Abstract
Backgound:
Bacterial or viral infections often cause acute and severe systemic inflammation, which affects the lungs lipopolysaccharide (LPS), a pathogenic component of the membrane of gram-negative bacteria, stimulates active innate immune cells, monocytes, macrophages to produce inducible nitric oxide synthase (iNOS). Excess production of this compound occurs in COVID-19 resulting in inflammatory cascade and thromboembolism. We intend to propose the use of sildenafil to reduce this production.
Method:
The analysis of biochemical pathways shows that viral infection produces a high amount of nitric oxide (NO), with an acute inflammatory process.
Results:
In the case of COVID-19 infection we verified that numerous biochemical processes activate a cascade of inflammatory processes through the activation of iNOS with uncontrolled generation of (NO).
Conclusions:
iNOS is the cause of damage to host cells with a consequent pulmonary thromboembolic lung phenomenon in a contest of interstitial pneumonia. This study proposes the use of sildenafil to counter the inflammatory cascade and thromboembolic episodes.
Keywords: COVID-19, nitric oxide, sildenafil citrate
Introduction
Recent observations suggest that respiratory failure in patients with COVID-19 is not driven by the development of acute respiratory distress syndrome alone, but that microvascular thrombotic processes may play a role. This may have important consequences for the diagnostic and therapeutic management of these patients. Pathological and imaging investigations have confirmed that COVID-19 syndrome is a thrombo-inflammatory process that initially affects pulmonary perfusion, but consecutively affects all organs in the body.[1] (NO) is a biomarker of nitrosative stress, involved in the pathogenesis of interstitial pneumonia (IP). A recent study[2] shows that the evaluation of exhaled NO levels (NO Es) in patients with IP correlates alveolar concentrations of NO (CalvNO) with exhaled fractionated of (NO). Patients with IP had higher, (NO Es), and higher CalvNO levels than healthy controls The treatment of patients with interstitial pulmonary diseases at risk of pulmonary hypertension, with a process of inhalation of NO[3] produced significant improvements in activity that were maintained over the long term. This study shows that the administration of NO improved moderate to vigorous physical activity by 34% compared to placebo as well as the long-term maintenance of the main parameters of motor and respiratory activity. These results suggest that NO inhalation is a potentially effective treatment option for patients at risk of pulmonary hypertension, which is associated with poor results in various forms of interstitial lung disease. This study proposes the use of sildenafil to counter the inflammatory cascade and thromboembolic episodes.
Our study method analyses the biochemical processes leading to inflammatory cascade and thromboembolic complications on the basis of the literature on PUBMED and to check whether this results from excessive NO production by inducible NO Synthase.
Results
Our study and/or proposal starts from the concept that the so-called inflammatory cascade can be derived from a breakdown of cellular metabolism with the role of AMPK induced by cytokines that disturb the balance of cellular metabolism, to promote a high permeability of vessels and tissue injury. Inhibiting the production of inflammatory cytokines is an important strategy for the treatment of inflammatory diseases. The AMP-activated protein kinase (AMPK), a metabolically sensitive serin/threonine protein kinase, is composed of α, β, and γ subunits and is known as the main metabolic regulator of cellular homeostasis. This activity is mainly regulated by the AMP/ATP ratio in cells.[4] AMPK is also a negative mediator of inflammation. AMPK improves inflammation and inflammation-associated diseases by inhibiting the nuclear potentiating factor of the light chain kappa of activated B cells (NF-κB) that targets gene expression in vivo and in vitro. To activate of the erythroid-factor 2 (Nrf2) related anti-oxidative pathway by AMPK is the rule of LPS-induced macrophage inflammation. Studies showed that AMPK has a connection to other inflammatory signalling pathways, including Janus kinase (JAK)/signal transducer and transcription 3 activator STAT3.[5] We can much more simply schematize the principal stages of this process with the proposed diagram below where sGC is soluble guanylate cyclase, and cGMP is guanosine monophosphate cyclic [Figure 1].
Figure 1.
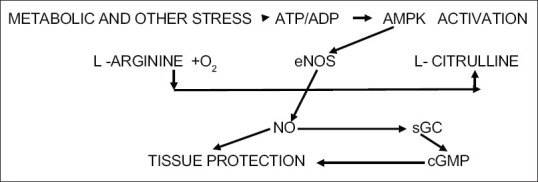
Metabolic pathways for no equilibrium restoration by activation of endothelial no synthase
In the above figure we can clearly see the very close link between the activation of AMPK and the production of eNOS (Nitric oxide endothelial), the natural and necessary NO compound. Furthermore, AMPK inhibits the synthesis of iNOS that by such increase in its concentration, it becomes one of the main parameters responsible for the inflammatory cascade. We can schematize this process as in Figure 2. Therefore the reduction of iNOS and the simultaneous activation of (eNOS) would lead to the elimination of the inflammatory cascade. Our study hypothesis is to use sildanafil viagra, so as to also have a reduction of thrombotic effects that appear in the radiological pictures of COVID patients and can be traced back to a process of the type illustrated in Figure 3. In fact we know that sildanafil inhibits iNOS and numerous studies show the process to inhibit hemodynamic effects of inducible NO synthase, with sildenafil during acute pulmonary embolism. This leads us to propose the use of sildenafil in this disease although practical studies on the use of sildenafil in COVID-1 have not yet been carried out It is also important to underline the importance of inactivating the production of iNOS and thus activating and increasing the concentration of AMPK, because this leads to an improvement in the autophagy process via mTOR This clearly leads to an improvement in the disposal of toxic products in COVID disease metabolism.
