Abstract
Lung perfusion scintigraphy is done as a part of preoperative evaluation in lung cancer patients for the prediction of postoperative forced expiratory volume in the first second (FEV1). This study was performed to see the accuracy of prediction of postoperative FEV1 by perfusion scintigraphy for patients undergoing lobectomy/pneumonectomy by comparing it with actual postoperative FEV1 obtained by spirometry 4–6 months after surgery. We retrospectively reviewed 50 surgically resected lung cancer patients who underwent preoperative spirometry, lung perfusion study, and postoperative spirometry. Pearson’s correlation coefficient was used to evaluate the relationship between predicted postoperative FEV1 (PPO FEV1) by lung perfusion scintigraphy and postoperative actual FEV1 measured by spirometry. Agreement between the two methods was analyzed with Bland–Altman method. The correlation between the PPO FEV1 and actual postoperative FEV1 was statistically significant (r = 0.847, P = 0.000). The correlation was better for pneumonectomy compared to lobectomy (r = 0.930 [P = 0.000] vs. 0.792 [P = 0.000]). The agreement analysis showed a mean difference of −0.0558 with a standard deviation (SD) of 0.284. The limits of agreement vary over a wide range from −−0.625 to 0.513 L (mean ± 2 SD) for the entire group. For pneumonectomy, the mean difference was −0.0121 and SD 0.169 with limits of agreement varying between −0.30 L and 0.30 L. For lobectomy, the mean difference was −0.0826 and SD 0.336 with limits of agreement varying between −0.755 L and 0.590 L. Postoperative FEV1 predicted using lung perfusion scintigraphy shows good correlation with actual postoperative FEV1 and shows reasonably good agreement in patients undergoing pneumonectomy. The limits of agreement appear to be clinically unacceptable in patients undergoing lobectomy, where single-photon emission computed tomography (SPECT) or SPECT/CT techniques may improve prediction.
Keywords: Lung perfusion scintigraphy, observed forced expiratory volume in the first second, predicted postoperative forced expiratory volume in the first second
INTRODUCTION
Lung cancer is the most common cancer in the world and is the leading contributor to cancer-related deaths.[1] It is increasingly being recognized in India and is ranked fourth in terms of incidence.[2] Curative surgical resection remains the best therapy for patients with localized non small cell lung cancer, and commonly performed operations include lobectomy and pneumonectomy.[3] Prior to surgical resection, investigations are carried out to evaluate the respiratory sufficiency. The aim of preoperative pulmonary assessment is to identify patients who are at increased risk of having perioperative complications and long-term disability from surgical resection of lung cancer using the least invasive tests available.[4] The incidence of complications varies depending on the extent of resection, the pulmonary reserve, and the presence of any comorbid conditions.
Spirometry and the diffusing capacity of lung for carbon monoxide (DLCO) are the commonly performed studies for the evaluation of lung function.[4,5] Forced expiratory volume in the first second (FEV1) obtained from spirometry is widely used to assess respiratory sufficiency in clinical practice. Patients with FEV1 values >1500 ml for lobectomy and 2000 ml for pneumonectomy, or values >80% predicted for gender, age, height, and body mass are considered to have low risk of complications.[4,6] When the values are below this, it suggests an increased risk of perioperative complications and demands investigations predicting postoperative FEV1 value.[7] Predicted postoperative FEV1 (PPO FEV1) has been shown to be an independent predictor of perioperative mortality and morbidity.[8] When PPO FEV1 is >800–1000 ml, the risk of complications following surgical resection is considered low.[6,9] PPO FEV1 <40% predicted is considered high risk for perioperative complications.[4,10] The assessment of regional lung function forms an integral part of the preoperative evaluation of patients.
