Abstract
Myocardial infarction with non-obstructive coronary arteries or any acute coronary syndrome (ACS) with normal or near-normal (non-obstructive) coronary arteries (ACS-NNOCA) is an heterogeneous clinical entity, which includes different pathophysiology mechanisms and is challenging to treat. Sudden cardiac death (SCD) is a catastrophic manifestation of ACS that is crucial to prevent and treat urgently. The concurrence of the two conditions has not been adequately studied. This narrative review focuses on the existing literature concerning ACS-NNOCA pathophysiology, with an emphasis on SCD, together with risk and outcome data from clinical trials. There have been no large-scale studies to investigate the incidence of SCD within ACS-NNOCA patients, both early and late in the disease. Some pathophysiology mechanisms that are known to mediate ACS-NNOCA, such as atheromatous plaque erosion, anomalous coronary arteries, and spontaneous coronary artery dissection are documented causes of SCD. Myocardial ischaemia, inflammation, and fibrosis are probably at the core of the SCD risk in these patients. Effective treatments to reduce the relevant risk are still under research. ACS-NNOCA is generally considered as an ACS with more ‘benign’ outcome compared to ACS with obstructive coronary artery disease, but its relationship with SCD remains obscure, especially until its incidence and effective treatment are evaluated.
Keywords: ACS-NNOCA, MINOCA, Sudden cardiac death, Acute myocardial infarction, Acute coronary syndrome, Ventricular arrhythmias
Introduction
The majority of myocardial infarctions (MIs) are associated with obstructive coronary artery disease (CAD). In the decade of 1980s, the pioneering angiographic studies by De Wood et al. had already discovered that almost 5% of patients with acute MI did not have obstructive CAD.1,2 This disease was subsequently named MI with non-obstructive coronary arteries (MINOCA) or MI with normal coronary arteries. Furthermore, there are patients who present with non-MI acute coronary syndrome (ACS) who also have no significant CAD detected at coronary angiography; all these patients have been recently grouped under the newly coined term of ACS with normal or near-normal (non-obstructive) coronary arteries (ACS-NNOCA).3
Sudden cardiac death (SCD) is responsible for over 4 million deaths worldwide every year, affecting both older and younger people.4 Identifying the cause of SCD is challenging, because in older individuals multiple cardiovascular (CV) and non-CV conditions exist, making the attribution of the event to one of them difficult, while in younger persons, conditions such as inherited channelopathies or arrhythmias might be missed even on autopsy.4
There is currently a lack of any dedicated studies assessing the incidence of SCD within MINOCA/ACS-NNOCA patients, before and after the initiation of symptoms, and the risk of potentially lethal ventricular arrhythmias late after the index event. Large cohort studies presenting predictors of adverse outcomes (e.g. for MI, heart failure, stroke, total death, CV death) do not address the specific outcome of SCD.5,6 Society guidelines and scientific statements have ostensibly neglected this important issue.7–9 The purpose of this narrative review is to concentrate current knowledge concerning MINOCA/ACS-NNOCA and its pathophysiology, with a special focus on the clinical presentation of SCD in this setting.
MINOCA and ACS-NNOCA
Myocardial infarction with non-obstructive coronary arteries patients comprise about 5–6% of all patients with acute MI who are referred for coronary angiography.9,10 The diagnosis of MINOCA is made in patients with MI (compatible with the fourth universal definition of MI) that are found to have non-obstructive coronary arteries on angiography (absence of stenosis ≥50% in any major epicardial vessel). No specific alternate diagnosis responsible for the clinical presentation should be obvious (such as sepsis, pulmonary embolism, and myocarditis).8,9 Myocardial infarction with non-obstructive coronary arteries patients can fulfil the criteria for both MI type 1 and type 2.7 Almost 50% of these patients are women, a higher prevalence compared to obstructive CAD-MI.9,10 The mean age of MINOCA patients is 55 years.10 In general, there is a lower prevalence of dyslipidaemia and other traditional CAD risk factors, such as diabetes mellitus, hypertension, smoking, and a family history in MINOCA patients.6,9
The diagnosis of MINOCA is a working diagnosis, made upon a normal or near-normal coronary angiography, but not a definite one, and physicians should be prompted to seek an underlying pathophysiologic mechanism. Furthermore, the term MINOCA should be attributed only to patients with an ischaemic basis for their symptoms and only when obstructive CAD has not been inadvertently overlooked.9,11 The electrocardiogram may feature development of Q waves or new ischaemic changes, such as ST-segment elevation, ST-segment depression, T-wave inversion, or new bundle branch blocks. Myocardial infarction with non-obstructive coronary arteries is diagnosed more frequently in non-ST-elevation MI (NSTEMI) than in ST-elevation MI (STEMI) patients.11
Myocardial infarction with non-obstructive coronary arteries patients should be differentiated into two separate groups: those with angiographically normal coronary arteries and those with angiographically mild to moderate (30–50%) stenoses (non-obstructive CAD). This is of importance, because there is evidence supporting a poorer prognosis with a heavier atheromatous burden in patients with non-obstructive CAD.9 The prevalence of angiographically smooth vessels was 51% in a meta-analysis.10
The 2017 European Society of Cardiology (ESC) guidelines for the management of STEMI included for the first time a reference to MINOCA, suggesting the use of additional diagnostic testing, aside from coronary angiography, to investigate the underlying pathophysiology, such as echocardiography, left ventricular (LV) angiogram, or cardiac magnetic resonance (CMR) imaging.7
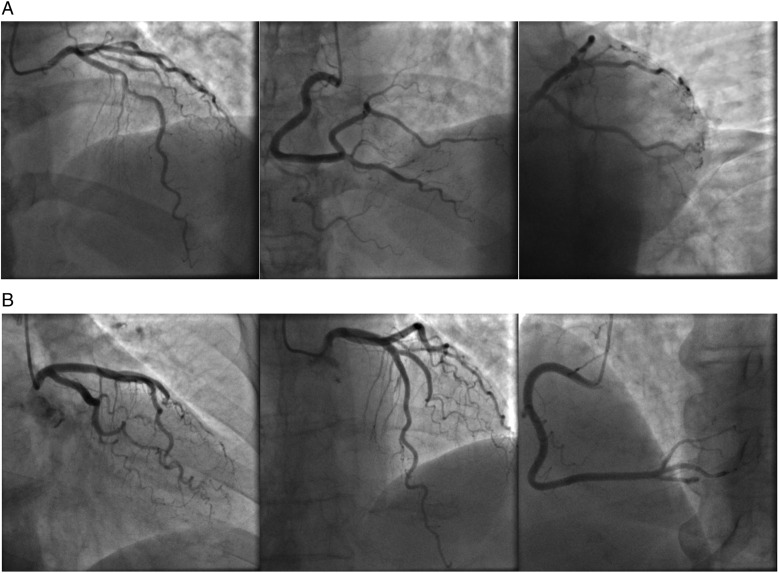
Recently, the subset of MINOCA was expanded to include all patients presenting with ACS who turn out to have normal or near-normal coronary arteries at coronary angiography, in a more inclusive patient group with the term of ACS-NNOCA.3 ACS-NNOCA includes both STEMI and NSTEMI, and patients suffering from unstable angina with no infarction who have minimal or no CAD. Figure 1 presents the coronary angiographies of two recent cases who presented to our department with typical ACS symptoms and were subsequently found to have ACS-NNOCA.
