Abstract
Introduction
In drug discovery and development, it is of high interest to characterize the potential for intestinal drug-drug interactions to alter bioavailability of a victim drug. For drugs that are substrates of both intestinal transporters and enzymes, estimating the relative contribution of each process has proved challenging, especially since the susceptibility of drug to uptake or efflux transporters in vitro does not always translate to clinically significant in vivo involvement. Here we introduce a powerful methodology to implicate intestinal transporters in drug-drug interactions based on the theory that clinically relevant intestinal transporter interactions will result in altered rate of absorption of victim drugs.
Methods and Materials
We present exemplary clinical drug-drug interaction studies that utilize well-characterized clinical substrates and perpetrators to demonstrate how mean absorption time (MAT) and time to maximum concentration (tmax) are expected to change (or remain unchanged) when either intestinal transporters or metabolic enzymes were/are altered. Apixaban was also selected to demonstrate the utility of the methodology, as the purported involvement of both intestinal enzymes and transporters has been suggested in its FDA package insert.
Results and Discussion
Acute inhibition of gut efflux transporters resulted in decreased MAT and tmax values, induction increased these values, while inhibition of intestinal metabolic enzymes did not result in altered MAT or tmax. Involvement of intestinal efflux transporters in apixaban disposition is unlikely.
Conclusion
Utilization of this simple but powerful methodology to implicate intestinal transporter involvement will have significant impact on how drug-drug interactions are interpreted.
Keywords: Intestinal DDIs, Absorption Rate, MAT, tmax
Introduction
Bioavailability is one of the most important factors in determining the dosing regimens of orally dosed drugs. Oral bioavailability (F) is defined as the fraction of an oral dose that reaches systemic circulation intact and therefore F is influenced by the extent of absorption of drug from the intestinal gut lumen into the enterocyte (which may vary depending on both gut uptake and efflux transporters), the degree of intestinal metabolism within the enterocyte, as well as the extent of first-pass hepatic elimination, as defined by the relationship in Eq. 1:
| (1) |
Where FA is the cumulative fraction of dosed drug arriving intact into the enterocytes, FG is the fraction of dose that escapes intestinal metabolism within the enterocyte and enters the portal vein, and FH is the fraction that is not metabolized on first pass through the liver. Bioavailability is directly proportional to drug exposure (AUC, area under the concentration-time curve) (Eq. 2):
| (2) |
where CL is clearance. Therefore, the extent to which an orally dosed drug can reach the systemic circulation, defined by its degree of intestinal absorption and ability to avoid intestinal and hepatic first pass metabolism, directly defines the dose required to achieve therapeutically effective drug concentrations.
Consequently, in the discovery and development of new chemical entities, it is of high interest to not only predict intestinal bioavailability, but also to characterize the potential for intestinal drug-drug interactions to alter bioavailability of a victim drug (1–4). Since CL measurements are inherently confounded by F following oral dosing, measurement of F can be achieved by comparing dose-normalized AUC values following oral and intravenous dosing of drug (assuming CL has not changed between studies). Recently, we published a methodology that allows discrimination of changes in CL from changes in F in metabolic DDIs (5). This is possible due to the recognition that volume of distribution (Vss) remains unchanged in metabolic DDIs (6,7) and therefore changes in apparent Vss (Vss/F) will reflect the change in F alone, allowing one to differentiate changes in F from CL in oral metabolic DDIs. This methodology may not be appropriate for use in clinically significant systemic transporter DDIs, since Vss is expected to change in such interactions (8,9).
Metabolic drug-drug interactions (DDIs) will have the potential to alter total bioavailability via increasing or decreasing drug metabolism in the intestine and/or liver, thereby altering the extent of FG and/or FH. Interactions involving xenobiotic transporters, however, will have the potential to not only alter the extent of absorption (FA) by allowing or disallowing entry of drug from the gut lumen into the enterocyte, but also can result in alterations of the rate of absorption (ka). Efflux transporters expressed on the apical side of the enterocytes, such as P-glycoprotein (P-gp) and Breast Cancer Resistance Protein (BCRP), are able to pump drug from inside the enterocyte back into the gut lumen, where drug may then re-enter the enterocytes. Thus, for clinically significant transporter substrates, inhibition of intestinal efflux transporters would prevent drug cycling between the enterocytes and gut lumen, thereby decreasing absorption time, while induction would increase absorption time. For apically expressed intestinal uptake transporters, such as organic anion transporting polypeptide (OATP) 2B1, inhibition would result in prolonged absorption, while induction would potentially decrease absorption time for those drugs that are clinically significant substrates. Therefore, it is expected that clinically significant intestinal transporter DDIs will result in noteworthy changes in mean absorption time (MAT), the inverse of the first order absorption rate constant (ka) and time of maximal concentration (tmax).
Often in complex DDIs, those in which both metabolic enzymes and transporters may be implicated, it is difficult to discern the contribution of each process to overall disposition (10,11). This is true not only for understanding the contribution of metabolism versus transportermediated elimination to systemic clearance, but also their individual impact on bioavailability, and both sets of parameters contribute directly to changes in observed drug exposure. Understanding of both of these complimentary aspects will allow investigators to anticipate the magnitude of a potential DDI when either transporters or enzymes (or both) are affected. We are concerned that a number of papers, and even approved drug labeling, have proposed that drug interactions leading to changes in AUC are the result of intestinal transporter interactions based primarily on in vitro measures of the interaction potential when, in fact, no changes in MAT and tmax are observed. In this investigation, we further explore how to interpret changes in MAT and tmax to implicate intestinal absorptive transporter involvement in oral drug-drug interactions.
Materials and Methods
To determine if intestinal absorptive transporters are involved in an oral DDI, changes in MAT and tmax were examined for the interaction versus control phases of published clinical DDI studies. In addition, AUC, apparent clearance (CL/F), mean residence time (MRT), terminal half life (t1/2,z ), andVss/F were also examined and all parameters were reported as ratios of interaction/control. Percent AUC extrapolation was also examined as a potential indication of accuracy of parameters derived from AUC, with understanding that a high percent extrapolation does not necessarily indicate inaccuracies if the elimination phase is accurately represented. All reported ratios of AUC are dose-normalized. For studies in which victim drug was dosed to steady-state, the AUC within the dosing interval (from 0 to τ) was utilized since this value is mathematically equivalent to AUC extrapolated to infinity for a single dose (12), and in these cases percent AUC extrapolation was not reported.
Clinical studies routinely publish tmax values, however, MAT or ka are less frequently reported. Therefore, in cases where MAT values were not available, these values were calculated by one of two means: (A) calculation by Eq. 3 or Eq. 4 using reported t1/2,z and tmax values, or (B) estimation from published concentration-time profiles via compartmental fitting of the data.
| (3) |
| (4) |
where ke is the elimination rate constant (that reflects the slope of the terminal half-life) and τ is the dosing interval. Equation 3 describes the tmax relationship for a one-compartment model with first order absorption following a single dose while Eq. 4 reflects the multiple-dose relationship at steady state (13), where here ke is the elimination rate constant determined during a dosing interval, not necessarily the terminal elimination rate constant after dosing has stopped (14). Equations 3 and 4 ignore the drug distribution that almost all drugs will experience following a single dose, therefore less faith can be attributed to calculations of ka using Eq. 3. In contrast, at steady-state, peripheral compartments will contain accumulated drug and there will be far less distribution following oral dosing so that utilization of Eq. 4 is reasonably appropriate for any drug that has been dosed to steady-state, since all drugs approximate a one-compartment model at steady-state regardless of how many compartments are required to describe its kinetics following a single dose (14). Therefore, more credence can be attributed to ka estimation based on Eq. 4. There are no explicit solutions of Eqs. 3 or 4, however, they can be solved iteratively for ka with known ke and tmax values.
