Background:
Preoperative planning software and a robotic device facilitate the placement of pedicle screws, especially in patients with difficult anatomy, thereby increasing the feasibility, accuracy, and efficiency of the procedure. The robot functions as a semiactive surgical assistive device whose goal is not to substitute but to offer the surgeon a set of versatile tools that can broaden his or her ability to treat patients1.
Description:
The robotic guidance system consists of a bed-mounted surgical arm and a workstation. We used the Mazor X Stealth Edition Robotic Guidance System by Medtronic for spine surgery, which has been previously described2-5. Unlike other systems that are navigation-based and require an optical tracking mechanism, this system relies on the preoperative plan to be referenced using the intraoperative registration. The workstation runs an interface software that facilitates preoperative planning, intraoperative image acquisition and registration, kinematic calculations, and real-time robot motion control. The robotic arm is mounted onto the bed as well as rigidly attached to the patient’s spine. It can move in 6 degrees of freedom to provide the preplanned screw trajectory and entry point thereby allowing the surgeon to manually perform the drilling and screw insertion through either an open or percutaneous procedure by first seating a drill tube and then drilling and tapping the hole as needed.
Alternatives:
Other robotic systems include the ROSA robot by Medtech, the ExcelsiusGPS robot by Globus Medical, and the SurgiBot and ALF‐X Surgical Robotic systems (both from TransEnterix). The Da Vinci Surgical System (Intuitive Surgical) has been utilized for laparoscopic anterior lumbar interbody fusion (ALIF), but it has not been approved by the U.S. Food and Drug Administration for actual spinal instrumentation. Alternative surgical techniques for pedicle screw placement include the freehand fluoroscopy-guided technique and intraoperative image-assisted computer navigation techniques, including isocentric C-arm (Iso-C) 3D (3-dimensional) navigation (Siemens), computed tomography (CT) navigation, O-arm navigation (Medtronic), CT-magnetic resonance imaging co-registration technology, and a 3D-visual guidance technique6-8.
Rationale:
The robotic-guided pedicle screw placement offers the following benefits over conventional dorsal instrumentation techniques: improved accuracy and safety in pedicle screw insertion2-4,9-13; precision in screw size selection and planned screw positioning2; a reduction in exposure to radiation for the surgeon, the patient, and the operating-room staff9,11,12,14-19; simplicity and user-friendliness with a moderate learning curve10,11,20,21; ease of registration and reduction of operating time2; significant enhancement of the surgeon’s ergonomics and dexterity for repetitive tasks in pedicle screw placement15,22-24; and a wider coverage in function to include utilization during minimally invasive surgery where applicable11,25.
Expected Outcomes:
Accuracy rates between 94.5% and 99%, comparable with those in our study10, have been reported with the robotic-guided pedicle screw insertion technique, even in studies involving complex deformities and revision surgeries for congenital malformations, degenerative disorders, destructive tumors, and trauma2-4,9-13. The safety of this technique, in terms of reduced complications and intraoperative radiation exposure, has also been documented as higher than that for freehand fluoroscopic guidance or other navigation techniques9,11,12,14-19. The feasibility of this procedure has been further extended to minimally invasive procedures and to use in the cervical region, with replication of its advantages. It is associated with a reasonable learning curve, with consistent successful results after 25 to 30 patients.
Important Tips:
The principles of robotic-guided pedicle screw placement are similar irrespective of the system used.
Although initially utilized mainly for thoracolumbar pedicle screw insertion, the latest robots and software have been adapted for use in the cervical spine with equivalent efficiency and accuracy.
Robotic guidance can be employed in non-pedicle-screw-insertion procedures.
Challenges include radiation exposure, trajectory failure, equipment and software failure, failed registration, logistics, time, and high cost.
Introductory Statement
The use of preoperative planning software and robotic guidance for the placement of pedicle screws facilitates the execution of the preoperative surgical plan, thereby increasing the feasibility and accuracy of the plan and helping to mitigate the risk of disastrous complications associated with the surgery because of the complex and variable pedicle anatomy and nearby structures.
Indications & Contraindications
Indications
Severe spinal deformities or osteoporosis and patients having repeat or revision surgery. Preoperative planning and robotic guidance have proven their necessity in many of these cases, with the ability to assist the surgeon to “visualize the invisible” difficult pedicle anatomy13,21.
Distorted or difficult pedicle anatomy as well as patients having minimally invasive surgery, those having less demanding open surgery involving pedicle screw insertion, and for cannula placement during vertebral cement augmentation.
Kyphosis, scoliosis, and other spinal deformities and patients with a history of spine surgery.
Spondylolisthesis, stenosis, spondylolysis, ankylosing spondylitis, fracture, neurofibromatosis, neuromuscular scoliosis, osteomyelitis, Scheuermann kyphosis, and spinal tumors.
Biopsy of spinal tumors and vertebral augmentation (vertebroplasty and kyphoplasty). Hu et al.26 highlighted the positive contribution and suitability of the use of robotic assistance in pedicle screw insertion in patients with spinal tumors, the results of which are in agreement with those of earlier studies for accuracy and safety with pedicle screw insertion3,4,9,10,27. They also highlighted the relevance of the technique for non-pedicle-screw-insertion procedures as well, such as biopsies and vertebral augmentation (vertebroplasty and kyphoplasty)26.
Pathologic entities including degenerative disorders, tumors, and trauma. Molliqaj et al.11 emphasized the challenge in the insertion of pedicle screws, especially in spinal deformity due to degeneration, tumors, and trauma, and in revision surgery because of the alteration of anatomical landmarks. They noted the solution provided in the robotic technique for these cases as evidenced by their results in terms of the accuracy and safety of the technique in comparison with the conventional freehand fluoroscopic technique.
Besides the use of the robotic-guided technique in targeting biopsies, other non-pedicle-screw-insertion indications for the technique include gaining access to extraforaminal disc herniation in distorted anatomic spaces and to arteriovenous fistulas with obscured vascular entry points into the spinal canal12.
In addition to the stabilization of spinal segments using pedicle screws, the robotic technology can facilitate the use of translaminar facet screws2,15.
The cervical spine is unique because of the limited surgical access in the face of naturally complex anatomy. The target bone volume for insertion of the screws is much smaller in the cervical spine, with important blood vessels and the sensitive neural structures running intimately within the same bone complex. At the least, there is a more crucial need for precision in pedicle screw insertion in this region, and robotic guidance technology provides the feasibility and accuracy relevant to the procedure12. Although the robotic technique initially was used mainly for thoracolumbar pedicle screw insertion, its role has been extended for use in the cervical spine. The latest robots and software have been adapted for use in this region with equivalent levels of efficiency and accuracy of placement. We used the Mazor X Stealth Edition Robotic Guidance System by Medtronic (Fig. 1) for spine surgery, which has been previously described2-5.