Figure 1.
(A) Coronary angiography of a male 53-year-old patient who presented to our department with typical and recurrent rest angina radiating to the neck and shoulders and was found to have near-normal coronary arteries (ACS-NNOCA). (B) Coronary angiography of a female 74-year-old patient who was admitted with typical angina radiating to the neck and left shoulder. The patient also had elevated troponin with a rise-and-fall pattern. The angiography revealed near-normal coronary arteries (ACS-NNOCA). ACS-NNOCA, acute coronary syndrome with normal or near-normal coronary arteries.
Patients with MINOCA or ACS-NNOCA generally have a better prognosis than patients with MI or ACS and obstructive CAD, both in-hospital and long-term, although this finding is not consistent among all studies.3,9,10,12,13 Furthermore, as mentioned, two distinct subgroups of patients are encountered in the ACS-NNOCA group, those with normal coronaries and those with near-normal coronaries (non-obstructive CAD), where major adverse cardiac event (MACE) and mortality rates may differ.
One study included 4793 consecutive STE-ACS patients and divided them as having obstructive CAD (88%), non-obstructive CAD (6%), and normal coronary arteries (5%). Short-term (≤30 days) mortality was lower in both patients with non-obstructive CAD [hazard ratio (HR) 0.49, 95% confidence interval (CI) 0.27–0.89; P = 0.018] and normal arteries (HR 0.31, 95% CI 0.11–0.83; P = 0.021), compared to those with obstructive CAD. In contrast, the long-term mortality (>30 days) was similar in patients with non-obstructive CAD (HR 1.15, 95% CI 0.77–1.72; P = 0.487) compared to those with obstructive CAD and higher in patients with normal arteries (HR 2.44, 95% CI 1.58–3.76; P < 0.001) compared to those with obstructive CAD. Causes of death were CV in 70% of patients with obstructive CAD, 38% with non-obstructive CAD and 32% with normal coronary arteries.6 In this study, SCD accounted for 3% (n = 1) of deaths of patients with non-obstructive CAD and 18% (n = 5) of deaths of patients with normal coronaries.6 Significant non-cardiac mortality in patients with MINOCA was noted in the ACUITY trial that also highlighted the fact that troponin elevation in these patients may prevent a non-cardiac primary condition from being diagnosed. Moreover, standard ACS treatment is of questionable utility in this setting.12
Sudden cardiac death
Sudden cardiac death is a frequent cause of death worldwide, accounting for 25% of all CV deaths.4 It is defined as an unexpected death, a result of known or unknown cardiac causes and occurring within 1 h of the onset of symptoms.14 The incidence ranges from 36 to 128 deaths per 100 000 people/year, according to the method of reporting.15 Coronary artery atherosclerosis is the most frequent cause of SCD (59–86% of the cases).16 Despite community and hospital-based interventions, both pre-hospital and in-hospital patient survival has not improved significantly over the past decades. In this regard, recognition of high-risk individuals and development of effective strategies for preventing and treating ventricular arrhythmias is of great importance.17
Acute coronary syndrome, both in the acute phase and later, are the aetiology of a significant percentage of SCD events. Up to 6% of patients with ACS develop ventricular tachycardia (VT) or ventricular fibrillation (VF) within the first 48 h from the onset of symptoms.4 The analogy of these percentages, as well as the real risk for a first episode or recurrence of SCD, has not yet been studied in MINOCA or ACS-NNOCA patients as a group. Interventions to reduce that risk in these patients are also yet to be supported by evidence, in contrast with obstructive CAD-MI, where prompt revascularization and additional pharmacologic and non-pharmacologic treatments are well documented to reduce the risk of malignant ventricular arrhythmias.4
In a study of 131 MINOCA patients with normal LV ejection fraction, 18 of them (13.8%) had a ventricular arrhythmia during hospitalization, while aborted SCD occurred in 6%. Late gadolinium enhancement transmural extent on CMR and ST-segment elevation were independent predictors of ventricular arrhythmias.18
In VIRGO study, 299 MINOCA patients were representing ∼11% of the total MI group. Four of them (1.3%) presented in cardiac arrest and received an automatic implantable cardioverter-defibrillator.19 From case reports, it is known that MINOCA presenting as STEMI may be complicated with heart failure and recurrent VT, even after ablation,20 or with VF soon after hospital discharge.21
In a study of 178 patients admitted to a coronary care unit with non-MI ACS-NNOCA, the final diagnosis comprised true NSTE-ACS (i.e., coronary thrombosis on an unstable plaque; n = 1, 0.6%), microvascular NSTE-ACS (56.2%), variant angina (10.1%), myocarditis (8.9%), Takotsubo cardiomyopathy (7.9%), tachyarrhythmia-related chest pain (6.7%), and non-cardiac pain (9.6%).22 At 24.5-month follow-up, 21 deaths (11.8%) had occurred, 9 (5.1%) from CV causes, including 1 (0.6%) SCD in a patient with non-obstructive CAD.