The second methodology relies on digitization of published concentration-time profiles of victim drug to estimate the MAT ratio, and this methodology was also used to supplement any unreported pharmacokinetic ratios. In such cases, the mean plasma-concentration time profiles of victim drug were digitized with WebPlotDigitizer Version 4.2 (San Francisco, CA) and subsequently analyzed using WinNonlin® Professional Edition Version 2.1 (Pharsight, Mountain View, CA). Mean absorption time (MAT) was calculated as the reciprocal of the first-order absorption rate constant (ka) from fitting of the victim drug oral concentration-time data to a 2-compartment model with absorption from the gut as we have previously described (15). If mean residence time (MRT) was not reported, MRT was calculated by Eq. 5:
| (5) |
where AUMC is the area under the moment curve, and both AUC and AUMC are extrapolated to infinity for single dose studies. For steady state studies, the AUC within the dosing interval from 0 to τ (AUC 0→τ) without extrapolation to infinity is mathematically equivalent to AUC extrapolated to infinity for a single dose (AUC 0→∞) (12). However, the AUMC within a dosing interval at steady-state (AUMC0→τ) is less than AUMC0→∞ for a single dose (16,17), therefore the relationship for orally dosed drugs presented in Eq. 6 was utilized to calculate MRT for steady state studies (18):
| (6) |
where AUCτ→∞refers to the extrapolation of steady-state AUC from the end of the dosing interval to infinity, which is calculated as the quotient of the concentration at the end of the dosing interval divided by the terminal phase rate constant. Calculation of Vss/F was achieved using Eq 7 (19):
| (7) |
where CL/F was calculated by dividing dose by AUC0→∞ for single dose studies, and by dividing dose by AUC 0→τ for steady-state studies.
All pharmacokinetic ratios were calculated using published data in priority, and supplemented with data derived from digitized values only when necessary. The source of all data used in calculated pharmacokinetic ratios are noted in footnotes in Tables II – V.
Table II:
Changes in Pharmacokinetic Parameters (Expressed as Ratios of Interaction / Control) in Oral Drug-Drug Interaction Studies that Affect Intestinal Xenobiotic Transporters
| Victim Drug: | Perpetrator Drug: | Major Enzyme or Transporter | Population | Percent AUC Extrapolation (DDI/Con) | Reference | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rosuvastatin (Single Dose) | Rifampin (Single Dose) | BCRP (Inhibition) | Whites (n=7) | 0.47 | 0.50 | 3.37 | 0.28 | 0.082 | 0.30a | 0.64 | 1%/8% | (21) |
| Rosuvastatin (Single Dose) | Rifampin (Single Dose) | BCRP (Inhibition) | Asians (n=8) | 0.34 | 0.55 | 3.21 | 0.31 | 0.11 | 0.34a | 0.59 | 1%/7% | (21) |
| Talinolol (Multiple Dose) | Rifampin (Multiple Dose) | P-gp (Induction) | Healthy Men (n=8) | 1.70b | 1.35 | 0.65 | 1.54 | 1.24c | 0.79c | 0.85 | n/a (steady-state) | (22) |
Pharmacokinetic values reported in the table are based on published average values, unless otherwise noted
Abbreviations: AUC, area under the curve; BCRP, breast cancer resistance protein; Con, control; CL/F, apparent clearance; DDI, drug-drug interaction; MAT, mean absorption time; MRT, mean residence time; n/a, not applicable; P-gp, P-glycoprotein; tmax, time to maximal concentration; t1/2,z, terminal half-life; Vss/F, apparent volume of distribution at steady state
MRT was calculated using Equation 7 and average reported values of CL/F and Vss/F
MAT was calculated by utilization of the steady-state relationship between reported tmax and t1/2,z (Equation 4)
Ratios are calculated by digitization of published average plasma concentration-time profiles and performing compartmental analysis, where steady-state MRT was calculated using Eq 6
Table V:
Utilization of the Sodhi and Benet (5) Methodology to Discriminate Clearance from Bioavailability Changes for Orally Dosed Apixaban (Victim) and Rifampin (Perpetrator) from the study of Vakkalagadda et al. (25)
| Victim | Perpetrator | Reference | ||||||
|---|---|---|---|---|---|---|---|---|
| Apixaban (IV) (Single Dose) | Rifampin (Multiple Dose) | Observed: 0.61 | – | Observed: 0.87 | Observed: 0.76 | – | Observed: 1.64 | (25) |
| Apixaban (Oral) (Single Dose) | Rifampin (Multiple Dose) | Observed: 0.48 | Observed: 1.42a | Assumed: 1 | Estimated: 0.70 | Observed: 2.14 | Estimated: 1.50 | (25) |
Pharmacokinetic values reported in the table are based on published average values, unless otherwise noted
Abbreviations: AUC, area under the curve; CL, clearance; CL/F, apparent clearance; DDI, drug-drug interaction; F, bioavailability; Vss, volume of distribution at steady state; Vss/F, apparent volume of distribution at steady state
Ratios are calculated by digitization of published average plasma concentration-time profiles and performing non-compartmental and/or compartmental analysis
A number of substrates of metabolic enzymes and transporters were selected for evaluation of the proposed methodology, with clinically recommended in vivo index substrates used in priority (20). The studies investigated here were selected to include an example of (A) inhibition of intestinal transporters (BCRP; rosuvastatin with single-dose rifampin) (21), (B) induction of intestinal transporters (P-gp; talinolol with multiple-dosed rifampin) (22), (C) inhibition of intestinal / hepatic metabolic enzymes (CYP3A4; triazolam with fluconazole) (23), and (D) inhibition of primarily hepatic metabolic enzymes only (CYP2C19; omeprazole with clarithromycin) (24). In addition, the proposed methodology was used to evaluate the purported involvement of intestinal efflux transporters in apixaban disposition (25), a drug for which involvement of both metabolic enzymes and efflux transporters has been suggested throughout the literature. All selected clinical studies had a crossover design, in order to minimize the impact of any potential inter-individual variability between treatment and control groups, which we have recently highlighted (7), and with the assumption that within the same individual, the dissolution and distribution of drug within the intestinal lumen is similar for both arms of the clinical study.
Analysis of involvement of intestinal transporters proceeded via examination of ratios of change in MAT and tmax. Ratios that indicated greater than 30% change (i.e. ratios outside of the range of 0.77 and 1.30) were considered to be a potentially clinically significant intestinal transporter interaction.
Simulations were conducted based on Eqs. 3 and 4 to examine the relationship between MAT and tmax for a rapidly versus more slowly absorbed drug with MAT values of 0.5 hr and 2 hr, respectively. The impact of 15 min changes in MAT on single-dose and steady-state tmax were examined.
Results
We identified and analyzed orally dosed clinical DDI studies from the literature in which intestinal transporters or metabolic enzymes were affected, as well as for an additional drug apixaban to further evaluate the utility of this methodology to implicate intestinal transporter involvement in oral DDIs. Intestinal transporter DDI studies were selected to include examples of both inhibition (21) and induction (22) by rifampin. Metabolic DDI studies were selected to highlight a significant intestinal metabolic interaction of the victim drug triazolam (23) versus a metabolic interaction that primarily occurs in the liver for omeprazole (24). Details of these drug interaction studies, including the substrate and inhibitory specificities of victim and perpetrator drugs, respectively, are outlined in Table I.
