Abstract
Background:
Currently, no reliable predictive clinical or laboratory tests exist that can accurately distinguish between benign and malignant pheochromocytomas or paragangliomas (PPGLs). The aim of this study was to investigate if serum microRNA-210 (miR-210) levels could be a marker of malignancy in patients with PPGLs.
Methods:
Preoperative serum from patients with PPGLs was collected on the day of surgery. Clinical demographics, germline mutation status, primary tumor size, postoperative biochemical response, and the development of malignant disease were prospectively collected. Total microRNA was extracted from preoperative serum samples, and miR-210 levels were measured by quantitative real-time reverse transcription-polymerase chain reaction and normalized to miR-16. Prognostic variables were compared using univariable and multivariable analyses.
Results:
Of the 35 patients, 10 (29%) were diagnosed with malignant PPGLs and 25 patients (71%) were diagnosed with benign PPGLs (median follow-up 72.5 mo). Sixty-nine percent of patients had a pheochromocytoma (n = 24/35) compared with 31% of patients with paraganglioma (n = 11/35). The most common germline mutation was succinate dehydrogenase complex subunit B (SDHB) (n = 10). On univariable analysis, lower serum miR-210 expression level (2.3 ± 0.5 versus 3.1 ± 1.2, P = 0.013) and larger primary tumor size (6.7 ± 5.0 cm versus 4.1 ± 2.3 cm, P = 0.043) were significantly associated with malignant disease. No significant prognostic variables were found on multivariable analysis.
Conclusions:
In this pilot study, low serum miR-210 expression levels and large primary tumors were identified to be markers of PPGL malignancy on univariable analysis. Given the initial encouraging results in a small cohort, further investigation is warranted to determine if serum miR-210 levels are prognostic.
Keywords: Pheochromocytoma, Paraganglioma, Adrenal, Malignancy, MicroRNA, miR-210
Introduction
Pheochromocytomas and paragangliomas (PPGLs) are neuroendocrine tumors derived from chromaffin cells arising in the adrenal medulla and along the paravertebral axis outside the adrenal gland, respectively.1 These rare tumors have a prevalence of 2–8 per million people per year and can be sporadic or associated with a genetic syndrome.2,3 Up to one-third of patients with PPGLs have germline DNA mutations, including mutations in succinate dehydrogenase complex subunit B or D (SDHB or SDHD) associated with the hereditary paraganglioma syndrome 4 and 1, respectively, RET mutations leading to multiple endocrine neoplasia 2 (MEN2), germline VHL gene mutation causing von Hippel-Lindau (VHL) disease, NF1 mutation causing neurofibromatosis type 1, and others.2
Current diagnostic workup of PPGLs includes biochemical analysis of urinary and plasma catecholamines and urine-fractionated and plasma-free metanephrines, as well as anatomic and functional imaging for tumor localization.1 According to Lenders et al, plasma-free and urinary-fractionated metanephrines have been reported to have the highest sensitivity (99% and 97%, respectively) for patients with pheochromocytomas.4 However, neither of these diagnostic tests nor histology of primary tumors can differentiate between benign and malignant PPGLs. In fact, there are currently no reliable clinical or laboratory tests to predict malignancy, although some positive associations have been shown with germline SDHB mutation, extra-adrenal site of disease, persistent postoperative hypertension, and tumor diameter greater than 5 cm.5 Because there are no histopathologic criteria, malignant PPGLs are defined by the presence of local invasion by the primary tumor and/or metastatic disease.6 Patients can develop recurrence as long as 15 y after initial surgical intervention; therefore, long-term follow-up is imperative.7
MicroRNAs (miRNAs) are small, nonprotein coding RNAs that help to maintain intracellular physiological balance through cell-cycle checkpoints, cell proliferation, and apoptosis. Because multiple studies have demonstrated a link between alteration in miRNAs and the development of cancer, miRNA profiling has been investigated as a potential diagnostic, prognostic, and therapeutic tool in a wide range of cancers (e.g., thyroid, lung, breast, cervical, soft tissue sarcoma, and adrenocortical tumors).8–10 A comprehensive miRNA analysis of benign and malignant pheochromocytoma tumors by Patterson et al demonstrated statistically significant higher expression of miR-483–5p, miR-183, and miR-101 in malignant samples, suggesting that these markers can serve as a diagnostic tool. Although expression of these three miRNAs was detected above background in serum samples, there was no difference in serum levels between patients with benign and those with malignant tumors.11 However, serum levels of microRNA-210 (miR-210), a miRNA that previously was found to be induced under hypoxic stress and linked to adverse prognosis in some cancer types, were not examined in the microarray analysis by Patterson et al.12,13 In addition, miR-210 has been shown to be overexpressed in SDHB-related PPGL tumor samples compared with normal adrenal tissue and in malignant tumors compared with benign.14
Because miR-210 is an important regulator of hypoxia and associated with SDHB-related PPGLs, a targeted approach examining the role of miR-210 as a serum biomarker to distinguish benign and malignant PPGLs was hypothesized.14 The aim of this study was to investigate whether there was a difference in preoperative serum miR-210 expression levels between patients with malignant and those with benign PPGLs who underwent resection.