Table 1 lists the main pathophysiology mechanisms known to cause MINOCA/ACS-NNOCA. Plaque disruption was found in up to 40% of MINOCA patients with intravascular ultrasound in two studies.23,24 Myocardial necrosis could be mediated by thrombosis, vasospasm, or a combination of these, along with subsequent spontaneous thrombolysis.8 Coronary angiography may underestimate these plaques. Furthermore, early ACS treatment may cause the thrombus to dissolve, and only an angiographically insignificant lesion remains that is, however, highly unstable. These patients may be falsely classified as low risk and receive less than optimal secondary prevention treatment.12 An eccentric plaque with positive remodelling is a mechanism that could possibly result in MINOCA with non-obstructive lesion seen in angiography. These plaques often are vulnerable to rupture and a subsequent transient thrombosis may cause distal embolization and myocardial necrosis.28 A lesion of this kind should be sought with intravascular imaging and treated as an obstructive CAD-MI, as the follow-up risk seems to be comparable between the two.30 Dysfunction of the coronary microvessels can be recognized in 30% to 50% of patients with chest discomfort and non-obstructive CAD on coronary angiography.31 ACS-NNOCA can also result from coronary thrombi and emboli, such as in hypercoagulation states, atrial fibrillation, valvular heart disease, paradoxic embolism, cardiac tumours, and vegetations.8
Table 1.
Main pathophysiology mechanisms known to cause MINOCA/ACS-NNOCA
| Mechanism | Assisting diagnostic modules | Incidence | |
|---|---|---|---|
| 1 | Plaque disruption or eccentric plaque with positive remodelling | Intracoronary imaging (OCT or IVUS) | Up to 40% of MINOCA23,24 |
| 2 | Coronary microvascular spasm or dysfunction | Intracoronary acetylcholine | 25% of ACS-NNOCA25 |
| 3 | Coronary thrombi and emboli | Coronary angiography, identification of an embolic source | 4.3% of STEMI26 |
| 4 | Coronary artery spasm (including substance abuse and smoking) | Intracoronary ergonovine or acetylcholine (not routinely performed) | Up to 27% of MINOCA10 |
| 5 | Spontaneous coronary artery dissection | Intracoronary imaging (OCT or IVUS) | 1.7–4% of ACS27 |
| 6 | Takotsubo cardiomyopathy | LV angiogram, ECHO, CMR | 1.2–2.2% of ACS28 |
| 7 | Myocarditis | Endomyocardial biopsy, CMR | 33% of MINOCA29 |
ACS-NNOCA, acute coronary syndrome with normal or near-normal coronary arteries; CMR, cardiac magnetic resonance; ECHO, echocardiogram; IVUS, intravascular ultrasound; LV, left ventricle; MINOCA, myocardial infarction with non-obstructive coronary arteries; OCT, optical coherence tomography.
Coronary angiography performed immediately after admission in 300 consecutive patients with non-apparent non-cardiac cause of out of hospital cardiac arrest, revealed coronary artery spasm in 10 cases. Repetitive provocation tests were performed to guide treatment and eliminate residual spasm, an intervention that reduced recurrent incidents of cardiac arrest.17 Up to 27% of MINOCA patients have positive provocative vasospasm testing.10 Substance abuse and smoking might precipitate coronary artery spasm and should be always sought when taking the medical history in such cases.3,32 The contribution of coronary artery spasm in SCD is probably underestimated as provocation studies are not routinely performed.17,32
In a retrospective observational study, 130 out of 645 patients with suspected ACS had a coronary artery stenosis less than 75%, and 70 of them were diagnosed to have coronary artery spasm. Of note, 6% of these patients developed aborted SCD.32 Indeed, some patients with vasospastic angina may experience SCD due to ischaemia-induced polymorphic VT degenerating into VF; when patients are successfully resuscitated, they will need an implantable cardioverter-defibrillator. It is, therefore, of importance to incorporate secondary SCD prevention in coronary artery spasm, since the prognosis is not favourable in some cases, as shown above. However, risk stratification for primary prevention of SCD in these patients remains unexplored.33
Spontaneous coronary artery dissection (SCAD) is more common among women and may cause an MI with or without vessel lumen obstruction that is visible on angiography, thus suggesting a diagnosis of MINOCA in several cases.8 Spontaneous coronary artery dissection is regarded responsible for 0.5% of SCD. This is probably an underestimation, as out of hospital SCD might not be attributed to coronary dissection.34 However, on admission, ∼8–10% present with VT/VF. In a series of 327 SCAD cases, all patients with MI, 8.9% developed VT or VF.34,35 Over a median long-term follow-up of 3.1 years, recurrent SCD occurred in 10.4%, while the death rate was 1.2%. In another series of 196 SCAD patients, again all presenting with MI (24% STEMI), VT/VF occurred in 8.1% (16/196), with 1% having cardiac arrest.36
Transient ischaemia is a central feature of MINOCA and ACS-NNOCA pathophysiology. A lot of these cases represent the result of coronary obstruction that evokes symptoms and sings of ischaemia but is then resolved through various mechanisms. The return of blood flow to previously ischaemic myocardial tissue is analogous to a STEMI that is treated with thrombolysis or percutaneous coronary intervention. Prompt restore of blood flow is crucial to reduce infarct size, but it also induces reperfusion injury. This injury can present as ventricular arrhythmias, consisting of ventricular premature beats, accelerated idioventricular rhythms and even VT/VF. Once thought as a sign of successful reperfusion, they are now considered to be associated with bigger infarcts and worse ventricular function.37,38 The exact pathophysiology is not fully understood, but delayed afterdepolarizations, resulting from intracellular calcium overload is likely the most common mechanism.39 The extent to which reperfusion arrhythmias in MINOCA or ACS-NNOCA patients might contribute to SCD is yet to be evaluated.