Methods mostly used for the prediction of postoperative FEV1 include anatomic calculation (segment counting), radionuclide perfusion/ventilation imaging, quantitative computed tomography (CT), and dynamic perfusion magnetic resonance imaging (MRI). Anatomic segment counting assumes that each segment contributes equally to the total pulmonary function, but the functional contribution of each segment can vary among patients, especially with underlying interstitial lung disease or emphysema.[11] In patients with completely obstructed airways, the underlying lung may be totally nonfunctional. In such cases, anatomical counting tends to underestimate residual lung function. The quantitative CT though provides more anatomical details, cannot account for regional perfusion heterogeneity, especially in patients with chronic obstructive airway disease.[12] The dynamic perfusion MRI has shown good predictive capability, but it is expensive, technically difficult, and affected by motion and severe susceptibility artefacts.[13] Radionuclide perfusion scintigraphy is easily available, cost-effective, can noninvasively evaluate the regional lung function and is the most commonly used method to predict postoperative FEV1.[7] Many studies have shown good correlation between the actual postoperative FEV1 and PPO FEV1 obtained by lung perfusion scintigraphy.[10,12,14] Lung perfusion scintigraphy is routinely done in our hospital as a part of preoperative evaluation for lung cancer patients for the PPO FEV1. We conducted this study to see whether the prediction of the postoperative FEV1 by perfusion scintigraphy was accurate for patients undergoing lobectomy/pneumonectomy by comparing it with actual postoperative FEV1 obtained by spirometry at 4–6 months after surgery. We also wanted to see the correlation between PPO FEV1 obtained from planar perfusion scan, where the lung is divided into zones with the actual postoperative FEV1 after lobectomy.
MATERIALS AND METHODS
Patient characteristics
The study was carried out after approval from the Institutional Review Board (Project No 1035/2012). It was a retrospective study of patients who underwent lung surgery (lobectomy/bilobectomy/pneumonectomy) and had a preoperative lung perfusion scintigraphy using 99mTc macro aggregated albumin (MAA) for the prediction of postoperative pulmonary function. Since the study involved only analysis of collected data, without any additional patient contact or intervention, the need for informed consent was waived. We enrolled 50 consecutive eligible lung cancer patients who underwent curative surgical resection. All patients underwent spirometry and lung perfusion study as per standard guidelines prior to their lung surgery for the prediction of postoperative FEV1. The data were compared with the postoperative spirometric study (4–6 months’ postsurgery) to assess the accuracy of the prediction of the postoperative pulmonary function by the preoperative scintigraphic study. The actual postoperative spirometric FEV1 (4–6 months’ postsurgery) was collected from the patient database. We excluded patients undergoing nonanatomical lung resections such as wedge resections or metastatectomy, patients with permanent tracheotomy or orofacial anomalies (unable to perform spirometry), and patients undergoing repeat surgeries of the thorax before 6 months.
Procedure
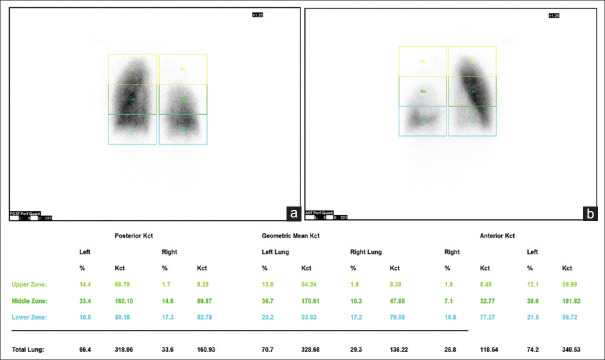
The prerequisite for lung perfusion scintigraphy study was the availability of spirometric results for assessing preoperative pulmonary function. No prior patient preparation was necessary. The scan was acquired as per the Society of Nuclear Medicine Guidelines for lung perfusion scintigraphy (2009). All patients were scanned using a dual-head gamma camera (Infinia Hawkeye by GE Healthcare, Chicago, IL, USA) after slow intravenous injection of 185 MBq of 99mTcMAA over several respiratory cycles with the patient in supine position. Planar scans were acquired in anterior and posterior projections in 256 × 256 matrix. Single-photon emission CT (SPECT) or SPECT/CT images were not routinely acquired. The images were processed by using automatic quant perfusion analysis software available on Xeleris workstation, GE Healthcare, Chicago, IL, USA. It divides both the lungs into three equal zones in both anterior and posterior images and calculates the geometric mean of counts in each zone with percentage perfusion [Figure 1]. On the basis of the amount of lung tissue to be removed (lobectomy/ bilobectomy/pneumonectomy), PPO FEV1 was calculated as percentage and absolute values depending on the functional lung tissue left after surgery. The preoperative spirometric value of actual FEV1 was taken as reference on which the PPO FEV1 was calculated by the following formula:
Figure 1.
A 61-year-old man with biopsy proven adenocarcinoma of the right lung. Posterior (a) and anterior (b) planar perfusion images showing division of lungs into three equal zones. Geometric mean of counts in each zone with percentage perfusion is displayed
PPO FEV1 = preoperative FEV1× (1−fraction of total perfusion for the lung to be resected).