Table I:
Substrate Specificities of Victim Drugs and Inhibitory Potential of Perpetrator Drugs for Metabolic Enzymes and Xenobiotic Transporters from Investigated Oral Drug-Drug Interactions
| Victim Drug | Dose | BDDCS Class | Substrate Specificity | Perpetrator Drug | Dose | BDDCS Class | Inhibitory Specificity | Reference |
|---|---|---|---|---|---|---|---|---|
| Rosuvastatin (Single Dose) | 20 mg PO | 3 |
BCRP OATP1B1 OATP1B3 |
Rifampin (Single Dose) | 600 mg IV infusion | 2 |
BCRP OATP1B1 OATP1B3 CYP3A |
(20,21) |
| Talinolol (Multiple Dose) | 100 mg PO 7 Days | 3 | P-gp | Rifampin (Multiple Dose) | 600 mg PO q.d. 9 Days | 2 | (Inducer) CYP3A CYP2C9 P-gp |
(22,54) |
| Triazolam (Single Dose) | 0.25 mg PO | 1 | CYP3A | Fluconazole (Multiple Dose) | 100 mg PO q.d. 4 Days | 3 |
CYP3A CYP2C19 CYP2C9 |
(20,23) |
| Omeprazole (Multiple Dose) | 40 mg PO 6 Days | 1 | CYP2C19 CYP3A (minor) |
Clarithromycin (Multiple Dose) | 500 mg PO t.i.d 5.33 Days | 3 |
CYP3A CYP2C19 P-gp |
(20,24) |
| Apixaban (Single Dose) | 10 mg PO | 1 |
CYP3A P-gp |
Rifampin (Multiple Dose) | 600 mg PO q.d. 11 Days | 2 | (Inducer) CYP3A P-gp |
(25,54) |
Intestinally expressed enzyme and transporters are highlighted in bold
Abbreviations: BCRP, Breast Cancer Resistance Protein; BDDCS, Biopharmaceutics Drug Disposition Classification System; CYP, Cytochrome P450; IV, Intravenous; OATP, Organic Anion-Transporting Protein; P-gp, P-glycoprotein
Table II displays the ratios of change in oral pharmacokinetics in the DDI studies that affect intestinal transporters, namely, acute inhibition of intestinal transporters (BCRP) with single dose rifampin (victim drug rosuvastatin) (21) and the induction of intestinal transporters (P-gp) with multiple dose rifampin (victim drug talinolol) (22). In the single dose rifampin study, 600 mg IV rifampin caused a 3.37-fold and 3.21-fold increase in AUC in White and Asian subjects, respectively. Additionally, a significant decrease in MAT and tmax was observed in both groups; Whites showed a 53% decrease in MAT and 50% decrease in tmax and Asians displayed a 66% decrease in MAT and 45% decrease in tmax. In the multiple-dose rifampin study, 600 mg PO rifampin for 9 days resulted in a 35% reduction in talinolol AUC, accompanied with a marked increase in MAT (1.70-fold) and tmax(1.35-fold).
Table III displays the ratios of change in oral pharmacokinetics in the DDI studies that affect metabolic enzymes, outlining (1) a triazolam–fluconazole interaction in which CYP3A4 (both intestinally and hepatically expressed) is inhibited (23) and (2) an omeprazole-clarithromycin DDI in which the primary interaction is due to CYP2C19 (primarily expressed in the liver with minor intestinal expression) (24). In the CYP3A4 inhibition study, multiple doses of PO fluconazole resulted in a 2.46-fold increase in oral exposure of single-dosed triazolam. This was accompanied by minimal changes in MAT (ratio of 0.87) and tmax (ratio of 1.11). In the CYP2C19 inhibition study, multiple doses of clarithromycin resulted in a 1.91-fold increase in steady-state omeprazole AUC, while MAT only decreased by 8% and tmax increased 1.11-fold.
Table III:
Changes in Pharmacokinetic Parameters (Expressed as Ratios of Interaction / Control) in Oral Drug-Drug Interaction Studies that Affect Metabolic Enzymes
| Victim Drug: | Perpetrator Drug: | Enzyme or Transporter | Population | Percent AUC Extrapolation (DDI/Con) | Reference | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Triazolam (Single Dose) | Fluconazole (Multiple Dose) | CYP3A4 | Healthy Subjects (n=12) | 0.87c | 1.11 | 2.46 | 0.41b | 0.79a | 1.91a | 1.84 | 20%/6% | (23) |
| Ompeprazole (Multiple Dose) | Clarithromycin (Multiple Dose) | CYP2C19 CYP3A4 (minor) |
Healthy Men (n=23) | 0.92d | 1.11 | 1.91 | 0.52b | 1.11a | 2.08a | 1.33 | n/a steady-state | (24) |
Pharmacokinetic values reported in the table are based on published average values, unless otherwise noted
Abbreviations: AUC, area under the curve; Con, control; CL/F, apparent clearance; CYP, cytochrome P450; DDI, drug-drug interaction; MAT, mean absorption time; MRT, mean residence time; n/a, not applicable; tmax, time to maximal concentration; t1/2,z, terminal half-life; Vss/F, apparent volume of distribution at steady state
Ratios are calculated by digitization of published average plasma concentration-time profiles and performing non-compartmental and/or compartmental analysis, where single dose MRT was calculated using Eq. 5 and steady-state MRT was calculated using Eq. 6
CL/F was calculated using known dose, reported AUC and Equation 2
MAT was calculated by utilization of the single-dose relationship between reported tmax and t1/2,z (Equation 3)
MAT was calculated by utilization of the steady-state relationship between reported tmax and t1/2,z (Equation 4)
Table IV displays the ratios of change in oral pharmacokinetics of apixaban dosed with multiple doses of rifampin, and shows a 52% decrease in apixaban exposure (25). This change is accompanied with minimal change in MAT (8% decrease) and an unchanged tmax ratio.
Table IV:
Changes in Pharmacokinetic Parameters (Expressed as Ratios of Interaction / Control) for Orally Dosed Apixaban (Victim) and Rifampin (Perpetrator)
| Victim Drug: | Perpetrator Drug: | Enzyme or Transporter | Population | Percent AUC Extrapolation (DDI/Con) | Reference | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Apixaban (Single Dose) | Rifampin (Multiple Dose) | CYP3A4 P-gp (Induction) |
Healthy Subjects (n=20)a | 0.92b | 1.00 | 0.48 | 2.14 | 1.42b | 0.65b | 1.03 | 10%/9% | (25) |
Pharmacokinetic values reported in the table are based on published average values, unless otherwise noted
Abbreviations: AUC, area under the curve; Con, control; CL/F, apparent clearance; CYP, cytochrome P450; DDI, drug-drug interaction; MAT, mean absorption time; MRT, mean residence time; P-gp, P-glycoprotein; tmax, time to maximal concentration; t1/2,z, terminal half-life; Vss/F, apparent volume of distribution at steady state
Interaction phase was n=18
Ratios are calculated by digitization of published average plasma concentration-time profiles and performing non-compartmental and/or compartmental analysis where single dose MRT was calculated using Eq. 5
Discussion
The methodology proposed here is a simple but powerful tool to evaluate the clinically significant involvement of intestinal transporters for orally dosed drugs. Utilization of this simple methodology will allow pharmaceutical scientists to better predict when an intestinal DDIs is expected to occur, as well as anticipate the degree to which exposure may change based on an improved understanding of potential determinants of F for a drug-of interest. In this manuscript, we present exemplary clinical DDI studies that utilize well-characterized clinical substrates and perpetrators to understand how MAT and tmax are expected to change (or remain unchanged) when either intestinal transporters or metabolic enzymes were/are altered.