Takotsubo cardiomyopathy’s presentation often mimics that of a STEMI, including reversible heart failure without the presence of obstructive CAD. Performance of an LV angiogram and/or early CMR can assist to establish the diagnosis.8 Although generally considered a benign cardiomyopathy due to its reversibility, Takotsubo has been shown to cause life-threatening arrhythmias in over 10% of patients in the early stage of the disease, along with other known acute phase complications.40,41 The long-term occurrence of SCD in the survivors is <2% and may be associated with recurrence of takotsubo syndrome.41
Myocarditis is the underlying pathophysiology in 33% of patients initially classified as having MINOCA according to a meta-analysis29 and is confirmed typically by CMR.3,8 In patients presenting with acute myocarditis, the occurrence of VT/VF depends on whether the myocarditis is fulminant or not, with higher incidence in the former group (∼17% vs. ∼3%).42 Myocarditis as a cause of SCD is probably underdiagnosed in non-autopsied patients compared to autopsied patients.43 In the latter group, SCD is attributed to myocarditis in 2% of infants, 5% of children, and ≤10% to 20% of young adults.44,45 Nevertheless, clinical data indicated that VT events may also occur during long-term follow-up of patients with myocarditis at a rate of ∼5%.46 A recent study of myocarditis patients (n = 235) with either active (n = 123) or previous myocarditis (n = 62) found that the first group more frequently had irregular and polymorphic ventricular arrhythmia, while the latter had more regular and monomorphic ventricular arrhythmia, highlighting the dynamic arrhythmogenic substrate created by acute inflammation. Overall, however, the arrhythmic burden was not different between the two groups.47
In an autopsy series of 72 CV deaths (of which 12 were SCD cases), lymphocytic infiltrate and a proinflammatory phenotype were found in the myocardium and coronary arteries of patients dying suddenly, shortly or late after coronary thrombosis, indicating a crucial role of pre-existing inflammation in this regard and supporting the theory that it can lead to susceptible plaque rupture.48 Furthermore, ACS and arrhythmia presentation can be the result of combined pathophysiology, as in the cases of parvovirus B19 myocarditis which has been shown to induce severe coronary vasospasm.49
In summary, life-threatening ventricular arrhythmias do occur in patients presenting with ACS-NNOCA, attributable to acute or transient myocardial ischaemia and/or reperfusion injury, as in cases where non-obstructive CAD, coronary spasm, microvascular dysfunction, or SCAD are diagnosed and to inflammation and other mechanisms in non-ischaemic causes of ACS-NNOCA (myocarditis, Takotsubo cardiomyopathy). This is in keeping with the fact that myocardial ischaemia, independent of the cause and the duration and even after it is resolved, independently of the degree of the underlying coronary artery stenosis, is notorious for producing VT/VF, either acutely or at a later time if it ends up with formation of myocardial scar, which is the most common mechanism of re-entry responsible for late occurrence of sustained VT and SCD.
Recent advances in genetic polymorphism testing have shown that certain alleles of ion channel genes, such as SCN5A, KCNQ1, and KCNH2, even if subclinical, are related to a predisposition for VF, torsade de pointes and other ventricular arrhythmias during or after an acute myocardial infarction.50–52 This mechanism might explain the occurrence of SCD despite the ischaemic burden being low, as in some cases of ACS-NNOCA.
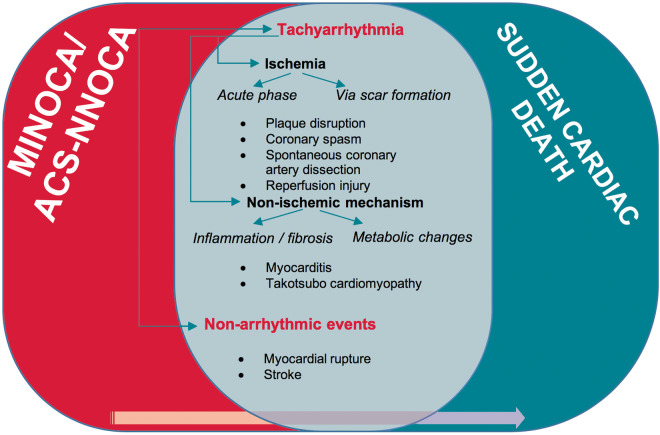
The late occurrence of SCD is commensurate with the specific diagnosis rendered in each case of ACS-NNOCA. In patients where myocardial fibrosis has occurred (MI, myocarditis), the risk is predictably higher than in cases where the myocardium has not been injured (e.g. short-lasting spasm) or has fully recovered (e.g. healed myocarditis, Takotsubo cardiomyopathy). Figure 2 summarizes the overlap between MINOCA or ACS-NNOCA and SCD and the possible mediating mechanisms. Table 2 presents all scientific research work with series of MINOCA or ACS-NNOCA patients regarding SCD occurrence and cause.