Fig. 1.
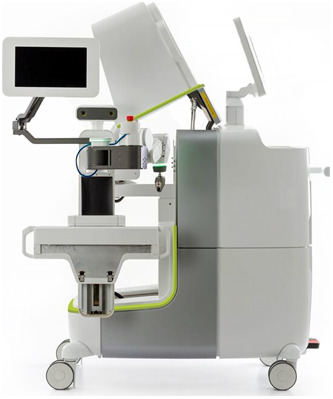
The Mazor X Stealth Robotic guidance system for spine surgery (Medtronic). (Image courtesy of Dr. Isador H. Lieberman.)
Contraindications
The inability to obtain accurate registration because of massive obesity or severe osteoporosis.
A lack of familiarity with standard open techniques of pedicle screw placement.
Step-by-Step Description of Procedure
Step 1: Confirm Indications for Surgery and Obtain Informed Consent
Ensure that the patient and family members understand the risks and benefits of operative and nonoperative treatment and, if operative treatment is appropriate, explain the risks and benefits of the use of the robotic guidance procedure.
Discuss extensively with the patient and the family members the risks, benefits, and alternatives to nonoperative and operative treatment options.
If operative treatment is appropriate, determine the utility of the robotic guidance procedure and explain that to the patient.
Obtain informed consent from the patient and/or family members.
Step 2: Preoperative Planning
Obtain the appropriate preoperative imaging, including radiographs and computed tomography (CT) scans, and upload them to the robotic surgical assistance system software to plan the pedicle screw placement and trajectories, and on the day of surgery, transfer the preoperative plan to the robotic workstation.
Obtain the appropriate preoperative spine imaging, including full-length spine radiographs, appropriate bending and flexion-extension radiographs, and a thin-slice (1-mm) CT scan of the contemplated surgical levels and the immediately adjacent vertebral spine levels.
Upload the CT images to the robotic surgical assistance preplanning program software system to facilitate the planning process.
On the basis of these images and using the software, simulate different implants by selecting the length, diameter, orientation, and angle of insertion, on a virtual 3-dimensional (3D) model of the spine, to plan the desired trajectories and placement of the pedicle screws and rods (Figs. 2-A through 2-D, Video 1).
On the day of the surgery and at the beginning of the operation, transfer this preoperative plan via a storage device to the robot workstation connected to both the robotic guidance device and the operating room C-arm, to facilitate the plan intraoperatively.
Alternatively, in lieu of a preoperative CT scan, use an intraoperative 3D cone beam CT scan (O-arm; Medtronic) to obtain registration imaging. Then create the plan in the operating room on the robot console. The matching process performed by the software is the same regardless of the use of fluoroscopic images or intraoperative CT images.
Figs. 2-A through 2-D Planning the placement of the pedicle screws and rods in a virtual 3D model of the spine. Blue (right) and yellow (left) markings indicate the planned rod and screw trajectories and measurements. Figs. 2-C and 2-D Planned screw trajectories.
Figs. 2-A.
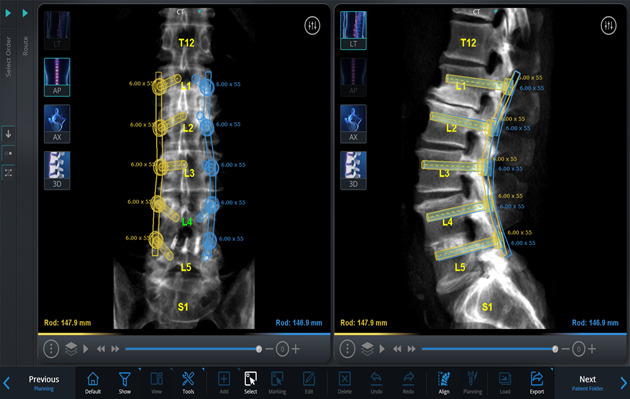
Coronal and midsagittal views of the lumbar spine showing all the planned rods, screw trajectories, and measurements from L1 to L5.
Figs. 2-B.
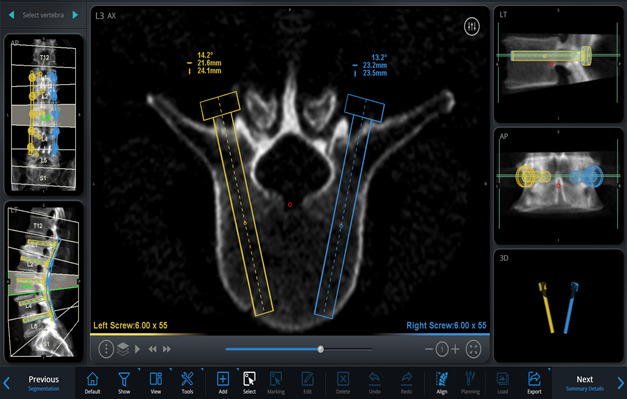
Axial view of L3 vertebral body at the level bisecting the planned screw trajectories along their length.
Figs. 2-C.
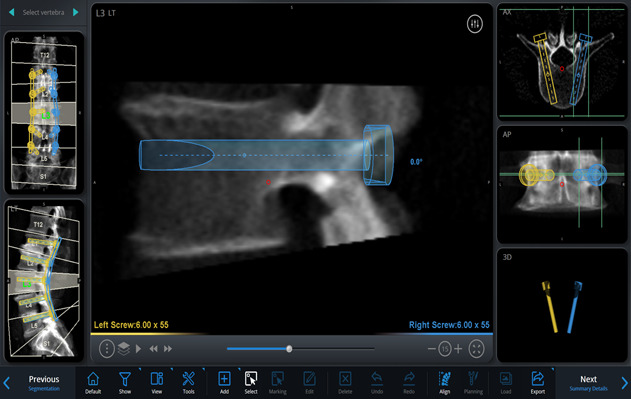
Right-sided sagittal view showing the planned right screw trajectory completely within the right L3 pedicle.
Figs. 2-D.