Materials and methods
Serum samples
Demographic, clinical, and pathologic information and tissue samples were prospectively collected under an institutional review board–approved protocol (NCT01005654). Fasting whole blood samples collected from patients with PPGL tumors were obtained on the day of surgery, and serum was extracted and stored at −80°C. Gender, race, body mass index (BMI), age at time of operation, primary tumor site, germline mutation status of genes associated with hereditary PPGL, postoperative biochemical response, and tumor size were all collected in a prospective manner. A complete postoperative biochemical response was defined as all serum and urine biochemical data returning to normal range after the operation. Patients were included into the malignant cohort if their blood drawn at the time of operation had metastatic or recurrent disease with local invasion or positive lymph nodes. Patients were included into the benign cohort if their blood drawn at the primary operation did not have metastatic disease, local invasion, or positive lymph nodes and did not develop malignant disease during the follow-up period. Patients who developed malignant disease during follow-up but did not have it at the time of sampling were excluded from the study (n = 5).
Serum miRNA extraction
MicroRNA was extracted from the serum using the Qiagen miRNeasy Kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. In brief, total RNA was isolated from 200 μL of the serum using QIAzol (Qiagen). After chloroform extraction, 1.5 times the volume of 100% ethanol was added to the aqueous phase. MicroRNA was purified from this mixture using the Qiagen miRNeasy Kit (Qiagen), enriched column purification system. Total miRNA was eluted in 28 μL of RNase-free water.
Real-time quantitative RT-PCR analysis
Serum levels of miR-210 were measured using TaqMan quantitative real-time reverse transcription-polymerase chain reaction (RT-PCR; Applied Biosystems, Foster City, CA). Single-stranded cDNA was synthesized from total miRNA using specific miRNA primers (TaqMan MicroRNA Assay, PN 4427975; Applied Biosystems) and the TaqMan MicroRNA Reverse Transcription Kit (PN 4366596; Applied Biosystems). 2.5 μL of cDNA was used as a template in a 10 μL PCR reaction. PCR products were amplified using specific primers (TaqMan MicroRNA Assay) and the TaqMan Universal PCR Master Mix (PN 4324018; Applied Biosystems) and detected using the 7900HT Fast Real-Time PCR System (Applied Biosystems). PCR reactions for each sample were run in triplicate. Control reactions included cDNA synthesized without reverse transcriptase enzyme (RNA only) and no cDNA template. MicroRNA-210 expression was measured using TaqMan MicroRNA Assay (catalog number 4427975; Applied Biosystems). MicroRNA-16 (001006) was used as an endogenous control for data normalization by measuring expression in all serum samples.15 Serum miRNA expression levels were calculated according to the 2−ΔCt method.
Statistical analysis
Categorical variables were compared using the chi-square test. Continuous variables were compared using the independent t-test if the D’Agostino-Pearson test demonstrated normality or the Mann-Whitney test if normality was not demonstrated. Linear regression was used to look for any correlations between miR-210 expression levels and preoperative laboratory values (serum dopamine, epinephrine, norepinephrine, fractionated metanephrines, fractionated normetanephrines, and urine-fractionated metanephrines). Variables with a P value < 0.1 on univariable analysis were included in multivariable binary logistic regression analysis. A two-tailed P value of < 0.05 on multivariable analysis by binary logistic regression was considered statistically significant. All calculations were performed using SPSS, version 25 (IBM, Armonk, NY).
Results
Patient characteristics
A total of 35 patients who underwent resection for PPGLs between 2009 and 2013 were included in this study. Seventy-one percent (n = 25) of the patients had benign disease, and 29% (n = 10) had malignant disease. Median follow-up time was 72.5 mo (range 0.4–434.1 mo).
Patients with benign PPGLs
Patients with benign PPGLs were predominantly Caucasian (80%, n = 20), female (60%, n = 15), had a mean BMI of 27.9 + 8.4, and a mean age of 38 + 20 y at the time of surgery. Primary tumors localized to the adrenal gland (68%, n = 17), retroperitoneum (16%, n = 4), periaortic region (12%, n = 3), and neck/paratracheal region (4%, n = 1). Mean tumor size was 4.1 ± 2.3 cm. Most patients had no known germline mutation in known susceptible genes (52%, n = 13). The most common mutation was SDHB (24%, n = 6). Postoperatively, most patients had a complete biochemical response (80%, n = 20). Mean serum miR-210 level was 3.1 ± 1.2 (Table 1).