Figure 2.
Common pathophysiology mechanisms between MINOCA/ACS-NNOCA and sudden cardiac death with the terminal event involving arrhythmic, more commonly tachy-arrhythmic (VT/VF), and non-arrhythmic (e.g. myocardial rupture, stroke) events. The exact incidence of the overlap is yet to be discovered. Ischaemia seems to be the final common pathway that leads to fatal arrhythmias in most circumstances. ACS-NNOCA, acute coronary syndrome with normal or near-normal coronary arteries; MINOCA, myocardial infarction with non-obstructive coronary arteries; VF, ventricular fibrillation; VT, ventricular tachycardia.
Table 2.
Scientific research providing data for SCD in AMI or MINOCA/ACS-NNOCA patients
| Authors | Year | Population | Main findings | |
|---|---|---|---|---|
| 1 | Lynge et al.43 | 2019 | Autopsies of 14 294 nationwide unselected deaths | Myocarditis was the cause of 6% of all autopsied SCD cases, corresponding to an SCD-myocarditis incidence of 0.16 (95% CI 0.11–0.21) per 100 000 person-years. |
| 2 | Wang et al.42 | 2019 | Meta-analysis of studies comprising patients with fulminant myocarditis (n = 158) and non-fulminant myocarditis (n = 388) | VT and VF were early characteristics of fulminant myocarditis. |
| 3 | Safdar et al.19 | 2018 | AMI patients aged 18–55 years (n = 2690), of which after coronary angiography 299 were classified as MINOCA | Four patients with MINOCA presented in cardiac arrest and received ICD. At 12 months, eight women with MINOCA had died (one with vasospasm, two with SCAD, and five with undefined MINOCA aetiology). No men with MINOCA had died. |
| 4 | Jesel et al.40 | 2018 | Cases of Takotsubo cardiomyopathy were followed up for 8 years (n = 214) | Life-threatening arrhythmias occurred in 10.7% of patients mainly in the first 24 h of hospitalization (VT/VF/cardiac arrest). In hospital and 1 year mortality were significantly reduced in this patient group. No VA recurrence was noted during follow-up. |
| 5 | Andersson et al.6 | 2018 | Patients with STE-ACS (n = 4793) triaged for acute coronary angiography | At median follow-up time of 2.6 years, SCD was the cause of death in 34 patients with obstructive CAD (6% of deaths in this group, n = 592), one patient with non-obstructive CAD (3% of deaths in this group, n = 40) and five patients with normal coronary arteries (18% of deaths in this group, n = 28). |
| 6 | El-Battrawy et al.41 | 2018 | Cases of Takotsubo cardiomyopathy were followed up for 3 years (n = 114) | Life-threatening arrhythmias were occurred in 11.4% of patients and the prognosis of these patients was significantly worse. The short-term recurrence rate of a life-threatening arrhythmia episode was 15.3% while the long-term recurrence rate was 5%. |
| 7 | Te et al.46 | 2017 | Patients with a history of myocarditis (n = 13 250) and a same size control group | After a median follow-up period of 10.4 ± 2.94 years, patients with a history of myocarditis had higher incidence of new VT events compared with healthy controls (5.4% vs. 0.47%; adjusted HR 16.1, 95% CI 2.14–2.73; P < 0.001). CV death was also more frequent in the myocarditis group (6.52% vs. 3.18%; HR 2.42, 95% CI 2.14–2.73; P < 0.001). |
| 8 | Saw et al.35 | 2017 | Patients with SCAD (n = 327) | In 8.9% VT/VF occurred (2.8% required cardioversion or ICD). |
| 9 | Luong et al.36 | 2017 | Patients with SCAD (n = 196) | In 8.1% VT/VF occurred, with 1% having cardiac arrest. |
| 10 | Bière et al.18 | 2017 | MINOCA patients with normal EF (n = 131) | 13.8% or patients had VA during hospitalization and 1 had VF. At 1-year follow-up, there were no SCD or VA recurrence. LGE transmural extent on CMR and ST-segment elevation at admission were risk factors for early VA. |
| 11 | Harmon et al.44 | 2016 | High school United States athletes with SCD or aborted SCD (n = 107) | Myocarditis was the diagnosis in 14% of autopsied cases (7 of 50). |
| 12 | Lanza et al.22 | 2016 | Patients with NSTE-ACS (n = 178) that were found to have no obstructive CAD and were followed up for 24.5 months | There were 12 deaths (6.7%) from non-CV causes and nine deaths (5.1%) from CV causes, including two (1.12%) coronary deaths, one resulting from ST elevation AMI and one from SCD. |
| 13 | Satoh et al.32 | 2013 | Patients with suspected ACS (n = 645) and with vasospastic angina (n = 90) | Incidence of aborted SCD due to VT/VF before arrival to the hospital was higher in spastic ACS than in organic ACS (with obstructive CAD) patients. Aborted SCD occurred in 6% of spastic ACS patients. |
| 14 | Bowker et al.16 | 2013 | Autopsy cases of SCD, white Caucasians, aged 16–64 (n = 692) | From cases with myocardial tissue available for examination (n = 564), the cause of SCD was found to be acute ischaemia (acute infarction with or without coronary thrombosis) in 43.1% (most frequent cause), myocardial scarring (without acute ischaemia/infarction) in 19.1%, coronary atheroma only (without acute ischaemia or scarring) in 20.2%, myocarditis in 0.7% and anomalous coronary artery in 0.2%. |
| 15 | Hill et al.53 | 2010 | Persons with SCD (n = 1647) that were referred for autopsy | 50 persons’ (3%) SCD was associated with non-atherosclerotic coronary pathology. Of these, 48% had anomalous coronary arteries, 16% had SCAD and 12% had spasm. |
| 16 | Meune et al.17 | 2003 | Patients with no obvious non-cardiac cause of out of hospital cardiac arrest (n = 300) | Coronary artery spasm was demonstrated in 10 patients (3%) after an initial coronary angiography and a second one with provocation test to those with minimal or no stenoses. |
ACS, acute coronary syndrome; ACS-NNOCA, acute coronary syndrome with normal or near-normal coronary arteries; AMI, acute myocardial infarction; CAD, coronary artery disease; CI, confidence interval; CMR, cardiac magnetic resonance; CV, cardiovascular; EF, ejection fraction; HR, hazard ratio; ICD, implantable cardioverter-defibrillator; LGE, late gadolinium enhancement; MINOCA, myocardial infarction with non-obstructive coronary arteries; NSTE-ACS, non-ST-elevation acute coronary syndromes; SCAD, spontaneous coronary artery dissection; SCD, sudden cardiac death; STE-ACS, ST-elevation acute coronary syndromes; VA, ventricular arrhythmia; VF, ventricular fibrillation; VT, ventricular tachycardia.