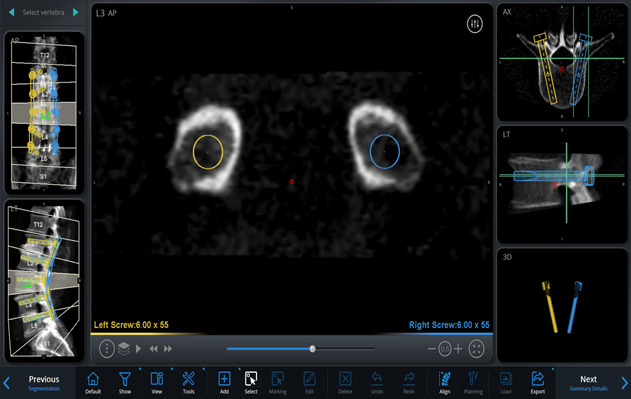
Transpedicular coronal view showing both the right and left screw trajectories completely within their respective pedicles at the L3 vertebral body level.
Video 1.
Preoperative planning for a patient with degenerative spondylolisthesis.
Step 3: Patient Positioning, Preparation, and Neurophysiological Monitoring
Correct patient positioning, preparation, and draping, and precise intraoperative neurophysiological monitoring are paramount for the success of the operation.
After the patient is brought to the operating room, place him or her under general anesthesia with hemodynamic monitoring.
Position the patient prone on the Jackson table (Mizuho OSI 5803 Jackson Table Base) with a 4-post frame.
Initiate spinal cord monitoring with somatosensory evoked potentials, motor evoked potentials, and electromyography monitoring.
Obtain the best baseline signals.
Prepare and drape the patient in a typical sterile fashion.
Drape the surgical system and the surgeon’s screen with sterile sleeves (Fig. 3).
Fig. 3.
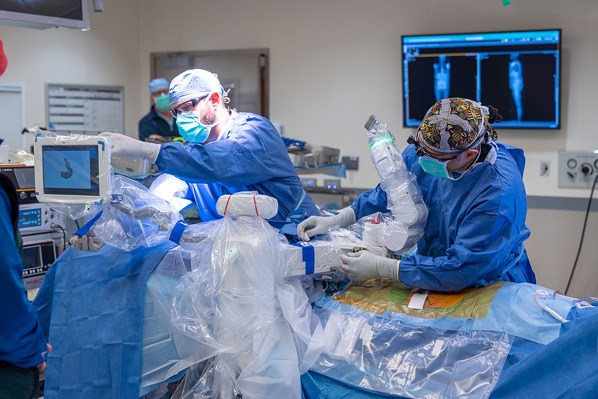
Draping the surgical system and the surgeon’s screen with sterile sleeves.
Step 4: Exposure
For open procedures, dissect the skin through a midline incision and expose the spine over the proposed segments, and for minimally invasive procedures, create a small incision directly over a spinous process or place Schanz pins into the posterior superior iliac spine.
For open procedures, dissect the skin down sharply through a midline incision and into the subcutaneous tissue to expose the spine over the proposed segments. Observe meticulous hemostasis. Extend the dissection subperiosteally over the facet joints to the tips of the transverse processes. Remove the remaining soft tissue off the osseous areas using a Cobb elevator and pituitary rongeur. Confirm the level of the spine both clinically and radiographically with fluoroscopic images. Apply the retractors to maintain the exposure.
For minimally invasive or percutaneous procedures, create a small incision directly over a spinous process or place Schanz pins into the posterior superior iliac spine.
Step 5: Attaching the Robot, Image Registration, and Referencing
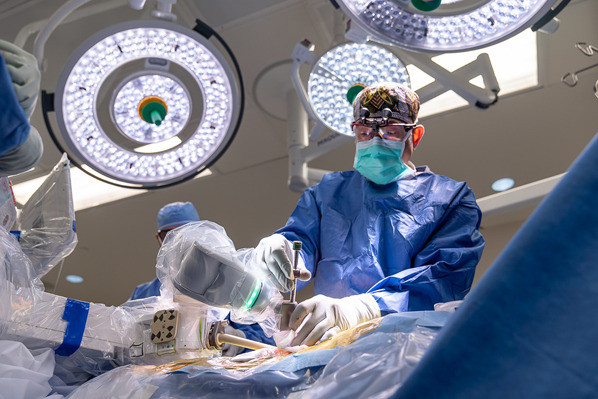
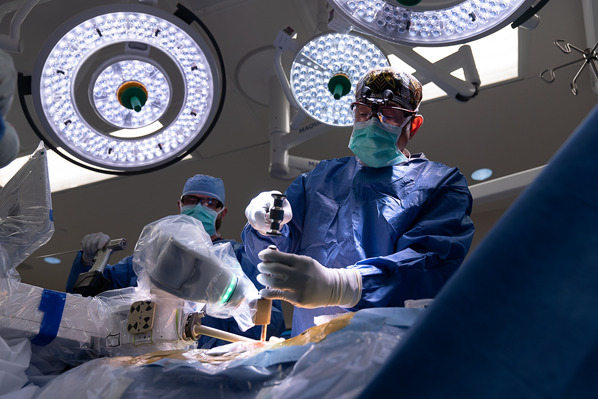
Rigidly affix the robotic guidance system to the patient’s skeletal anatomy through a secure, solid platform and perform automatic image registration and referencing (Video 2).
Mount the robotic platform clamp onto the spinous process of the accessible vertebrae over the proposed levels of the fusion or the Schanz pin inserted in the posterior superior iliac spine. For open cases, use the clamp platform attached to the exposed spinous processes, whereas for implantation of S2 alar-iliac screws or iliac bolts, use iliac crest pins. Either configuration can be used for minimally invasive or percutaneous screw placement.
Bring the robot into the sterile field and attach the robot bridge to the clamps or pins to facilitate the link between the robot and spine. This optimizes the stability of the robot and spine and minimizes the risk of drill skiving.
Rigidly secure the components to one another using bolts and a driver.
Move the fluoroscope into the surgical field.
Send the robot arm with the reference frame into position over the segments to be instrumented and obtain anteroposterior and oblique radiographs for registration and referencing (Figs. 4 and 5); this step serves to define the position of each vertebra in 3D spaces with respect to the mounting platform.
The images are then matched by the system software (Mazor X Align) to the preoperative CT scans containing the preoperative plan.
Once the images are made and the registration is verified, remove the reference frame from the robotic arm, and move the fluoroscope out of the surgical field.
Fig. 4.
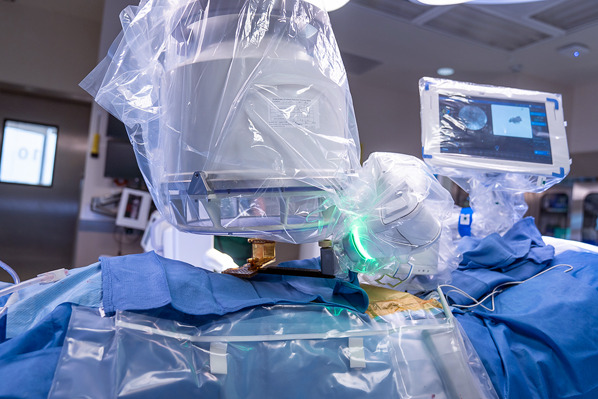
Making the anteroposterior radiograph with the reference frame strategically positioned over the segments to be instrumented for registration and referencing.