Statistical analysis
Statistical analysis was performed using Statistical Package for the Social Sciences software, Version 21, IBM, Chicago, IL, USA. Pearson’s correlation coefficient was used to evaluate the relationship between PPO FEV1 by lung perfusion scintigraphy and postoperative (4–6 months’ postsurgery) actual FEV1 measured by spirometry. Agreement between the two methods was analyzed with Bland–Altman method by plotting the difference between the predicted and postoperative actual FEV1. Limits of agreement were defined as mean of difference ± 2 standard deviation (SD).
RESULTS
The characteristics of patients are shown in Table 1. The correlation between the PPO FEV1 by lung perfusion scintigraphy and measured actual postoperative FEV1 by spirometry for the entire patient group showed a statistically significant correlation (r = 0.847, P = 0.000). There was better correlation between the two methods of measurement of pulmonary function for pneumonectomy patients compared to those who underwent lobectomy (r = 0.930 [P = 0.000] vs. 0.792 [P = 0.000]).
Table 1.
Patient characteristics
| Patient characteristics | Value |
|---|---|
| Median age (range) | 59 (32-77) |
| Sex | |
| Male | 39 |
| Female | 11 |
| Histology | |
| Adenocarcinoma | 21 |
| Squamous | 18 |
| Others | 11 |
| Laterality | |
| Left | 29 |
| Right | 21 |
| Type of surgery | |
| Lobectomy | 31 |
| Pneumonectomy | 19 |
| PPO FEV1 (L) mean (range) | 1.67 (0.59-3.03) |
| Actual PO FEV1 (L) mean (range) | 1.61 (0.50-2.60) |
FEV1: Forced expiratory volume in the first second; PPO FEV1: Predicted postoperative FEV1; PO FEV1: postoperative FEV1
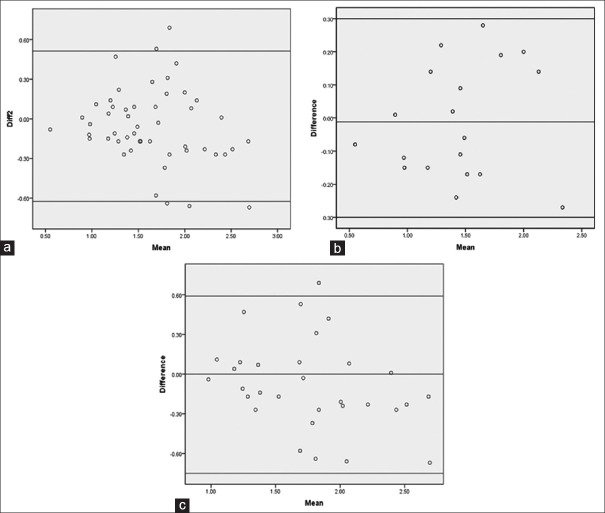
The agreement analysis between the two methods of evaluation of pulmonary function using Bland–Altman method showed a mean difference of −0.0558 with a SD of 0.284. The limits of agreement vary over a wide range from −0.625 to 0.513 L (mean ± 2SD) for the entire group [Figure 2a]. The agreement between the two methods of evaluation of pulmonary function showed a mean difference of −0.0121 and SD of 0.169 for patients who underwent pneumonectomy (n = 19) with limits of agreement varying between −0.30 and 0.30 L [Figure 2b]. The agreement between the two methods, for patients who underwent lobectomy (n = 31) showed a mean difference of −0.0826 and SD of 0.336 with limits of agreement varying between −0.755 and 0.590 L [Figure 2c]. Statistical results are summarized in Table 2.
Figure 2.
Agreement between the predicted postoperative forced expiratory volume in the first second and observed forced expiratory volume in the first second 4–6 months’ postsurgery for the entire group (a), pneumonectomy (b), and lobectomy (c)
Table 2.