Table II outlines two clinical studies in which the major apical efflux transporters BCRP or P-gp were either inhibited (21) or induced (22) by rifampin. In the single-dose rifampin study, intestinal inhibition of BCRP resulted in a greater than 3-fold increase in exposure of the BCRP substrate rosuvastatin in both Whites and Asians that were wild-type carriers for both BCRP and OATP1B1 (21). This significant interaction was accompanied by decreases in MAT and tmax (ranging from approximately 2- to 3-fold reduction) as would be expected for inhibition of an intestinal efflux transporter. In the multiple-dose rifampin study, induction of P-gp resulted in a 35% decrease in talinolol exposure and both MAT and tmax markedly increased (1.70- and 1.35fold, respectively) (22). In summary, inhibition of efflux transporters results in decreased MAT and induction of efflux increases MAT values. Changes in tmax trend in the same direction, although not always to the same degree since changes in elimination half-life will also have an impact on tmax, as evidenced by Eqs. 3 and 4. Table III displays two metabolic DDIs in which the interaction either occurs due to CYP3A4 in both the intestine and liver (23), or due to CYP2C19 (with minor CYP3A4 contribution) that is primarily expressed in the liver with minimal intestinal involvement (24). No change in MAT values was observed in either study, as would be expected when transporters are not involved in absorption processes. These observations further demonstrate the utility of our MAT methodology to implicate intestinal transporter involvement.
In order to identify clinically significant transporter involvement in DDIs, we have recently published guides to understanding DDIs involving transporters (8) and metabolic enzymes (6). In clinically significant transporter interactions, the magnitude of change in Vss can often be larger than the change in CL, resulting in counterintuitive changes in t1/2,z and MRT (i.e., decreases in CL can be associated with a shorter elimination half-life). This trend can be observed in the rosuvastatin – rifampin DDI, where an approximate 70% reduction in CL is associated with shorter t1/2,z and MRT values due to an approximate 90% reduction in Vss/F as a result of the inhibition of the hepatic uptake transporters OATP1B1/1B3. This is in contrast to the classic pharmacokinetic trend where changes in CL are associated with an equal but opposite change in t1/2,z and MRT (due to unchanged Vss in metabolic DDIs), which we have recently reviewed for a large number of metabolic DDIs (7). These guiding concepts, in addition to the MAT methodology proposed here, can help discern transporter involvement in purported complex DDIs and were applied to the drug apixaban.
Apixaban is an anticoagulant factor Xa inhibitor that is primarily metabolized by CYP3A4. The involvement of the efflux transporters P-gp and BCRP has also been suggested throughout the literature as well as in the apixaban FDA label (26–28). Multiple dosing of rifampin resulted in an approximate 2-fold reduction in apixaban exposure, however, there was no change in MAT (ratio of 0.92) and tmax (ratio of 1.00) (Table IV), suggesting that the in vitro susceptibility of apixaban to P-gp is not clinically significant. Additionally, increase in clearance is associated with a decrease in MRT of similar magnitude, as would be expected for a metabolic interaction (7).
These results are consistent with the Biopharmaceutics Drug Disposition Classification System (BDDCS), a simple drug classification system based on permeability rate and solubility that can anticipate which drugs may be susceptible to transporters in vivo (29). Apixaban is a BDDCS Class 1 drug with favorable membrane permeability characteristics and high solubility, allowing free passage across biological membranes via passive processes (rather than reliance on xenobiotic transporters to cross membranes). It is theorized that due to the rapid membrane permeability and high solubility of BDDCS Class 1 drugs, these drugs can rapidly cross biological membranes at concentrations high enough to either saturate active transport or render the active uptake to only be a minimal part of total uptake. Thus, the clinically relevant involvement of transporters in vivo may be negligible even if demonstrated to be a substrate in in vitro studies (29). BDDCS Class 2 drugs are also highly permeable, however due to their low solubility it is thought that the lower soluble concentrations available for passive diffusion may (in some cases) either be incapable of saturating transporters or passive uptake due to the low solubility does not outweigh the active process, and therefore transporters may or may not be involved for these primarily metabolized BDDCS Class 2 drugs. BDDCS Class 3 and 4 have unfavorable membrane permeability characteristics and thus rely on transporters to cross membranes, and this theory is supported by the fact that Class 3 and 4 drugs are primarily eliminated in the urine or bile (i.e. transporter-dependent processes) rather than being metabolized. The BDDCS classes of the victim drugs investigated here are displayed in Table I and nicely highlights that the transporter interactions are associated with BDDCS class 3 victim drugs, while the metabolic interactions are associated with BDDCS class 1 drugs. We propose that BDDCS can be utilized for development compounds (that are inherently less well-studied than the index substrates highlighted here) to help anticipate contributing factors in prediction of intestinal DDIs.
Knowledge that Vss is unchanged in strictly metabolic interactions can help differentiate changes in CL from changes in F in metabolic DDIs, a very useful finding to allow investigators to understand the contribution of each parameter in overall observed exposure changes (5). For the two metabolic DDIs investigated here, the CL and F differentiation methodology estimated that in the CYP3A4 triazolam-fluconazole DDI, the observed 2.46-fold increase in exposure was due to a 48% reduction in CL and a 1.27-fold increase in F, while in the omeprazole-clarithromycin CYP2C19 DDI, the observed 1.91-fold increase in exposure was due almost entirely to a 53% decrease CL (with a minor 10% reduction in F ). Although confirming IV data were not available, these estimates are consistent with the fact that CYP3A4 is expressed extensively in the intestine and liver, whereas CYP2C19 expression is minor in the intestine, therefore it is expected that the interaction would primarily occur hepatically. In the apixaban-rifampin DDI investigated above, the DDI study was conducted after both PO and IV dosing of apixaban, allowing for confirmation (from the IV interaction study) of the estimated change in CL versus F based on the oral interaction data. Table V displays the ratios of change in IV and oral apixaban pharmacokinetics, showing that the observed 52% reduction in apixaban oral exposure following multiple dosing of rifampin was estimated to be a result of a 30% reduction in F and a 1.5-fold increase in CL based on oral interaction data. These estimates were remarkably close to the observed changes in F (24% reduction) and CL (1.64-fold observed), with estimated and observed values only differing by 9% for each parameter.
To appropriately guide use of the MAT methodology, it is important to highlight its assumptions and limitations to prevent any misinterpretations of interaction data. First, following oral dosing, changes in MAT can only implicate modulation of those transporters that are expressed in the intestine, but will not necessarily provide information on involvement of transporters that are only expressed in the liver and/or kidney (but not the intestine). Table VI outlines the regional expression of major xenobiotic transporters in the intestine, liver, kidney and brain adapted from the International Transporter Consortium’s recommendations on clinically significant xenobiotic transporters (30).