Fig. 5.
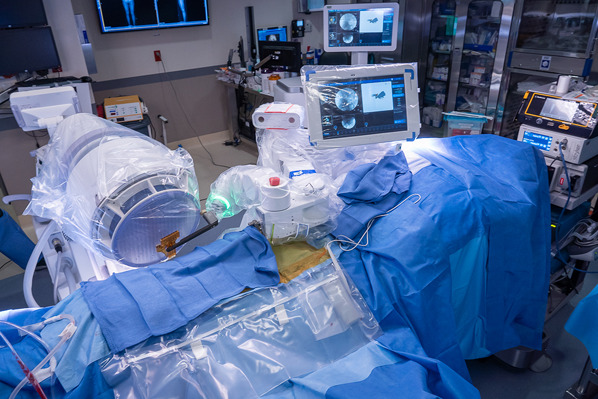
Making the oblique radiograph with the reference frame strategically positioned over the segments to be instrumented for registration and referencing.
Video 2.
Mounting the robot, securing the robot to the spine, and image registration and referencing.
Step 6: Robotic-Assisted Pedicle Screw Placement
Manually place the pedicle screws along the preplanned trajectory under robotic guidance (Video 2 and 3).
Activate the surgical arm.
Dispatch the robot to the previously planned, precise position for pedicle screw placement at each of the planned levels.
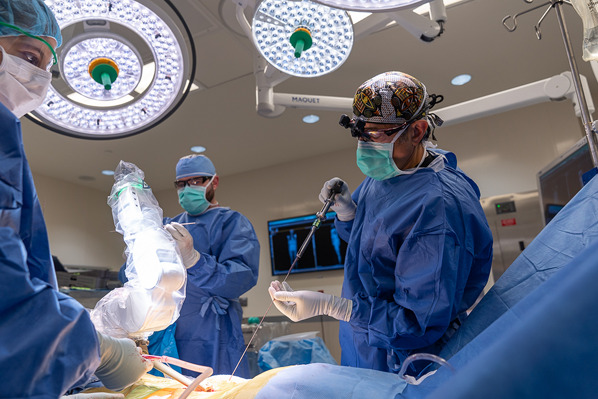
Once the robot reaches 1 of its final positions, pass the drill tube with trocar through the end effector of the arm (Figs. 6-A and 6-B).
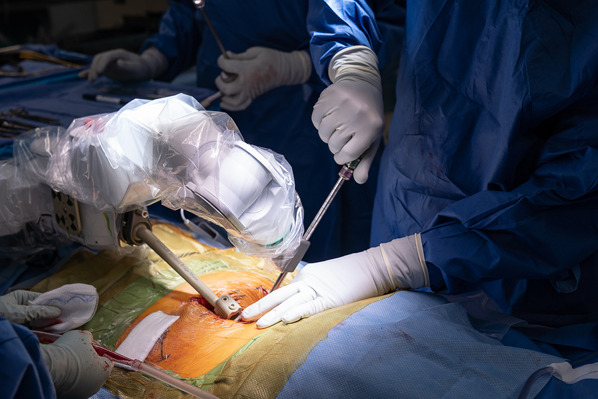
Remove the trocar and replace it with a serrated drill guide (Figs. 7-A and 7-B).
Place the drill into the guide and perform the drilling process in oscillation mode (Fig. 8).
Once drilling is complete, place an exchange tube into the drill guide and remove the arm together with the drill guide.
Pass a Kirschner wire through the exchange tube, remove the exchange tube, then tap the channel, and insert the pedicle screw (Figs. 9-A and 9-B).
Repeat this procedure for all screw positions until all of the screws are in position, then make a final radiograph to verify the position of all of the screws.
Depending on the implant system, the use of Kirschner wires may be optional. The instruments of some pedicle screw systems can fit directly through the end effector of the robotic arm.
Alternatively, all of the Kirschner wires could be placed at the desired screw trajectory positions under robotic guidance before the actual placement of the screws. This is particularly relevant for minimally invasive surgical procedures.
Figs. 6-A and 6-B Once the robot reaches 1 of its final positions, the drill tube with the blunt trocar is passed through the end effector of the arm.
Fig. 6-A.
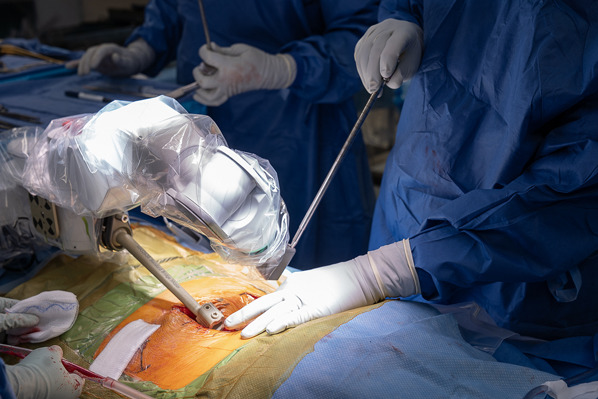
Beginning the insertion of the drill tube cannula with the blunt trocar.
Fig. 6-B.
Removing the trocar.
Figs. 7-A and 7-B Passing the second drill cannula and tapping it in place.
Fig. 7-A.
Passing the second drill cannula, which is serrated at its end and with a narrower lumen, through the initial drill tube cannula.
Fig. 7B.
The serrated second drill cannula with a narrower lumen is tapped into place to maintain its position and perform its function as the drill guide.
Fig. 8.
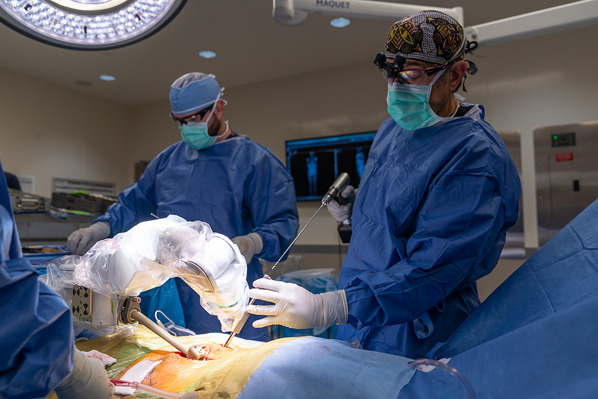
Placing the drill into the guide and performing the drilling process in oscillation mode for the pilot hole.