Summary of statistical results
| Test | Result |
|---|---|
| Pearson correlation (r) | |
| Entire group | 0.847 |
| Pneumonectomy | 0.930 |
| Lobectomy | 0.792 |
| Bland Altman agreement, mean (mean±2SD) | |
| Entire group | −0.0558 (−0.625L-0.513L) |
| Pneumonectomy | −0.0121 (−0.30L-0.30L) |
| Lobectomy | −0.0826 (−0.755L-0.590L) |
SD: Standard deviation
DISCUSSION
Three aspects of lung function are assessed prior to resection – respiratory mechanics, gas exchange, and cardiorespiratory interaction. Spirometry is performed to assess respiratory mechanics. Gas exchange function is assessed by DLCO, and it correlates with the total functioning area of the alveolocapillary membrane. Cardiorespiratory interaction is assessed by exercise tests such as stair climb, Shuttle Walk test, or cardiopulmonary exercise test with the estimation of maximal oxygen consumption (VO2max). According to the third edition of the American College of Chest Physicians Clinical Practice Guidelines, in all patients both PPO FEV1 and PPO DLCO are calculated, and if both PPO FEV1 and PPO DLCO are >60% predicted, no further tests are recommended. If either the PPO FEV1 or PPO DLCO is <60% predicted, exercise tests are recommended for risk stratification.[5]
Tests such as spirometry and DLCO do not differentiate between functioning and nonfunctioning lung tissue. PPO values may be falsely low if the lung to be resected is nonfunctioning or minimally functioning. The functional contribution of the lung segments which are to be resected can be evaluated by assessing the fractional contribution of perfusion of that lung. The single most valid test for post thoracotomy respiratory complications is the PPO FEV1. The preoperative lung perfusion scanning can be used for the prediction of postoperative complications and the short and long-term performance in lung resection candidates at increased risk for complications. To minimize perioperative pulmonary complications, respiratory care (prophylaxis and therapy) adequate for the functional risk of the patient is necessary.
The preoperative evaluation and prediction of postoperative lung function in patients with lung cancer is a challenging problem. Several studies have shown a good correlation between predicted and observed postoperative pulmonary function values. The direct scintigraphic quantification of the contribution of each lobe to the overall pulmonary function is prone to difficulties due to the geometric overlapping of the lobes and to the cross-talk in the different views. The role of SPECT imaging for this purpose is under evaluation. Tumors obstructing the airway may create a ventilation-perfusion mismatch, and this should be taken into consideration when evaluating patients for pulmonary resection. The segments or lobes which are partially or completely atelectatic, surgical resection may not have the anticipated negative impact on postoperative function. At times, one may even improve pulmonary function with resection by decreasing the ventilation-perfusion mismatch, provided that the atelectatic parenchyma is well perfused. However, the main aim is to identify those patients at greatest risk for complications and those who would not benefit from surgery.
Many studies have tried to predict the postoperative pulmonary function on the basis of reduced perfusion of the involved lung which is marked for resection.[6,14,15] Bolliger et al. showed that perfusion and quantitative CT-based predictions of postoperative pulmonary function are useful irrespective of the extent of resection, but perfusion-based results were the most accurate. They also concluded that anatomical segment counting calculations for resection should be reserved for resection not exceeding lobectomy.[16] Few studies have shown that combined ventilation and perfusion scintigraphy is better for PPO FEV1, especially in patients with borderline low respiratory reserve.[12,17,18]
However, the lung ventilation study comes with disadvantages such as difficult to perform, expensive, time-consuming, additional radiation exposure, and nonavailability. Many studies in literature have shown a good correlation of the actual postoperative FEV1 with prediction made from perfusion only studies.[6,12,18] Researchers also evaluated the role of SPECT and SPECT/CT methods in predicting the postoperative lung function. Kovacević-Kuśmierek et al. showed that performing planar perfusion scintigraphy is not inferior to tridimensional methods such as SPECT and SPECT/CT in the quantification of split lung function.[6] Many other authors claimed that tridimensional method is more appropriate than performing planar images, especially in calculating relative lobar perfusion.[12,19,20]
In our study, we analyzed the accuracy of the quantitative planar lung perfusion scintigraphy to predict the lung function post 4–6 months lung surgery and compared the predicted against the observed actual postoperative FEV1 evaluated by spirometry. We found a significant correlation between actual and predicted values (using percentage zonal distribution of counts on planar perfusion images) in terms of absolute FEV1 in liter. Our study showed a significant correlation coefficient of 0.93 for patients who underwent pneumonectomy and correlation coefficient of 0.79 for lobectomy, with an overall correlation coefficient of 0.847. However, in assessing the agreement between the PPO FEV1 by lung perfusion scintigraphy and actual postoperative FEV1 by spirometry 4–6 months’ postsurgery, we found a mean difference of −0.0558 between the two methods for all patients with 95% limits of agreement ranging from −0.625 to 0.513 L. This 95% limit of agreement appears much wider (−0.75–0.59 L vs. −0.30–0.30 L) in patients underwent lobectomy compared to those with pneumonectomy.