Table VI:
Regional Expression of Clinically Significant Efflux and Uptake Transporters in the Intestine, Liver, Kidney and Brain
| Transporter Type | Transporter | Intestinal Localization | Hepatic Localization | Renal Localization | Brain Localization |
|---|---|---|---|---|---|
| Efflux | BCRP | Apical | Bile Canaliculi | Apical (Blood) | |
| MDR1 (P-gp) | Apical | Bile Canaliculi | Apical (Urine) | Apical (Blood) | |
| MRP2 | Apical | Bile Canaliculi | Apical (Urine) | ||
| MRP3 | Basolateral | Sinusoidal (Basolateral) | |||
| THTR1 | Basolateral | Basolateral | |||
| BSEP | Bile Canaliculi | ||||
| MDR3 | Bile Canaliculi | ||||
| MATE1 | Bile Canaliculi | Apical (Urine) | |||
| MATE2-K | Apical (Urine) | ||||
| MRP4 | Sinusoidal (Basolateral) | Apical (Urine) | Apical (Blood) | ||
| MRP6 | Sinusoidal (Basolateral) | ||||
| Uptake | OATP2B1 | Apical | Sinusoidal (Basolateral) | ||
| ASBT | Apical | ||||
| MCT1 | Apical | ||||
| PEPT1/PEPT2 | Apical | Apical (Urine) | |||
| THTR2 | Apical | Apical (Urine) | |||
| OCT1 | Sinusoidal (Basolateral) | ||||
| OAT2 | Sinusoidal (Basolateral) | Basolateral | |||
| OAT7 | Sinusoidal (Basolateral) | ||||
| OATP1A2 | Apical (Blood) | ||||
| OATP1B1 | Sinusoidal (Basolateral) | ||||
| OATP1B3 | Sinusoidal (Basolateral) | ||||
| NTCP | Sinusoidal (Basolateral) | ||||
| OAT1/3 | Basolateral | ||||
| OATP4C1 | Basolateral | ||||
| OCT2 | Basolateral | ||||
| URAT1 | Apical (Urine) | ||||
| Bidirectional | OSTα/β | Basolateral | Sinusoidal (Basolateral) | ||
| ENT1 | Basolateral | Bile Canaliculi Sinusoidal (Basolateral) | Apical (Urine) | Basolateral (Brain) | |
| ENT2 | Basolateral | Sinusoidal (Basolateral) | Basolateral | Basolateral (Brain) Apical (Blood) | |
| OAT4 | Apical | ||||
| OCTN1/2 | Apical (Urine) |
Second, in this investigation we examined commonly used index substrates and inhibitors with known specificities for transporters and enzymes, however this may not be the case for compounds in development. For victim drugs, in vitro metabolic stability and transporter assays can be conducted to characterize potential determinants of drug disposition, and in tandem with BDDCS theory, conclusions can be made on the clinical relevance of such results. For perpetrator drugs, the intestinal inhibitory potential can be calculated by comparing the maximum perpetrator concentration in the gut [Igut] to its inhibitory potential (IC50) for the major enzymes or transporters involved in intestinal disposition, where ratios of [Igut]/IC50 greater than 10 indicate potential for clinically significant inhibition (31). This aspect is quite important as currently there are a limited number of well-characterized (and specific) clinical inhibitors of transporters (20) and commonly-used metabolic inhibitors may have the potential to inhibit xenobiotic transporters (32). Further, consideration towards the rate of absorption of potential inhibitors relative to that of substrate drugs should be accounted for, as intestinal inhibition will only occur if inhibitor is still present in the intestine. It has been demonstrated that predictions of changes in overall exposure as a result of a DDI have been improved by incorporating the ka of perpetrator drug (33,34). Here, we extend this concept towards understanding the potential for an intestinal DDI to occur if perpetrator drug is more rapidly absorbed, and suggest that further investigation is warranted.
The third crucial aspect in utilization of the MAT methodology is ensuring that for analysis of rapidly absorbed drugs, there is sufficient clinical sampling in the absorption phase to adequately estimate MAT. For instance, the absorption rate of the CYP3A4 index substrate midazolam is extremely rapid, with reported ka values of 9.6 hr−1 (35) and greater than 5 hr−1 (36), which correspond to MAT values of 6.25 min and less than 12 min, respectively. The study by Smith and coworkers (1981) included intensive sampling up to 1 hour (8 points) and reported tmax was approximately 20 min (35), while the study by Heizmann et al. (1983) only included 4 time points up to 1 hour and tmax ranged from 15 – 30 min between individuals (36). However, in the large majority of DDI studies, average MAT or ka values are rarely reported, and in the absence of access to individual patient data, digitization of average concentration-time profiles introduces additional error for drugs with short MAT values. The reported pharmacokinetic profiles are generated from the average drug concentrations of all subjects at each time point, and therefore results in profiles that do not necessarily represent any single subject within the study. As a result, these profiles may not be able to adequately account for potential interindividual variability in aspects such as lag-time, absorption rate, secondary peaks, and thus we recommend that in practice, this methodology be carried out for each individual in a DDI study. Of the numerous midazolam DDI studies available in the literature, we were only able to identify one ketoconazole interaction study that not only had extensive absorption phase sampling (with time points at 10, 20, 30, 45, 60 and 90 min), but of equal importance, absorption rate was calculated for each subject and average values were reported (37), resulting in an MAT ratio of 1.19 but a 1.50-fold increase in tmax (due to a 15 min increase from 30 min to 45 min).
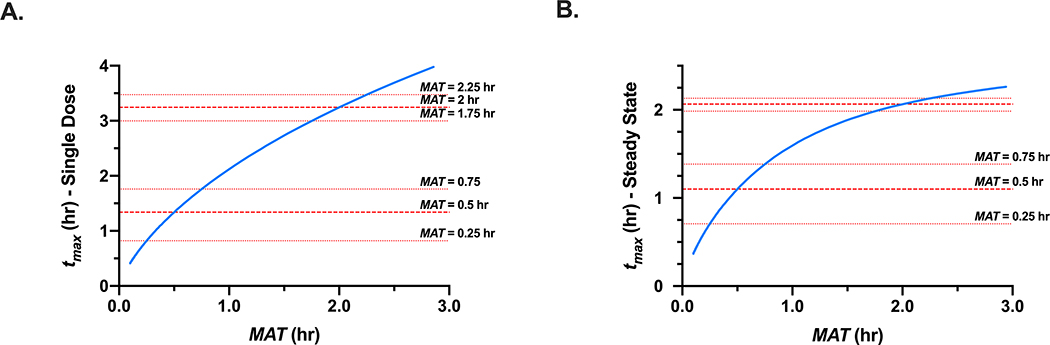
Simulations have been conducted investigating the impact of a reduced sampling schedule on estimations of MAT, confirming that minimal error was associated for a theoretical drug with an MAT of 1 hr, however, the resulting error in MAT estimation becomes increasingly larger for drugs that are more rapidly absorbed (for theoretical drugs for which the MAT was decreased to 0.33 hr and 0.2 hr) (38). The issue of estimating MAT when sampling is not adequate could potentially be overcome by using Eq. 3 (for a victim drug that follows one-compartment kinetics) or Eq. 4 (for any victim drug that is dosed to steady-state) in tandem with reported tmax and t1/2,z values. However, this still depends on adequate capture of t1/2,z, which is quite reasonable in most DDI studies, and tmax, which may pose a challenge for rapidly absorbed drugs as these drugs inherently have less time points describing the absorption phase as compared to drugs with larger tmax values. Figure I depicts the impact on tmax that 15 min changes in MAT have (which could occur due to minimal absorption phase sampling) for both a rapidly absorbed drug (MAT = 0.5 hr) and a drug that that is less-rapidly absorbed (MAT = 2 hr), for both a singledose of a drug that follows one-compartment kinetics and the relationship in Eq. 3 and for the steady-state relationship in Eq. 4. Clearly, a small change in MAT has a greater impact on tmax for rapidly absorbed drugs, compared to a drug with larger MAT values. This also highlights that if tmax is not adequately captured, calculated MAT values can display large differences for rapidly absorbed drugs, that may not reflect real changes in absorption. Examination of the midazolamketoconazole pharmacokinetic profiles reported by Lee and coworkers (1996) clearly demonstrates a significantly larger degree of variability with the absorption-phase time points as compared to the elimination phase (37), indicating the possibility that at inter-individual differences in drug absorption are more pronounced for rapidly absorbed drugs.