Figs. 9-A and 9-B Passing a Kirschner wire through the exchange tube, removing the exchange tube, tapping the channel, and inserting the pedicle screw.
Fig. 9-A.
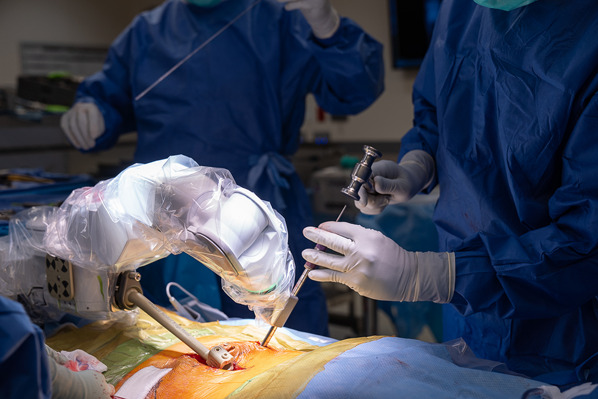
Passing a Kirschner wire through the exchange tube.
Fig. 9-B.
Inserting the pedicle screw over the Kirshner wire after removing the working drill cannulas, the robotic arm, and exchange tube only out of the way.
Video 3.
Placing pedicle screws using robotic technology.
Step 7: Segmental Correction, Decortication, and Bone-Grafting
Perform an appropriate segmental correction and securely lock the construct in place while closely monitoring the neurophysiological signals, followed by decortication and placement of morselized bone graft to enhance fusion.
Once all of the screws are placed and confirmed, remove the robotic apparatus.
Complete any other procedure-associated decompressions or osteotomies.
Measure, contour, insert, and fasten the rods proximally and then distally onto the heads of the screws in situ, and then perform segmental correction and derotation as needed.
Once the desired correction with strategic distraction and compression is achieved, lock the construct in place.
Check and confirm that there is no change in the neurophysiological monitoring signals.
Make another radiograph to verify the overall correction.
Decorticate the remaining laminae and transverse processes; amputate the spinous processes, and morselize and add them to the bone graft mixture.
Step 8: Closure and Immediate Postoperative Care
Close the incision in multiple layers and provide postoperative care as dictated by the extent of the procedure.
Close the incision in multiple layers with an absorbable subcutaneous stitch.
Postoperative care is dictated by the extent of the procedure and the usual preference of the surgeon.
Results
The importance of the robotic-guided pedicle screw insertion technique in spine surgery has been verified by the higher accuracy, safety, and feasibility rates of the procedure compared with its conventional alternatives as noted in several studies3,26,28. Accuracy rates between 94.5% and 99%, comparable with those in our study10, have been reported, even in studies involving complex severe deformity and/or revision surgeries due to congenital malformations, degenerative disorders, destructive tumors, and trauma2-4,9-13. The safety of this technique, in terms of reduced complications and intraoperative radiation exposure, has also been documented as higher than that reported for freehand fluoroscopic guidance or other navigation techniques9,11,12,14-19.
The feasibility of this procedure has been further extended to minimally invasive procedures, and in the cervical region, with replication of its advantages and outcomes10. It is associated with a reasonable learning curve, with consistent successful results seen after 25 to 30 patients.
Further review of the literature revealed that robotic guidance in percutaneous and minimally invasive approaches not only reduces radiation exposure substantially to the patient and the operating-room personnel during the procedure but also provides the surgeon with more confidence to carry out the procedure9,12,29.
It should be noted that in robotic-assisted spine surgery, the goal is not to replace the surgeon with the robot but rather to provide the surgeon with a versatile set of tools that can extend her or his ability to treat patients. The robot in this case only facilitates the execution of the preoperative plan, but the surgeon actually performs the surgery. One must therefore emphasize that the robot is a tool that helps to make a good surgeon more precise and efficient but will not make a bad surgeon good.
Lieberman et al., in their study on the feasibility and accuracy of the robotic technique, retrospectively reviewed 10 consecutive cases that underwent robotic-guided placement of cervical (C2 to C7) pedicle screws (unpublished data). No intraoperative complications related to the placement of cervical pedicle screws or the use of the robot were observed. They also found that only 10 (12.8%) of 78 cervical pedicle screws placed in the 10 patients had pedicle screw breaches, all of which were medial, <1 mm, of no clinical consequences, and were attributed to the narrow waist of the pedicle. (Fig. 10). The overall average deviation of the inserted screws from the preoperative plan was 1.25 mm in both axial and coronal planes.
Fig. 10.
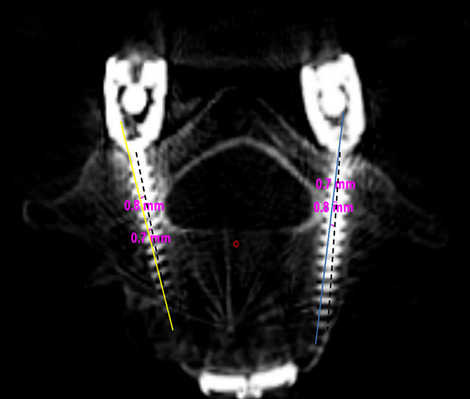
Robotic software image of fusion of the axial CT images of the preoperative planned screw trajectories and postoperative visualized screws. Blue (right) and yellow (left) markings indicate the planned trajectories, dashed black lines indicate the executed trajectory, and pink inscriptions indicate the deviation measurements from the planned preoperative trajectory. Pedicle screws shown are completely within the margins of the respective pedicles.
Robotic guidance has also been employed in non-pedicle-screw-insertion procedures such as biopsies and vertebral augmentation, especially in spinal tumor cases, and in targeting extraforaminal disc prolapses in distorted anatomic spaces or arteriovenous fistulas with obscured vascular entry points into the spinal canal12. The diversity in patient diagnosis, as well as the complexity of the spine surgeries that have been carried out with this technique, demonstrates the broad utility of the robotic-assisted system across patient populations.
Pitfalls & Challenges
-
Surgical technique
-
○
Despite the precision of pedicle screw placement with the robotic guidance procedure, the risk of complications, pitfalls, and challenges exists. A study noted issues at rates between 1% and 54%28 associated with the surgical technique, the robotic equipment, the software program, radiation exposure or logistical issues, or clinical complications. Therefore, it is imperative that the surgeon maintains a clear appreciation of the surgical anatomy as well as the ability to manage unexpected intraoperative events, such as opting to insert the screws by the conventional techniques despite the availability of the robotic system.
