussain SM, Ahmad M, Nageen B, et al. Malic enzyme 2 as a potential therapeutic drug target for cancer. IUBMB life. 2018;70:1076–1083. doi: 10.1002/iub.1930. [DOI] [PubMed] [Google Scholar]
- 159.Sharif F, Rasul A, Ashraf A, Hussain G, Younis T, Sarfraz I, et al. Phosphoglycerate mutase 1 in cancer: a promising target for diagnosis and therapy. IUBMB life. 2019;71:1418–1427. doi: 10.1002/iub.2100. [DOI] [PubMed] [Google Scholar]