Simulated changes in tmax (blue line) based on changes in MAT ranging from 10.1 to 3 hr for (A) a one-compartment drug with an elimination half-life of 4 hr following a single dose (Eq. 4) and (B) a drug dosed to steady-state with an elimination half-life of 4 hr and dosing interval of 6 hr. Horizontal (red) lines indicate the impact of 15 min changes in MAT on tmax (y-axis) for a rapidly absorbed drug (MAT = 0.5 hr) versus a less-rapidly absorbed drug (MAT = 2 hr)
Because absorption rate may be inherently different for different people, it is crucial that an analysis using this methodology proceeds only when the DDI was conducted within the same subjects using a crossover study design. We have recently investigated Vss differences for victim drugs in DDI studies conducted in multiple populations, and found significant differences that could not be accounted for by body weight (7). We expect the same to be true for MAT values in different people, depending on the differences in degree of transporter expression throughout the population, and indeed there is evidence in the literature of the association of MAT with age (39) and disease state potentially as a result of changes in blood flow, gut motility, pH or edema (40–42). Thus, we believe that this methodology should only be used qualitatively for parallel design studies to implicate transporter involvement; that is, for disease state and pharmacogenomic studies that are conducted with different subjects in each arm.
In situations where absorptive processes have the potential to be saturated, or in situations of dose-limited solubility of victim drug, the relationship between MAT and dose should be taken into account. This point is particularly relevant when different doses of victim drug are administered in the control versus interaction arms, as it is common for clinical DDI studies for which a significant systemic interaction is expected, and therefore a lower dose is given in the interaction arm. Such differences in victim drug dosage levels will result in significant differences in drug concentrations within the intestinal lumen, which can be particularly relevant to saturation of absorptive processes because drug concentrations in the intestinal lumen can approach concentrations in the mM range. For example, cefatrizine has been observed to display dose-dependent absorption characteristics (43) and incorporation by Reigner et al. (1989) (44) of saturable absorption by Michaelis-Menten type kinetics into compartmental models resulted in improved data fitting. These authors concluded that involvement of a carrier-mediated transporter system was the most likely explanation, and involvement of the intestinal uptake polypeptide transporter (PEPT) has more recently been implicated in cefatrizine absorption (45). An interesting point to mention here is that even if rate of absorption has the potential to be saturated, overall extent of absorption (reflected in bioavailability measurements) will only be decreased if absorption changes are such that there is insufficient time for absorption to occur. In other words, the overall rate of absorption may decrease, however, extent of absorption as reflected in total bioavailability measurements may or may not change. Indeed, in the cefatrizine bioavailability investigation mentioned above, a dose-dependent MAT increase was observed when dose was increased from 250 mg to 500 mg and 1000 mg, however, total bioavailability (approximately 75%) was unchanged between the two lower doses, whereas the 1000 mg dose was associated with a marked decrease in F of 46.8% (43).
It is important to note that there are situations in which MAT (and tmax) may change outside of transporter modulation, as was briefly mentioned above. Such scenarios primarily include modulation of gastric emptying (46) and food effects (47), but might also be due to viscosity (48), osmolality that can have an effect on luminal fluid volume (49), intestinal pH and solubility (50), which could potentially result in changes in absorption rate of victim drug. However, we would expect for the great majority of DDI studies these factors would be kept constant in both phases of the study when conducted within the same individual. It is possible that perpetrator drug may influence victim drug absorption, for instance, by altering intestinal pH or solubility of victim drug, resulting in changes in victim drug absorption rate that are not transporter-related. We propose that such false-positive results can be rationalized based on in vitro transporter studies or further understood if dose-dependent DDIs are conducted. Further, this approach relies on the reasonable assumption that within the same individual, dissolution and distribution of drug within the intestinal lumen remains relatively constant between both arms of the clinical study. As discussed in detail above, investigations into validating this assumption should rely heavily on utilization of individual subject data and intensive absorptive phase sampling, particularly for rapidly absorbed drugs.
Very recently, the efforts of many groups have been directed towards better predicting drug disposition related to complex DDIs and identifying clinical evidence of transportermediated DDIs (10, 51,52). Rodrigues et al. (2020) presented a review to identify clinical evidence of induction of hepatic and intestinal OATPs (51), while Yu et al. (2017) focused on intestinal OATP2B1 interactions for known substrates (52). In general, the basis of these investigations focuses on changes in AUC and/or Cmax, however, we propose that the potential for intestinal OATP-mediated DDIs would be strengthened by incorporation of the methodology presented here, that significant intestinal transporter interactions will alter rate of absorption. The localization of OATP2B1 (the primary intestinal OATP) in the apical versus basolateral membrane has been debated in recent years, as recently highlighted by the International Transporter Consortium (30). Even amongst those scientists who agree on basolateral expression of OATP2B1, the direction of transport has been further questioned (enterocyte to blood versus blood to enterocyte), and the evidence for both sides has been nicely summarized by McFeely et al. (53). In addition to the development of specific probe substrates and inhibitors of OATP2B1, we propose that the MAT methodology will provide our field with an additional tool to confirm the apical versus basolateral localization of OATP2B1, and such evaluations are currently of high interest to our laboratory. Alluri et al. (10) in their recent article “Transporter-enzyme interplay and the hepatic drug clearance: what have we learned so far?” outline approaches to predict potential complex DDIs, however, discussion of intestinal interactions is notably lacking. For orally dosed drugs, the contribution of intestinal interactions to overall exposure changes can be significant and is often overlooked. This highlights that improved methodologies to predict or characterize intestinal DDIs is an area that warrants further efforts by the field, and we recommend that such efforts be founded on the MAT and tmax theory presented here.
Conclusions
Here we have introduced a powerful methodology to implicate intestinal transporters in DDIs, based on the theory that clinically relevant intestinal transporter interactions will result in altered rate of absorption of victim drugs. We highlight interactions involving the two major intestinal drug transporters, BCRP and P-gp, and demonstrate that the expected directional changes in MAT and tmax are observed for both inhibition and induction of intestinally expressed transporters. If MAT and tmax remain unchanged in a DDI, it can be inferred that (A) intestinal absorptive transporters are not significantly involved clinically in the DDI and (B) any changes in F as a result of the interaction are not due to alteration in intestinal transporters activity or expression. It is also possible that equivalent effects on uptake and efflux transporters may be observed resulting in unchanged MAT, and this possibility is currently being investigated by our laboratory. Based on the relationships presented in Eqs. 3 and 4, changes in t1/2,zcan impact tmax values, therefore in such situations focus should be on MAT changes, although both of these parameters should trend in the same direction if MAT has been adequately captured. This simple but powerful methodology will allow investigators to implicate transporters in complex DDIs, allow clinical validation of additional transporter inhibitors due to the current lack of specific inhibitors, further investigate the potential for transporter induction, characterize emerging intestinal transporters, and helping the field solve transporter-related debates, such as the localization and/or direction of OATP2B1 within the enterocyte.
Acknowledgements
The authors would like to thank members of our laboratory for their thoughtful discussions in the development of this manuscript, including Dr. Annette Chu, Caroline Huang, Wen Kou, Dr. Ivan Kozachenko, Shuaibing Liu, and Dr. Yue Xiang.