-
○
-
Screw malposition
-
○
In their study in 2017, Molliqaj et al. indicated that the rate of clinically “unacceptable” screws (corresponding to Gertzbein-Robbins Grades C, D, and E) in the robot group was 6.6%11. Hu and Lieberman, in 2014, reported a mean rate of screw malposition of 0.66%28. However, they acknowledged that the use of intraoperative biplanar fluoroscopy and postoperative radiographs for assessment of screw placement accuracy instead of postoperative CT scans was the cause, in some cases, of the very low rate. A similar rate of 1.1% was reported by the same authors in an earlier study10. In studies in which postoperative CT scans were done systematically, screw malposition rates as high as 15.7% have been reported30. Roser et al., in 2013, reported suboptimal accuracy for 7.8% of the 244 robotic-assisted pedicle screws in 46 patients, 2.5% of which were medially deviated and needed revision surgery12. The screw malposition was attributed to the “skiving” phenomenon, in which the percutaneously inserted cannula, trocar, or drill bit skirts off an osseous surface, such as a facet osseous spur, and causes inaccuracy of the drilling procedure. During the preoperative planning, the surgeon must appreciate the potential for skiving and plan an entry point on a flat surface or in a “stable valley” to avoid skiving. Depending on the anatomic level and the size and the trajectory of the pedicle, the docking point can be variable. It is preferable to dock in a stable valley provided the screw can be safely positioned in the vertebral body and not violate the epidural space. Occasionally, an “in-out-in” trajectory is required for the screw, which means docking on the transverse process to achieve the ideal screw path.
-
○
The rate of malpositioning of pedicle screws has been shown to be higher at the sacral level than at the lumbar level, and more so in patients having traditional open surgery11,21. This is due, in part, to the large lever arm related to the robotic cannula (often needed for approaching the S1 entry point) and, laterally, the soft-tissue pressure exerted on the cannula itself. In deep robust patients (those with a high body-mass index or high muscle bulk [athletic], or substantial curvature at the lumbosacral region), an option to avoid this malpositioning is to use a combined open and percutaneous approach through a stab incision at the sacral level. Alternatively, maintaining great soft-tissue retraction, while inserting sacral pedicle screws, affords a more lateral entry point and a more medially directed trajectory to achieve the correct trajectory11.
-
○
Ringel et al., in 2012, proposed similar reasons for dislocations of the cannula from the planned trajectory, which included the hindrances caused by muscle bundles and other soft tissue25. Obstruction by the iliac crest, especially in the case of S1 level screws, was also reported.
-
○
Equipment and/or software failure or malfunction could force the surgeon to abandon the use of robotic guidance and resort to a conventional freehand technique.
-
Failed or poor registration
-
○
An earlier study on the lessons learned from the first 102 patients who underwent robotic-assisted pedicle screw placement by the same surgeon noted that the robot was not used as planned in 7 patients10. Instead, the screws were placed manually, and no intraoperative neurologic or vascular complications were encountered. Most manual placements were necessary, in part, because of underlying patient conditions. In 4 of these patients, the underlying pathology precluded adequate intraoperative anteroposterior and oblique fluoroscopic images used for registration. This included:
-
○
-
▪
Overly severe deformity due to a high degree of curvature leading to inadequate registration.
-
▪
Scheuermann kyphosis with underlying high body mass index (49.9 kg/m2), which precluded the attainment of good fluoroscopic radiographic images for registration.
-
▪
In an elderly female patient with severe osteoporosis, the robotic system was unable to recognize the vertebral anatomy from the poor-quality intraoperative fluoroscopic images.
-
▪
In a patient having a revision, movement of the previously implanted, loosened hardware caused difficulty during registration.
The factors responsible for manual placement in the remaining 3 cases were the following:
-
▪
In the fifth patient, who had L5-S1 spondylolysis, the difficulty in seating the drill bit on the mobile posterior elements of the lytic segment forced the surgeon to abort the use of the robot.
-
▪
In the other 2 patients, who both had degenerative scoliosis, the robot system failed because of technical issues, e.g., a software crash, even though the anatomy of the patients was well appreciated.
-
○
Roser et al., in 2013, also reported failed execution of the planned robotic procedure in 2 patients because of failure to match the intraoperative imaging to the preoperative CT scan12. This was due to bad bone quality on intraoperative fluoroscopic images in 1 patient and artifacts overlying the region of interest, such as a pacemaker cable or sternal wiring from previous cardiac surgery, in the other. Both resulted in failed registration, and the open procedure was performed instead.
-
○
Similar findings involving failed execution of the robotic procedure were reported by Joseph et al., in 2017, in their meta-analysis16.
-
○
-
Trajectory issues
-
○
Previously, the robotic system was fixed by the bed mount technique (a platform fixed to a cranial spinous process with a Kirschner wire and caudally attached to the operating table), which was insufficient and allowed movement of the robot relative to the patient, resulting in trajectory discrepancies and malpositioning of the screws10,25,31. Recent robotic system versions, however, are much more rigidly fixed to the spine in order to reinforce stability10,25,32.
-
○
In some instances, by virtue of the limits of arm excursion, the robot may not be able to reach the appropriate trajectory angles planned preoperatively3,10,16.
-
○
-
▪
In terms of anatomy, it is of note that the thoracic levels are associated with a smaller pedicle size. Also, the intrathoracic organs and rib cage make this region difficult to image. A higher level of failed registration and/or trajectory issues is much more common in this region10.
-
Radiation exposure
-
○
The robotic guidance technique provides a 40% to 70% reduction in the intraoperative radiation exposure rate for the patient, the surgeon, and the operating-room personnel in comparison with the open conventional freehand fluoroscopic technique9,10,12,14,19,29, with an average radiation exposure time of 34 and 77 seconds, respectively. Despite this fact, an average radiation exposure time of 34 seconds is still substantial9.
-
○
There is also a substantial risk of exposure for the patient for whom a preoperative CT scan is mandatory before the preoperative planning10.
-
○
It is important, however, to note that some studies have shown equal, or even greater, rates of radiation exposure associated with the robotic guidance technique in comparison with the freehand fluoroscopic technique25. It is hypothesized that the rate of radiation exposure will decrease with improved use of the robotic guidance technique.
-
○
-
Logistics (time, costs, etc.):
-
○
Time: The setting up of the computer system and the robotic arm, the software start-up process, and attaching the robotic clamp onto the spinous processes result in increased length of the surgical procedure in comparison to the conventional freehand fluoroscopic technique, most markedly in the surgeon’s initial cases11. This increased setup time, in addition to that required for preoperative planning, presents a setback for this novel system.