Figure 2.
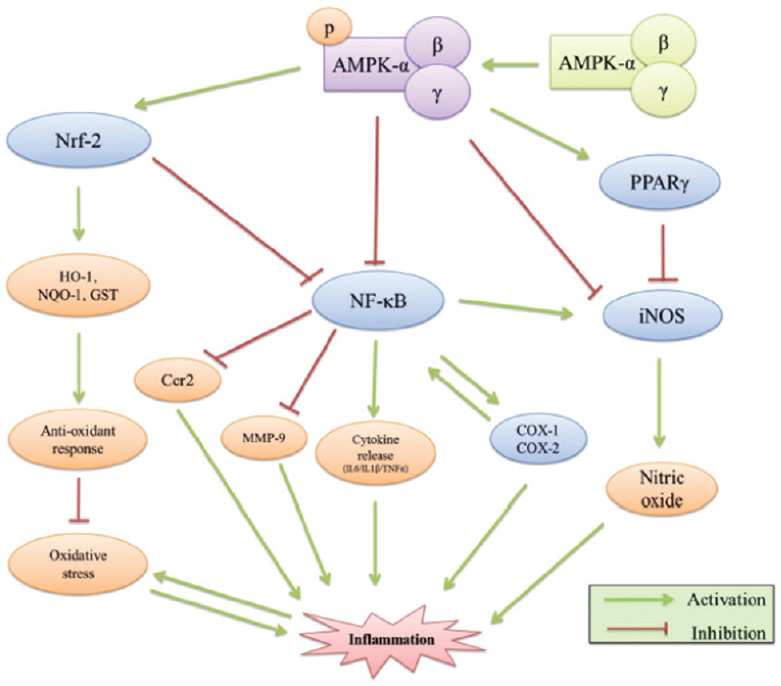
AMPK in inflammatory cascade
Figure 3.
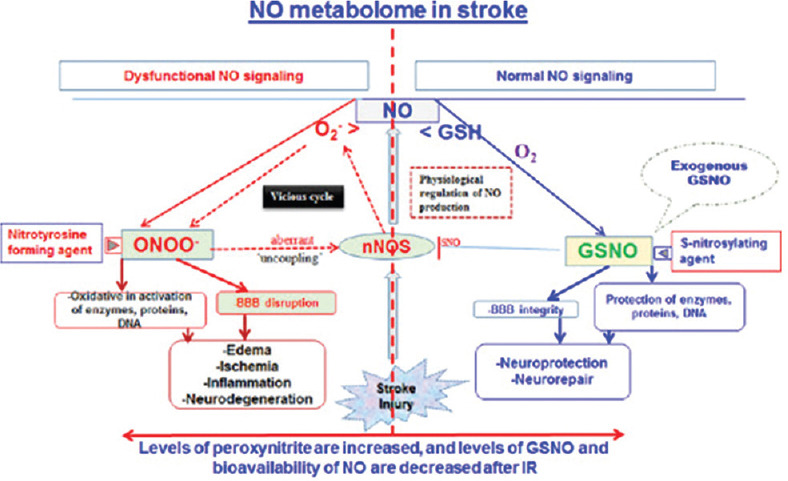
Biochemical pathways of no in thromboembolisms
Conclusions
iNOS is the cause of damage to host cells with a consequent pulmonary thromboembolic lung phenomenon in a contest of interstitial pneumonia. This study proposes the use of sildenafil to counter the inflammatory cascade and thromboembolic episodes.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.New Research Highlights Risk of Thromboembolic Complications in Patients With COVID-19. ASCO Post; 4/30/2020 [Google Scholar]
- 2.Pilon G, Dallaire P, Marette AJ. Inhibition of inducible nitric-oxide synthase by activators of AMP-activated protein kinase: A new mechanism of action of insulin-sensitizing drugs. Biol Chem. 2004;279:20767–74. doi: 10.1074/jbc.M401390200. [DOI] [PubMed] [Google Scholar]
- 3.Dias-Junior CA, Neto-Neves EM, Montenegro MF, Tanus-Santos JE. Hemodynamic effects of inducible nitric oxide synthase inhibition combined with sildenafil during acute pulmonary embolism. Nitric Oxide. 2010;23:284–8. doi: 10.1016/j.niox.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Viollet B, Andreelli F. AMP-activated protein kinase and metabolic control? Handb Exp Pharmacol. 2011:303–30. doi: 10.1007/978-3-642-17214-4_13. doi: 10.1007/978-3-642-17214-4_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gong H, Tai H, Huang N, Xiao P, Mo C, Wang X, et al. Nrf2-SHP cascade-mediated STAT3 inactivation contributes to AMPK-driven protection against endotoxic inflammation. Front Immunol. 2020;11:414. doi: 10.3389/fimmu.2020.00414. [DOI] [PMC free article] [PubMed] [Google Scholar]