This work was supported in part by a Mary Ann Koda-Kimble Seed Award for Innovation. Ms. Sodhi was supported in part by an American Foundation for Pharmaceutical Education Predoctoral Fellowship, NIGMS grant R25 GM56847 and a Louis Zeh Fellowship. Dr. Benet is a member of the UCSF Liver Center supported by NIH grant P30 DK026743
Abbreviations
- AUC
area under the curve
- AUC0→∞
area under the curve extrapolated to infinity for a single dose
- AUC0→τ
area under the curve during a dosing interval at steady-state
- AUCτ→∞
area under the curve extrapolated from the end of the dosing interval to infinity at steady-state
- AUMC
area under the moment curve
- AUMC0→∞
area under the moment curve extrapolated to infinity for a single dose
- AUMC0→τ
area under the moment curve during a dosing interval at steady-state
- BCRP
breast cancer resistance protein
- BDDCS
Biopharmaceutics Drug Disposition Classification System
- CL
clearance
- CL/F
apparent clearance
- CYP
cytochrome P450
- DDI
drug-drug interaction
- F
bioavailability
- FA
fraction absorbed
- FG
fraction escaping intestinal elimination
- FH
fraction escaping hepatic elimination
- Igut
maximum perpetrator concentration in the gut
- ka
absorption rate constant
- ke
elimination rate constant
- MAT
mean absorption time
- MRT
mean residence time
- OATP
organic anion transporting polypeptide
- P-gp
P-glycoprotein
- τ
dosing interval
- tmax
time at which maximal concentration is observed
- t1/2,z
terminal half-life
- Vss
volume of distribution at steady state
- Vss/F
apparent volume of distribution at steady state
References
- 1.Benet LZ, Cummins CL, Wu CY. Transporter-enzyme interactions: implications for predicting drug-drug interactions from in vitro data. Curr Drug Metab. 2003. 4:393–398. [DOI] [PubMed] [Google Scholar]
- 2.Shugarts S, Benet LZ. The role of transporters in the pharmacokinetics of orally administered drugs. Pharm Res. 2009. 26:2039–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van de Waterbeemd H, Jones BC. Predicting oral absorption and bioavailability. Prog Med Chem. 2003. 41:1–59. [DOI] [PubMed] [Google Scholar]
- 4.Yoshida K, Maeda K, Sugiyama Y. Hepatic and intestinal transporters: prediction of pharmacokinetic effects caused by drug-drug interactions and genetic polymorphisms. Annu Rev Pharmacol Toxicol. 2013. 53:581–612. [DOI] [PubMed] [Google Scholar]
- 5.Sodhi JK, Benet LZ. A simple methodology to differentiate changes in bioavailability from changes in clearance following oral dosing of metabolized drugs. Clin Pharmacol Ther. 2020. [E-pub ahead of print, March 9, 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benet LZ, Bowman CM, Koleske ML, Rinaldi CL, Sodhi JK. Understanding drug-drug interaction and pharmacogenomic changes in pharmacokinetics for metabolized drugs. J Pharmacokinet Pharmacodyn. 2019. 46:155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sodhi JK, Huang CH, Benet LZ. Volume of distribution is unaffected by drug-drug interaction studies for metabolized drugs. Clin Pharmacokinet 2020. [Under Review, Submitted April 1, 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benet LZ, Bowman CM, Sodhi JK. How transporters have changed basic pharmacokinetic understanding. AAPS J. 2019. 21:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grover A, Benet LZ. Effects of drug transporters on volume of distribution. AAPS J 2009. 11:250–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alluri RV, Li R, Varma MVS. Transporter-enzyme interplay and the hepatic drug clearance: what have we learned so far? Expert Opin Drug Metab Toxicol. 2020. [E-pub ahead of print, April 12, 2020]. [DOI] [PubMed] [Google Scholar]
- 11.Varma MV, Pang KS, Isoherranen N, Zhao P. Dealing with complex drug-drug interactions: towards mechanistic models. Biopharm Drug Dispos. 2015. 36:71–92. [DOI] [PubMed] [Google Scholar]
- 12.Wagner JG, Northam JI, Alway CD, Carpenter OS. Blood levels of drug at the equilibrium state after multiple dosing. Nature. 1965. 207:1301–1302. [DOI] [PubMed] [Google Scholar]
- 13.Greenberg HE, England MJ, Hellriegel ET, Bjornsson TD. Time of peak drug concentration after a single dose and a dose at steady state. J Clin Pharmacol. 1997. 27:480–485. [DOI] [PubMed] [Google Scholar]
- 14.Sahin S, Benet LZ. The operational multiple dosing half-life: a key to defining drug accumulation in patients and to designing extended release dosage forms. Pharm Res. 2008. 25:2869–2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lau YY, Huang Y, Frassetto L, Benet LZ. Effect of OATP1B1 transporter inhibition on the pharmacokinetics of atorvastatin in healthy volunteers. Clin Pharmacol Ther. 2007. 81,194–204. [DOI] [PubMed] [Google Scholar]
- 16.Cheng H, Jusko WJ. Noncompartmental determination of the mean residence time and steady-state volume of distribution during multiple dosing. J Pharm Sci. 1991. 80:202204. [DOI] [PubMed] [Google Scholar]
- 17.Pfeffer M Estimation of mean residence time from data obtained when multiple-dosing steady state has been reached. J Pharm Sci. 1984. 73:854–856. [DOI] [PubMed] [Google Scholar]
- 18.Rohatagi S, Kan S, Derendorf H. Non-compartmental analysis of pharmacokinetic data after multiple intravenous and oral administration. Pharmazie. 1997. 52:529–532. [PubMed] [Google Scholar]
- 19.Benet LZ, Galeazzi RL. Noncompartmental determinations of the steady-state volume of distribution. J Pharm Sci. 1979. 68:1071–1074. [DOI] [PubMed] [Google Scholar]
- 20.Tornio A, Filppula AM, Niemi M, Backman JT. Clinical studies on drug-drug interactions involving metabolism and transport: methodology, pitfalls and interpretation. Clin Pharmacol Ther. 2019. 105:1345–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu HF, Hristeva N, Chang J, Liang X, Li R, Frassetto L, Benet LZ. Rosuvastatin pharmacokinetics in Asian and White subjects wild type for both OATP1B1 and BCRP under control and inhibited conditions. J Pharm Sci. 2017. 106:2751–2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Westphal K, Weinbrenner A, Zschiesche M, Franke G, Knoke M, Oertel R, Fritz P, von Richter O, Warzok R, Hachenberg T, Kauffmann HM, Schrenk D, Terhaag B, Kroemer HK, Siegmund W. Induction of P-glycoprotein by rifampin increases intestinal secretion of talinolol in human beings: a new type of drug/drug interaction. Clin Pharmacol Ther. 2000. 68:345–355. [DOI] [PubMed] [Google Scholar]
- 23.Varhe A, Olkkola KT, Neuvonen PJ. Fluconazole, but not terbinafine, enhances the effects of triazolam by inhibiting its metabolism. Br J Clin Pharmacol. 1996. 41:319–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gustavson LE, Kaiser JF, Edmonds AL, Locke CS, DeBartolo ML, Schneck DW. Effect of omeprazole on concentrations of clarithromycin in plasma and gastric tissue at steady state. Antimicrob Agents Chemother. 1995. 39:2078–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vakkalagadda B, Frost C, Byon W, Boyd RA, Wang J, Zhang D, Yu Z, Dias C, Shenker A, LeCresta F. Effect of rifampin on the pharmacokinetics of apixaban, an oral direct inhibitor of factor Xa. Am J Cardiovasc Drugs. 2016. 16:119–127. [DOI] [PubMed] [Google Scholar]