-
○
Operating-room setup: The robotic equipment setup takes up a space equivalent to that needed for an instrument trolley. This extra requirement complicates the conventional workspace within the operating room11.
-
○
Operational costs: Extra apparatus, such as a fiducial marker for the C-arm, and sterile material that must be procured for the smooth running of the robotic guidance procedure, present an additional expense. It is important to assess the hypothetical clinical benefit of this procedure against the incurred expenses, especially in future studies11.
-
○
“New technology” phenomenon: Like any novel technology, robotic-assisted spine surgery faces the challenge of skill development and assessment, teaching, and translation into the operating room, as well as a learning curve for the surgeon28,33.
-
○
-
Clinical complications
-
○
Screw malposition could potentially result in injury to vessels, neural structures, and the dura, especially when the patient’s anatomy is altered. Misplaced screws could also lead to instability, fractures, biomechanically weaker constructs, and lower fusion rates2,10,21,30. Although some studies have noted pedicle screw-related complication rates ranging from 1% to 54%10,34-36, there is scant information to distinguish actual rates of screw malposition from individual clinical complications.
-
○
Clinical complications may also result from failure of the implants themselves.
-
○
-
Nerve impingement
-
○
According to a study by Hu et al., in 2013, 11 (1.1%) of 960 screws were malpositioned, and of those, 10 were recognized immediately and were manually repositioned intraoperatively10. In the patient in whom the malpositioned screw was not immediately recognized, an L3 radiculopathy developed in the immediate postoperative period. After a CT scan was done 3 days postoperatively to confirm the malpositioned screw, the patient underwent surgery to remove the loose screw.
-
○
Published outcomes of this procedure can be found at: Eur Spine J. 2013 Mar;22(3):661-6, Clin Orthop Relat Res. 2014 Jun;472(6):1839-44, and Int J Spine Surg. 2015 Feb 3;9:1-10.
Investigation performed at the Scoliosis and Spine Tumor Center, Texas Back Institute, Plano, Texas
Disclosure: The authors indicated that no external funding was received for any aspect of this work. On the Disclosure of Potential Conflicts of Interest forms, which are provided with the online version of the article, one or more of the authors checked “yes” to indicate that the author had a relevant financial relationship in the biomedical arena outside the submitted work (http://links.lww.com/JBJSEST/A294).
References
- 1.Hu X, Lieberman IH. Robotic-assisted spine surgery. In: Phillips FM, Lieberman IH, Polly DW, Jr, editors. Minimally invasive spine surgery: surgical techniques and disease management. New York: Springer; 2014. p 61-6. [Google Scholar]
- 2.Lieberman IH, Togawa D, Kayanja MM, Reinhardt MK, Friedlander A, Knoller N, Benzel EC. Bone-mounted miniature robotic guidance for pedicle screw and translaminar facet screw placement: part I—technical development and a test case result. Neurosurgery. 2006. September;59(3):641-50; discussion 641-50. [DOI] [PubMed] [Google Scholar]
- 3.Devito DP, Kaplan L, Dietl R, Pfeiffer M, Horne D, Silberstein B, Hardenbrook M, Kiriyanthan G, Barzilay Y, Bruskin A, Sackerer D, Alexandrovsky V, Stüer C, Burger R, Maeurer J, Donald GD, Schoenmayr R, Friedlander A, Knoller N, Schmieder K, Pechlivanis I, Kim IS, Meyer B, Shoham M. Clinical acceptance and accuracy assessment of spinal implants guided with SpineAssist surgical robot: retrospective study. Spine. 2010. November 15;35(24):2109-15. [DOI] [PubMed] [Google Scholar]
- 4.Pechlivanis I, Kiriyanthan G, Engelhardt M, Scholz M, Lücke S, Harders A, Schmieder K. Percutaneous placement of pedicle screws in the lumbar spine using a bone mounted miniature robotic system: first experiences and accuracy of screw placement. Spine. 2009. February 15;34(4):392-8. [DOI] [PubMed] [Google Scholar]
- 5.Togawa D, Kayanja MM, Reinhardt MK, Shoham M, Balter A, Friedlander A, Knoller N, Benzel EC, Lieberman IH. Bone-mounted miniature robotic guidance for pedicle screw and translaminar facet screw placement: part 2—evaluation of system accuracy. Neurosurgery. 2007. February;60(2)(Suppl 1):ONS129-39; discussion ONS139. [DOI] [PubMed] [Google Scholar]
- 6.Overley SC, Cho SK, Mehta AI, Arnold PM. Navigation and robotics in spinal surgery: where are we now? Neurosurgery. 2017. March 1;80(3S):S86-99. [DOI] [PubMed] [Google Scholar]
- 7.Liu H, Wang Y, Pi B, Qian Z, Zhu X, Yang H. Comparison of intraoperative O-arm- and conventional fluoroscopy (C-arm)-assisted insertion of pedicle screws in the treatment of fracture of thoracic vertebrae. J Orthop Surg (Hong Kong). 2017. January;25(1):2309499016684090. [Google Scholar]
- 8.Abe Y, Ito M, Abumi K, Kotani Y, Sudo H, Minami A. A novel cost-effective computer-assisted imaging technology for accurate placement of thoracic pedicle screws. J Neurosurg Spine. 2011. November;15(5):479-85. Epub 2011 Jul 15. [DOI] [PubMed] [Google Scholar]
- 9.Kantelhardt SR, Martinez R, Baerwinkel S, Burger R, Giese A, Rohde V. Perioperative course and accuracy of screw positioning in conventional, open robotic-guided and percutaneous robotic-guided, pedicle screw placement. Eur Spine J. 2011. June;20(6):860-8. Epub 2011 Mar 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu X, Ohnmeiss DD, Lieberman IH. Robotic-assisted pedicle screw placement: lessons learned from the first 102 patients. Eur Spine J. 2013. March;22(3):661-6. Epub 2012 Sep 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Molliqaj G, Schatlo B, Alaid A, Solomiichuk V, Rohde V, Schaller K, Tessitore E. Accuracy of robot-guided versus freehand fluoroscopy-assisted pedicle screw insertion in thoracolumbar spinal surgery. Neurosurg Focus. 2017. May;42(5):E14. [DOI] [PubMed] [Google Scholar]