Due to these wide limits of agreement, the results appear to be clinically unacceptable as there is a chance of underestimation of postoperative lung function as low as around 0.6 L and overestimation as high as around 0.5 L. The level of agreement between the PPO FEV1 and postoperative actual FEV1 by spirometry showed more clinically acceptable results in patients undergoing pneumonectomy compared to those who underwent lobectomy. It is mainly because the percentage zonal distribution of counts on planar perfusion images is not a true representation of lobar fraction due to the anatomical overlap of lobes. It could be possible that refinements in our technique might have yielded better postoperative predictions, especially in patients undergoing lobectomy. A recent study by Yoo et al. evaluated different planar acquisition methods and found that posterior oblique method performed better than conventional anterior-posterior planar method in predicting postoperative lung function. The posterior oblique view reflected the lobar anatomy more precisely than conventional anteroposterior images.[21] With the widespread availability of hybrid machines, it is now possible to combine radionuclide SPECT imaging techniques with the quantitative CT perfusion techniques. This technique can be successfully used to examine the zone-wise distribution of perfusion defect and to estimate the function of the remaining normally perfused lung parenchyma. Combining SPECT images with CT helps in delineating lung lobes more precisely and may predict postoperative lung function more accurately.
CONCLUSION
Quantitative planar lung perfusion scintigraphy is a simple, convenient, and easily available tool to calculate the PPO FEV1 and shows good correlation with actual postoperative (post 4–6 months’ surgery). It also shows reasonably good agreement in patients undergoing pneumonectomy. However, the limits of the agreement appear to be clinically unacceptable in patients undergoing lobectomy as there is a chance of overestimation or underestimation of PPO lung function. The application of SPECT or SPECT/CT techniques may improve prediction in such patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.World Health Organization. Geneva: World Health Organization; 2015. [Last accessed on 2017 Aug 22]. GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012 All Cancers (Excluding Nonmelanoma Skin Cancer) Estimated Incidence, Mortality and Prevalence Worldwide in 2012. Available from: http://globocaniarcfr/Pages/fact_sheets_canceraspx?cancer=lung . [Google Scholar]
- 2.Noronha V, Pinninti R, Patil VM, Joshi A, Prabhash K. Lung cancer in the Indian subcontinent. South Asian J Cancer. 2016;5:95–103. doi: 10.4103/2278-330X.187571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosen JE, Keshava HB, Yao X, Kim AW, Detterbeck FC, Boffa DJ. The natural history of operable non-small cell lung cancer in the national cancer database. Ann Thorac Surg. 2016;101:1850–5. doi: 10.1016/j.athoracsur.2016.01.077. [DOI] [PubMed] [Google Scholar]
- 4.Colice GL, Shafazand S, Griffin JP, Keenan R, Bolliger CT. American College of Chest Physicians Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: ACCP evidenced-based clinical practice guidelines (2nd edition) Chest. 2007;132:161S–77. doi: 10.1378/chest.07-1359. [DOI] [PubMed] [Google Scholar]
- 5.Brunelli A, Kim AW, Berger KI, Addrizzo-Harris DJ. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: Diagnosis and management of lung cancer, 3rd ed: American college of chest physicians evidence-based clinical practice guidelines. Chest. 2013;143:e166S–90. doi: 10.1378/chest.12-2395. [DOI] [PubMed] [Google Scholar]
- 6.Kovacević-Kuśmierek K, Kozak J, Pryt Ł, Bieńkiewicz M, Cichocki P, Kuśmierek J, et al. Perfusion lung scintigraphy for the prediction of postoperative residual pulmonary function in patients with lung cancer. Nucl Med Rev Cent East Eur. 2015;18:70–7. doi: 10.5603/NMR.2015.0018. [DOI] [PubMed] [Google Scholar]
- 7.Sawabata N, Nagayasu T, Kadota Y, Goto T, Horio H, Mori T, et al. Risk assessment of lung resection for lung cancer according to pulmonary function: Republication of systematic review and proposals by guideline committee of the Japanese association for chest surgery 2014. Gen Thorac Cardiovasc Surg. 2015;63:14–21. doi: 10.1007/s11748-014-0475-x. [DOI] [PubMed] [Google Scholar]