- 26.ELIQUIS (apixaban) [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2012. [Google Scholar]
- 27.Jacqueroux E, Mercier C, Margelidon-Cozzolino V, Hodin S, Bertoletti L, Delavenne X. In vitro assessment of P-gp and BCRP transporter-mediated drug-drug interactions of riociguat with direct oral anticoagulants. Fundam Clin Pharmacol 2020. 34:109–119. [DOI] [PubMed] [Google Scholar]
- 28.Zhang D, He K, Herbst JJ, Kolb J, Shou W, Wang L, Balimane PV, Han YH, Gan J, Frost CE, Humphreys WG. Characterization of efflux transporters involved in distribution and disposition of apixaban. Drug Metab Dispos. 2013. 41:827–835. [DOI] [PubMed] [Google Scholar]
- 29.Wu CY, Benet LZ. Predicting drug disposition via application of BCS: transport / absorbtion / elimination interplay and development of a Biopharmaceutics Drug Disposition Classification System. Pharm Res. 2005. 22:11–23. [DOI] [PubMed] [Google Scholar]
- 30.Zamek-Gliszczynski MJ, Taub ME, Chothe PP, Chu X, Giacomini KM, Kim RB, Ray AS, Stocker SL, Unadkat JD, Wittwer MB, Xia C, Yee SW, Zhang L, Zhang Y International Transporter Consortium. Transporters in drug development: 2018 ITC recommendations for transporters of emerging clinical importance. Clin Pharmacol Ther. 2018. 104:890899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.U.S. Food and Drug Administration, Center for Drug Evaluation and Research. In vitro metabolism- and transporter-mediated drug-drug interaction studies: guidance for industry. Silver Spring, MD: 2017. [Google Scholar]
- 32.Cheong J, Halladay JS, Plise E, Sodhi JK, Salphati L. The effects of drug metabolizing enzymes inhibitors on hepatic efflux and uptake transporters. Drug Metab Lett. 2017. 11:111–118. [DOI] [PubMed] [Google Scholar]
- 33.Brown HS, Ito K, Galetin A, Houston JB. Prediction of in vivo drug-drug interactions from in vitro data: impact of incorporating parallel pathways of drug elimination and inhibitor absorption rate constant. Br J Clin Pharmacol. 2005. 60:508–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ito K, Iwatsubo T, Kanamitsu S, Ueda K, Suzuki H, Sugiyama Y. Prediction of pharmacokinetic alterations caused by drug-drug interactions: metabolic interaction in the liver. Pharmacol Rev. 1998. 50:387–412. [PubMed] [Google Scholar]
- 35.Smith MT, Eadie MJ, Brophy TO. The pharmacokinetics of midazolam in man. Eur J Clin Pharmacol. 1981. 19:271–278. [DOI] [PubMed] [Google Scholar]
- 36.Heizmann P, Eckert M, Ziegler WH. Pharmacokinetics and bioavailability of midazolam in man. Br J Clin Pharmacol. 1983. 16:43S–49S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee JI, Chaves-Gnecco D, Amico JA, Kroboth PD, Wilson JW, Frye RF. Application of semisimultaneous midazolam administration for hepatic and intestinal cytochrome P450 3A phenotyping. Clin Pharmacol Ther. 2002. 72:718–728. [DOI] [PubMed] [Google Scholar]
- 38.Brazzell RK, Kaplan SA. Factors affecting the accuracy of estimated mean absorption times and mean dissolution times. J Pharm Sci. 1983. 72:713–715. [DOI] [PubMed] [Google Scholar]
- 39.Veering BT, Burm AGL, Vletter AA, van den Hoeven RAM, Spierdijk J. The effect of age on systemic absorption and systemic disposition of bupivacaine after subarachnoid administration. Anesthesiology. 1991. 74:250–257. [DOI] [PubMed] [Google Scholar]
- 40.Fredrick MJ, Pound DC, Hall SD, Brater DC. Furosemide absorption in patients with cirrhosis. Clin Pharmacol Ther. 1991. 49:241–247. [DOI] [PubMed] [Google Scholar]
- 41.Holt S, Heading RC, Clements JA, Tothill P, Prescott LF. Acetaminophen absorption and metabolism in celiac disease and Crohn’s disease. Clin Pharmacol Ther. 1981. 30:232238. [DOI] [PubMed] [Google Scholar]
- 42.Kitis G, Lucas ML, Bishop H, Sargent A, Schneider RE, Blair JA, Allan RN. Altered jejunal surface pH in coeliac disease: its effect on propranolol and folic acid absorption. Clin Sci. 1982. 63:373–380. [DOI] [PubMed] [Google Scholar]
- 43.Pfeffer M, Gaver RC, Ximenez J. Human intravenous pharmacokinetics and absolute oral bioavailability of cefatrizine. Antimicrob Agents Chemother. 1983. 24:915–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reigner BG, Couet W, Guedes JP, Fourtillan JB, Tozer TN. Saturable rate of cefatrizine absorption after oral administration to humans. J Pharmacokinet Biopharm. 1990. 18:17–24. [DOI] [PubMed] [Google Scholar]
- 45.Estudante M, Morais JG, Soveral G, Benet LZ. Intestinal drug transporters: an overview. Adv Drug Deliv Rev. 2013. 65:1340–1356. [DOI] [PubMed] [Google Scholar]
- 46.Morse BL, Alberts JJ, Posada MM, Rehmel J, Kolur A, Tham LS, Loghin C, Hillgren KM, Hall SD, Dickinson GL. Physiologically-based pharmacokinetic modeling of atorvastatin incorporating delayed gastric emptying and acid-to-lactone conversion. CPT Pharmacometrics Syst Pharmacol. 2019. 8:664–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Custodio JM, Wu CY, Benet LZ. Predicting drug disposition, absorption / elimination / transporter interplay and the role of food on drug absorption. Adv Drug Deliv Rev. 2008. 60:717–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Levy G, Jusko WJ. Effect of viscosity on drug absorption. J Pharm Sci. 1965. 54:219–225. [DOI] [PubMed] [Google Scholar]
- 49.Tanaka Y, Goto T, Kataoka M, Sakuma S, Yamashita S. Impact of luminal fluid volume on the drug absorption after oral administration: analysis based on in vivo drug concentration-time profile in the gastrointestinal tract. J Pharm Sci. 2015. 104:31203127. [DOI] [PubMed] [Google Scholar]
- 50.Martinez MN, Amidon GL. A mechanistic approach to understanding factors affecting drug absorption: a review of fundamentals. J Clin Pharmacol. 2002. 42:620–643. [DOI] [PubMed] [Google Scholar]
- 51.Rodrigues AD, Lai Y, Shen H, Varma MVS, Rowland A, Oswald S. Induction of human intestinal and hepatic organic anion transporting polypeptides: where is the evidence for its relevance in drug-drug interactions? Drug Metab Dispos. 2020. 48:205–216. [DOI] [PubMed] [Google Scholar]
- 52.Yu J, Zhou Z, Tay-Sontheimer J, Levy RH, Ragueneau-Majlessi I. Intestinal drug interactions mediated by OATPs: a systemic review of preclinical and clinical findings. J Pharm Sci. 2017. 106:2312–2325. [DOI] [PubMed] [Google Scholar]
- 53.McFeely SJ, Wu L, Ritchie TK, Unadkat J. Organic anion transporting polypeptide 2B1 – more than a glass-full of drug interactions. Pharmacol Ther. 2019. 1996:204–215. [DOI] [PubMed] [Google Scholar]
- 54.Niemi M, Backman JT, Fromm MF, Neuvonen PJ, Kivistö KT. Pharmacokinetic interactions with rifampicin. Clin Pharmacokinet. 2003. 42:819–850. [DOI] [PubMed] [Google Scholar]