Conclusion
Five percent of STEMIs is found to be MINOCA. Furthermore, additional ACS patients presenting with unstable angina or NSTEMI are found to have normal or near-normal coronary arteries that can all be grouped under the newly coined term of ACS-NNOCA. Several pathophysiology mechanisms contribute to this clinical presentation. Some of them are causes of SCD. Patients with normal coronaries compared to those with non-obstructive CAD have lower, but still alarming, MACE and mortality rates, although there are studies with opposing data. Whether SCD rates differ between these two subgroups remains unknown. Probably, myocardial ischaemia, inflammation and fibrosis is at the core of the SCD risk in these patients, although other overlapping mechanisms may also play a role in different subsets. The exact incidence of this overlap as well as effective treatments to reduce the relative risk need to be further investigated.
Conflict of interest: none declared.
References
- 1. DeWood MA, Spores J, Notske R, Mouser LT, Burroughs R, Golden MS. et al. Prevalence of total coronary occlusion during the early hours of transmural myocardial infarction. N Engl J Med 1980;303:897–902. [DOI] [PubMed] [Google Scholar]
- 2. DeWood MA, Stifter WF, Simpson CS, Spores J, Eugster GS, Judge TP. et al. Coronary arteriographic findings soon after non-Q-wave myocardial infarction. N Engl J Med 1986;315:417–23. [DOI] [PubMed] [Google Scholar]
- 3. Manolis AS, Manolis AA, Manolis TA, Melita H.. Acute coronary syndromes in patients with angiographically normal or near normal (non-obstructive) coronary arteries. Trends Cardiovasc Med 2018;28:541–51. [DOI] [PubMed] [Google Scholar]
- 4. Priori SG, Blomström-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J. et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by Association for European Paediatric and Congenital Cardiology (AEPC). Europace 2015;17:1601–87. [DOI] [PubMed] [Google Scholar]
- 5. Nordenskjöld AM, Baron T, Eggers KM, Jernberg T, Lindahl B.. Predictors of adverse outcome in patients with myocardial infarction with non-obstructive coronary artery (MINOCA) disease. Int J Cardiol 2018;261:18–23. [DOI] [PubMed] [Google Scholar]
- 6. Andersson HB, Pedersen F, Engstrøm T, Helqvist S, Jensen MK, Jørgensen E. et al. Long-term survival and causes of death in patients with ST-elevation acute coronary syndrome without obstructive coronary artery disease. Eur Heart J 2018;39:102–10. [DOI] [PubMed] [Google Scholar]
- 7. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H. et al. ; ESC Scientific Document Group. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 2018;39:119–77. [DOI] [PubMed] [Google Scholar]
- 8. Agewall S, Beltrame JF, Reynolds HR, Niessner A, Rosano G, Caforio ALP. et al. ESC working group position paper on myocardial infarction with non-obstructive coronary arteries. Eur Heart J 2017;38:143–53. [DOI] [PubMed] [Google Scholar]
- 9. Tamis-Holland JE, Jneid H, Reynolds HR, Agewall S, Brilakis ES, Brown TM. et al. ; On behalf of the American Heart Association Interventional Cardiovascular Care Committee of the Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Epidemiology and Prevention; and Council on Quality of Care and Outcomes Research. Contemporary diagnosis and management of patients with myocardial infarction in the absence of obstructive coronary artery disease: a scientific statement from the American Heart Association. Circulation 2019;139:1–18. [DOI] [PubMed] [Google Scholar]
- 10. Pasupathy S, Air T, Dreyer RP, Tavella R, Beltrame JF.. Systematic review of patients presenting with suspected myocardial infarction and nonobstructive coronary arteries. Circulation 2015;131:861–70. [DOI] [PubMed] [Google Scholar]
- 11. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA et al. Fourth universal definition of myocardial infarction. Circulation 2018;138:e618–e651. [DOI] [PubMed] [Google Scholar]
- 12. Planer D, Mehran R, Ohman EM, White HD, Newman JD, Xu K. et al. Prognosis of patients with non-ST-segment-elevation myocardial infarction and nonobstructive coronary artery disease: propensity-matched analysis from the acute catheterization and urgent intervention triage strategy trial. Circ Cardiovasc Interv 2014;7:285–93. [DOI] [PubMed] [Google Scholar]
- 13. Smilowitz NR, Mahajan AM, Roe MT, Hellkamp AS, Chiswell K, Gulati M. et al. Mortality of myocardial infarction by sex, age, and obstructive coronary artery disease status in the ACTION Registry-GWTG (Acute Coronary Treatment and Intervention Outcomes Network Registry-Get with the Guidelines). Circ Cardiovasc Qual Outcomes 2017;10:e003443. [DOI] [PubMed] [Google Scholar]
- 14. Wannamethee G, Shaper AG, Macfarlane PW, Walker M.. Risk factors for sudden cardiac death in middle-aged British men. Circulation 1995;91:1749–56. [DOI] [PubMed] [Google Scholar]