- 8.Magdeleinat P, Seguin A, Alifano M, Boubia S, Regnard JF. Early and long-term results of lung resection for non-small-cell lung cancer in patients with severe ventilatory impairment. Eur J Cardiothorac Surg. 2005;27:1099–105. doi: 10.1016/j.ejcts.2005.01.034. [DOI] [PubMed] [Google Scholar]
- 9.Block AJ, Olsen GN. Preoperative pulmonary function testing. JAMA. 1976;235:257–8. [PubMed] [Google Scholar]
- 10.British Thoracic Society Society of Cardiothoracic Surgeons of Great Britain and Ireland Working Party BTS guidelines: Guidelines on the selection of patients with lung cancer for surgery. Thorax. 2001;56:89–108. doi: 10.1136/thorax.56.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Papageorgiou CV, Antoniou D, Kaltsakas G, Koulouris NG. Role of quantitative CT in predicting postoperative FEV1 and chronic dyspnea in patients undergoing lung resection. Multidiscip Respir Med. 2010;5:188–93. doi: 10.1186/2049-6958-5-3-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toney LK, Wanner M, Miyaoka RS, Alessio AM, Wood DE, Vesselle H. Improved prediction of lobar perfusion contribution using technetium-99m-labeled macroaggregate of albumin single photon emission computed tomography/computed tomography with attenuation correction. J Thorac Cardiovasc Surg. 2014;148:2345–52. doi: 10.1016/j.jtcvs.2014.04.036. [DOI] [PubMed] [Google Scholar]
- 13.Ohno Y, Seki S, Koyama H, Yoshikawa T, Matsumoto S, Takenaka D, et al. 3D ECG- and respiratory-gated non-contrast-enhanced (CE) perfusion MRI for postoperative lung function prediction in non-small-cell lung cancer patients: A comparison with thin-section quantitative computed tomography, dynamic CE-perfusion MRI, and perfusion scan. J Magn Reson Imaging. 2015;42:340–53. doi: 10.1002/jmri.24800. [DOI] [PubMed] [Google Scholar]
- 14.Subramanyam P, Sundaram PS. Which is better – A standalone ventilation or perfusion scan or combined imaging to predict postoperative FEV in one seconds in patients posted for lung surgeries with borderline pulmonary reserve. Indian J Nucl Med. 2018;33:105–11. doi: 10.4103/ijnm.IJNM_149_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohno Y, Koyama H, Takenaka D, Nogami M, Kotani Y, Nishimura Y, et al. Coregistered ventilation and perfusion SPECT using krypton-81m and Tc-99m-labeled macroaggregated albumin with multislice CT utility for prediction of postoperative lung function in non-small cell lung cancer patients. Acad Radiol. 2007;14:830–8. doi: 10.1016/j.acra.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 16.Bolliger CT, Gückel C, Engel H, Stöhr S, Wyser CP, Schoetzau A, et al. Prediction of functional reserves after lung resection: Comparison between quantitative computed tomography, scintigraphy, and anatomy. Respir Int Rev Thorac Dis. 2002;69:482–9. doi: 10.1159/000066474. [DOI] [PubMed] [Google Scholar]
- 17.Mariano-Goulart D, Barbotte E, Basurko C, Comte F, Rossi M. Accuracy and precision of perfusion lung scintigraphy versus 133Xe-radiospirometry for preoperative pulmonary functional assessment of patients with lung cancer. Eur J Nucl Med Mol Imaging. 2006;33:1048–54. doi: 10.1007/s00259-006-0087-5. [DOI] [PubMed] [Google Scholar]
- 18.Mineo TC, Schillaci O, Pompeo E, Mineo D, Simonetti G. Usefulness of lung perfusion scintigraphy before lung cancer resection in patients with ventilatory obstruction. Ann Thorac Surg. 2006;82:1828–34. doi: 10.1016/j.athoracsur.2006.05.041. [DOI] [PubMed] [Google Scholar]
- 19.Provost K, Leblond A, Gauthier-Lemire A, Filion É, Bahig H, Lord M. Reproducibility of lobar perfusion and ventilation quantification using SPECT/CT segmentation software in lung cancer patients. J Nucl Med Technol. 2017;45:185–92. doi: 10.2967/jnmt.117.191056. [DOI] [PubMed] [Google Scholar]
- 20.Knollmann D, Meyer A, Noack F, Schaefer WM. Preoperative assessment of relative pulmonary lobar perfusion fraction in lung cancer patients A rather simple three-dimensional CT-based vs planar image-derived quantification. Nuklearmedizin. 2015;54:178–82. doi: 10.3413/Nukmed-0729-15-03. [DOI] [PubMed] [Google Scholar]
- 21.Yoo ID, Im JJ, Chung YA, Choi EK, Oh JK, Lee SH. Prediction of postoperative lung function in lung cancer patients using perfusion scintigraphy. Acta Radiol. 2019;60:488–95. doi: 10.1177/0284185118787355. [DOI] [PubMed] [Google Scholar]