- 12.Roser F, Tatagiba M, Maier G. Spinal robotics: current applications and future perspectives. Neurosurgery. 2013. January;72(Suppl 1):12-8. [DOI] [PubMed] [Google Scholar]
- 13.van Dijk JD, van den Ende RP, Stramigioli S, Köchling M, Höss N. Clinical pedicle screw accuracy and deviation from planning in robot-guided spine surgery: robot-guided pedicle screw accuracy. Spine. 2015. September 1;40(17):E986-91. [DOI] [PubMed] [Google Scholar]
- 14.Barzilay Y, Schroeder JE, Hiller N, Singer G, Hasharoni A, Safran O, Liebergall M, Itshayek E, Kaplan L. Robot-assisted vertebral body augmentation: a radiation reduction tool. Spine. 2014. January 15;39(2):153-7. [DOI] [PubMed] [Google Scholar]
- 15.Stüer C, Ringel F, Stoffel M, Reinke A, Behr M, Meyer B. Robotic technology in spine surgery: current applications and future developments. Acta Neurochir Suppl (Wien). 2011;109:241-5. [DOI] [PubMed] [Google Scholar]
- 16.Joseph JR, Smith BW, Liu X, Park P. Current applications of robotics in spine surgery: a systematic review of the literature. Neurosurg Focus. 2017. May;42(5):E2. [DOI] [PubMed] [Google Scholar]
- 17.Hyun SJ, Kim KJ, Jahng TA, Kim HJ. Minimally invasive robotic versus open fluoroscopic-guided spinal instrumented fusions: a randomized controlled trial. Spine. 2017. March 15;42(6):353-8. [DOI] [PubMed] [Google Scholar]
- 18.Keric N, Doenitz C, Haj A, Rachwal-Czyzewicz I, Renovanz M, Wesp DMA, Boor S, Conrad J, Brawanski A, Giese A, Kantelhardt SR. Evaluation of robot-guided minimally invasive implantation of 2067 pedicle screws. Neurosurg Focus. 2017. May;42(5):E11. [DOI] [PubMed] [Google Scholar]
- 19.Schoenmayr R, Kim IS. Why do I use and recommend the use of navigation? ArgoSpine News J. 2010. December;22(4):132-5. [Google Scholar]
- 20.Kim HJ, Lee SH, Chang BS, Lee CK, Lim TO, Hoo LP, Yi JM, Yeom JS. Monitoring the quality of robot-assisted pedicle screw fixation in the lumbar spine by using a cumulative summation test. Spine. 2015. January 15;40(2):87-94. [DOI] [PubMed] [Google Scholar]
- 21.Schatlo B, Martinez R, Alaid A, von Eckardstein K, Akhavan-Sigari R, Hahn A, Stockhammer F, Rohde V. Unskilled unawareness and the learning curve in robotic spine surgery. Acta Neurochir (Wien). 2015. October;157(10):1819-23; discussion 1823. Epub 2015 Aug 19. [DOI] [PubMed] [Google Scholar]
- 22.Kelly PJ. Neurosurgical robotics. Clin Neurosurg. 2002;49:136-58. [PubMed] [Google Scholar]
- 23.Louw DF, Fielding T, McBeth PB, Gregoris D, Newhook P, Sutherland GR. Surgical robotics: a review and neurosurgical prototype development. Neurosurgery. 2004. March;54(3):525-36; discussion 536-7. [DOI] [PubMed] [Google Scholar]
- 24.Bozzio A, Hu Z, Lieberman IH. Robotic-assisted spine surgery. In: Phillips FM, Lieberman IH, Polly DW, Wang MY, editors. Minimally invasive spine surgery. 2nd ed. Springer; 2019. p 93-100 [Google Scholar]
- 25.Ringel F, Stüer C, Reinke A, Preuss A, Behr M, Auer F, Stoffel M, Meyer B. Accuracy of robot-assisted placement of lumbar and sacral pedicle screws: a prospective randomized comparison to conventional freehand screw implantation. Spine. 2012. April 15;37(8):E496-501. [DOI] [PubMed] [Google Scholar]
- 26.Hu X, Scharschmidt TJ, Ohnmeiss DD, Lieberman IH. Robotic assisted surgeries for the treatment of spine tumors. Int J Spine Surg. 2015. February 3;9:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sukovich W, Brink-Danan S, Hardenbrook M. Miniature robotic guidance for pedicle screw placement in posterior spinal fusion: early clinical experience with the SpineAssist. Int J Med Robot. 2006. June;2(2):114-22. [DOI] [PubMed] [Google Scholar]
- 28.Hu X, Lieberman IH. What is the learning curve for robotic-assisted pedicle screw placement in spine surgery? Clin Orthop Relat Res. 2014. June;472(6):1839-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lieberman I, Hardenbrook M, Wang J, Guyer RD, Khanna AJ. P152. Radiation exposure using miniature robotic guidance for spinal surgery. Spine J. 2007;7:152S-3S. [Google Scholar]
- 30.Hicks JM, Singla A, Shen FH, Arlet V. Complications of pedicle screw fixation in scoliosis surgery: a systematic review. Spine. 2010. May 15;35(11):E465-70. [DOI] [PubMed] [Google Scholar]
- 31.Cahill KS, Wang MY. Evaluating the accuracy of robotic assistance in spine surgery. Neurosurgery. 2012. August;71(2):N20-1. [DOI] [PubMed] [Google Scholar]
- 32.Schatlo B, Molliqaj G, Cuvinciuc V, Kotowski M, Schaller K, Tessitore E. Safety and accuracy of robot-assisted versus fluoroscopy-guided pedicle screw insertion for degenerative diseases of the lumbar spine: a matched cohort comparison. J Neurosurg Spine. 2014. June;20(6):636-43. Epub 2014 Apr 11. [DOI] [PubMed] [Google Scholar]
- 33.Kaul S, Shah NL, Menon M. Learning curve using robotic surgery. Curr Urol Rep. 2006. March;7(2):125-9. [DOI] [PubMed] [Google Scholar]
- 34.Jutte PC, Castelein RM. Complications of pedicle screws in lumbar and lumbosacral fusions in 105 consecutive primary operations. Eur Spine J. 2002. December;11(6):594-8. Epub 2002 Oct 15. [DOI] [PubMed] [Google Scholar]
- 35.Kosmopoulos V, Schizas C. Pedicle screw placement accuracy: a meta-analysis. Spine. 2007. February 1;32(3):E111-20. [DOI] [PubMed] [Google Scholar]
- 36.Tian NF, Huang QS, Zhou P, Zhou Y, Wu RK, Lou Y, Xu HZ. Pedicle screw insertion accuracy with different assisted methods: a systematic review and meta-analysis of comparative studies. Eur Spine J. 2011. June;20(6):846-59. Epub 2010 Sep 23. [DOI] [PMC free article] [PubMed] [Google Scholar]