- 15. Priori SG, Aliot E, Blomstrom-Lundqvist C, Bossaert L, Breithardt G, Brugada P et al. Task force on sudden cardiac death of the European Society of Cardiology. Eur Heart J 2001;22:1374–450. [DOI] [PubMed] [Google Scholar]
- 16. Bowker TJ, Wood DA, Davies MJ, Sheppard MN, Cary NRB, Burton JDK. et al. Sudden, unexpected cardiac or unexplained death in England: a national survey. QJM Int J Med 2003;96:269–79. [DOI] [PubMed] [Google Scholar]
- 17. Meune C, Joly LM, Chiche JD, Charpentier J, Leenhardt A, Rozenberg A. et al. Diagnosis and management of out-of-hospital cardiac arrest secondary to coronary artery spasm. Resuscitation 2003;58:145–52. [DOI] [PubMed] [Google Scholar]
- 18. Bière L, Niro M, Pouliquen H, Gourraud J-B, Prunier F, Furber A. et al. Risk of ventricular arrhythmia in patients with myocardial infarction and non-obstructive coronary arteries and normal ejection fraction. World J Cardiol 2017;9:268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Safdar B, Spatz ES, Dreyer RP, Beltrame JF, Lichtman JH, Spertus JA. et al. Presentation, clinical profile, and prognosis of young patients with myocardial infarction with nonobstructive coronary arteries (MINOCA): results from the VIRGO study. J Am Heart Assoc 2018;7:e009174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li B, Ming Z, Wu J, Zhang M.. Nonobstructive coronary artery myocardial infarction complicated by heart failure, ventricular aneurysm, and incessant ventricular arrhythmia: a case report. Medicine (Baltimore )2019;98:e13995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Piels M, Faes T, Vainer J.. Extreme ST-segment elevations in seemingly no significant angiographic coronary artery abnormalities: a case report. BMC Cardiovasc Disord 2019;19:28–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lanza GA, Careri G, Stazi A, Villano A, Vita AD, Aurigemma C. et al. Clinical spectrum and outcome of patients with non-ST-segment elevation acute coronary syndrome and no obstructive coronary atherosclerosis. Circ J 2016;80:1600–6. [DOI] [PubMed] [Google Scholar]
- 23. Ouldzein H, Elbaz M, Roncalli J, Cagnac R, Carrié D, Puel J. et al. Plaque rupture and morphological characteristics of the culprit lesion in acute coronary syndromes without significant angiographic lesion: analysis by intravascular ultrasound. Ann Cardiol Angeiol (Paris) 2012;61:20–6. [DOI] [PubMed] [Google Scholar]
- 24. Reynolds HR, Srichai MB, Iqbal SN, Slater JN, Mancini GBJ, Feit F. et al. Mechanisms of myocardial infarction in women without angiographically obstructive coronary artery disease. Circulation 2011;124:1414–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mohri M, Koyanagi M, Egashira K, Tagawa H, Ichiki T, Shimokawa H. et al. Angina pectoris caused by coronary microvascular spasm. Lancet 1998;351:1165–9. [DOI] [PubMed] [Google Scholar]
- 26. Popovic B, Agrinier N, Bouchahda N, Pinelli S, Maigrat CH, Metzdorf PA. et al. Coronary embolism among ST-segment-elevation myocardial infarction patients. Circ Cardiovasc Interv 2018;11. [DOI] [PubMed] [Google Scholar]
- 27. Saw J, Mancini GBJ, Humphries KH.. Contemporary review on spontaneous coronary artery dissection. J Am Coll Cardiol 2016;68:297–312. [DOI] [PubMed] [Google Scholar]
- 28. Niccoli G, Scalone G, Crea F.. Acute myocardial infarction with no obstructive coronary atherosclerosis: mechanisms and management. Eur Heart J 2015;36:475–81. [DOI] [PubMed] [Google Scholar]
- 29. Tornvall P, Gerbaud E, Behaghel A, Chopard R, Collste O, Laraudogoitia E. et al. Myocarditis or “true” infarction by cardiac magnetic resonance in patients with a clinical diagnosis of myocardial infarction without obstructive coronary disease: a meta-analysis of individual patient data. Atherosclerosis 2015;241:87–91. [DOI] [PubMed] [Google Scholar]
- 30. Rossini R, Capodanno D, Lettieri C, Musumeci G, Limbruno U, Molfese M. et al. Long-term outcomes of patients with acute coronary syndrome and nonobstructive coronary artery disease. Am J Cardiol 2013;112:150–5. [DOI] [PubMed] [Google Scholar]
- 31. Ong P, Camici PG, Beltrame JF, Crea F, Shimokawa H, Sechtem U. et al. International standardization of diagnostic criteria for microvascular angina. Int J Cardiol 2018;250:16–20. [DOI] [PubMed] [Google Scholar]
- 32. Satoh S, Omura S, Inoue H, Mori T, Takenaka K, Numaguchi K. et al. Clinical impact of coronary artery spasm in patients with no significant coronary stenosis in acute coronary syndromes. J Cardiol 2013;61:404–9. [DOI] [PubMed] [Google Scholar]
- 33. Polytarchou K, Vouliotis A-I, Kappos K, Manolis AS.. Primary prevention of sudden cardiac death in Prinzmetal angina: the role of electrophysiology study in risk stratification. Hellenic J Cardiol 2016;57:205–9. [DOI] [PubMed] [Google Scholar]
- 34. Lebrun S, Bond RM.. Spontaneous coronary artery dissection (SCAD): the underdiagnosed cardiac condition that plagues women. Trends Cardiovasc Med 2018;28:340–5. [DOI] [PubMed] [Google Scholar]
- 35. Saw J, Humphries K, Aymong E, Sedlak T, Prakash R, Starovoytov A. et al. Spontaneous coronary artery dissection. J Am Coll Cardiol 2017;70:1148–58. [DOI] [PubMed] [Google Scholar]
- 36. Luong C, Starovoytov A, Heydari M, Sedlak T, Aymong E, Saw J.. Clinical presentation of patients with spontaneous coronary artery dissection. Catheter Cardiovasc Interv 2017;89:1149–54. [DOI] [PubMed] [Google Scholar]
- 37. Majidi M, Kosinski AS, Al-Khatib SM, Lemmert ME, Smolders L, van WA. et al. Reperfusion ventricular arrhythmia ‘bursts’ predict larger infarct size despite TIMI 3 flow restoration with primary angioplasty for anterior ST-elevation myocardial infarction. Eur Heart J 2008;30:757–64. [DOI] [PubMed] [Google Scholar]
- 38. Iliodromitis EK, Cohen MV, Dagres N, Andreadou I, Kremastinos DT, Downey JM.. What is wrong with cardiac conditioning? We may be shooting at moving targets. J Cardiovasc Pharmacol Ther 2015;20:357–69. [DOI] [PubMed] [Google Scholar]
- 39. van der WK, Prinzen FW, Gorgels AP.. Editor’s Choice—Reperfusion cardiac arrhythmias and their relation to reperfusion-induced cell death. Eur Hear J Acute Cardiovasc Care 2019;8:142–52. [DOI] [PubMed] [Google Scholar]
- 40. Jesel L, Berthon C, Messas N, Lim HS, Girardey M, Marzak H. et al. Ventricular arrhythmias and sudden cardiac arrest in Takotsubo cardiomyopathy: incidence, predictive factors, and clinical implications. Hear Rhythm 2018;15:1171–8. [DOI] [PubMed] [Google Scholar]
- 41. El-Battrawy I, Lang S, Ansari U, Tülümen E, Schramm K, Fastner C. et al. Prevalence of malignant arrhythmia and sudden cardiac death in takotsubo syndrome and its management. Europace 2018;20:843–50. [DOI] [PubMed] [Google Scholar]
- 42. Wang Z, Wang Y, Lin H, Wang S, Cai X, Gao D.. Early characteristics of fulminant myocarditis vs non-fulminant myocarditis: a meta-analysis. Medicine (Baltimore )2019;98:e14697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lynge TH, Nielsen TS, Gregers Winkel B, Tfelt-Hansen J, Banner J.. Sudden cardiac death caused by myocarditis in persons aged 1-49 years: a nationwide study of 14 294 deaths in Denmark. Forensic Sci Res 2019;4:247–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Harmon KG, Asif IM, Maleszewski JJ, Owens DS, Prutkin JM, Salerno JC. et al. Incidence and etiology of sudden cardiac arrest and death in high school athletes in the United States. Mayo Clin Proc 2016;91:1493–502. [DOI] [PubMed] [Google Scholar]
- 45. Maron BJ, Udelson JE, Bonow RO, Nishimura RA, Ackerman MJ, Estes NAM. et al. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: task force 3: hypertrophic cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy and other cardiomyopathies, and myocarditis. Circulation 2015;132. [DOI] [PubMed] [Google Scholar]
- 46. Te ALD, Wu T-C, Lin Y-J, Chen Y-Y, Chung F-P, Chang S-L. et al. Increased risk of ventricular tachycardia and cardiovascular death in patients with myocarditis during the long-term follow-up: a national representative cohort from the National Health Insurance Research Database. Medicine (Baltimore) 2017;96:e6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Peretto G, Sala S, Rizzo S, Palmisano A, Esposito A, Cobelli FD. et al. Ventricular arrhythmias in myocarditis. J Am Coll Cardiol 2020;75:1046–57. [DOI] [PubMed] [Google Scholar]
- 48. Abbate A, Bussani R, Liuzzo G, Biondi-Zoccai GGL, Barresi E, Mellone P. et al. Sudden coronary death, fatal acute myocardial infarction and widespread coronary and myocardial inflammation. Heart 2008;94:737–42. [DOI] [PubMed] [Google Scholar]
- 49. Yilmaz A, Mahrholdt H, Athanasiadis A, Vogelsberg H, Meinhardt G, Voehringer M. et al. Coronary vasospasm as the underlying cause for chest pain in patients with PVB19 myocarditis. Heart 2008;94:1456–63. [DOI] [PubMed] [Google Scholar]
- 50. Hu D, Viskin S, Oliva A, Cordeiro JM, Guerchicoff A, Pollevick GD. et al. Genetic predisposition and cellular basis for ischemia-induced ST-segment changes and arrhythmias. J Electrocardiol 2007;40:S26–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wang F, Liu Y, Liao H, Xue Y, Zhan X, Fang X. et al. Genetic variants on SCN5A, KCNQ1, and KCNH2 in patients with ventricular arrhythmias during acute myocardial infarction in a Chinese population. Cardiology 2020;145:38–45. [DOI] [PubMed] [Google Scholar]
- 52. Jabbari R, Glinge C, Jabbari J, Risgaard B, Winkel BG, Terkelsen CJ. et al. A common variant in SCN5A and the risk of ventricular fibrillation caused by first ST-segment elevation myocardial infarction. Song C, ed. PLoS One 2017;12:e0170193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hill SF, Sheppard MN.. Non-atherosclerotic coronary artery disease associated with sudden cardiac death. Heart 2010;96:1119–25. [DOI] [PubMed] [Google Scholar]