Table 1 –
Univariable analysis comparing clinical and tumor characteristics of patients with benign disease versus those with malignant disease.
| Characteristic | Benign cohort, n = 25 (%) | Malignant cohort, n = 10 (%) | P-value |
|---|---|---|---|
| Gender | 0.283 | ||
| Male | 10 (40) | 6 (60) | |
| Female | 15 (60) | 4 (40) | |
| Race | 0.717 | ||
| Caucasian | 20 (80) | 9 (90) | |
| African American | 4 (16) | 1 (10) | |
| Asian | 1 (4) | 0 (0) | |
| Unknown | 0 (0) | 0 (0) | |
| Age at time of operation (y) | 38 ± 20 | 38 ± 14 | 0.930 |
| Primary tumor site | 0.333 | ||
| Adrenal | 17 (68) | 7 (70) | |
| Periaortic | 3 (12) | 1 (10) | |
| Bladder | 0 (0) | 1 (10) | |
| Neck/paratracheal | 1 (4) | 1 (10) | |
| Retroperitoneal | 4 (16) | 0 (0) | |
| Genetics | 0.262 | ||
| No germline genetic mutation | 13 (52) | 2 (20) | |
| SDHB | 6 (24) | 4 (40) | |
| VHL | 3 (12) | 2 (20) | |
| MEN2A | 1 (4) | 0 (0) | |
| HIF2a | 0 (0) | 1 (10) | |
| MAX | 0 (0) | 1 (10) | |
| KIF1B | 1 (4) | 0 (0) | |
| NF1 | 1 (4) | 0 (0) | |
| Postoperative biochemical response | 0.207 | ||
| Partial biochemical response | 2 (8) | 2 (20) | |
| Complete biochemical response | 20 (80) | 5 (50) | |
| Unknown | 3 (12) | 3 (30) | |
| Body mass index at time of operation (kg/m2) | 27.9 ± 8.4 | 29.1 ± 6.3 | 0.692 |
| Serum miR-210 level (normalized) | 3.0987 ± 1.2278 | 2.3299 ± 0.5078 | 0.013 |
| Tumor size (cm) | 4.1 ± 2.3 | 6.7 ± 5.0 | 0.03 |
Categorical values expressed as n (%) rounded to the nearest percentage. Continuous variables expressed as mean ± standard deviation. The miR-210 expression level is normalized.
SDHB = succinate dehydrogenase complex subunit B mutation; VHL = von Hippel-Lindau disease; MEN2A = multiple endocrine neoplasia type 2A; HIF2a = hypoxia-inducible factor a mutation; MAX = myc-associated factor X mutation; KIF1B = kinesin family member 1B mutation; NF1 = neurofibromatosis 1.
Patients with malignant PPGLs
Patients with malignant PPGLs were predominantly Caucasian (90%, n = 9), male (60%, n = 6), had a mean BMI of 29.1 ± 6.4, and a mean age of 38 ± 14 y at the time of surgery. Primary tumors localized to the adrenal gland (70%, n = 7), periaortic region (10%, n = 1), bladder (10%, n = 1), and neck/paratracheal region (10%, n = 1). Mean tumor size was 6.7 ± 5.0 cm. Twenty percent (n = 2) of patients had no known germline mutation in known susceptible genes. The most common mutation was SDHB (40%, n = 4). Postoperatively, half of the patients had a complete biochemical response (50%, n = 5). Mean serum miR-210 level was 2.3 ± 0.5 (Table 1).
Predictors of malignant disease
On univariable analysis, there was no difference between the benign and malignant cohort with respect to gender, race, age, primary tumor site, germline mutation status, postoperative biochemical response, or BMI. Germline mutation status was compared as individual mutations compared to no germline mutation and as SDHB mutation versus all other germline mutations versus no germline mutation. Neither of these analyses demonstrated a significant difference. Univariable analysis showed that the malignant cohort had a lower miR-210 level (2.3 ± 0.5 versus 3.1 ± 1.2, P = 0.013, Figure A) and a higher mean tumor size (6.7 ± 5.0 cm versus 4.1 ± 2.3 cm, P = 0.03, respectively, Figure B). On multivariable analysis, neither tumor size (OR 1.294, CI 0.932–1.796, P = 0.124) nor miR-210 expression level (OR 0.413, CI 0.134–1.274, P = 0.124) was significantly associated with malignant disease (Table 2).
Fig –
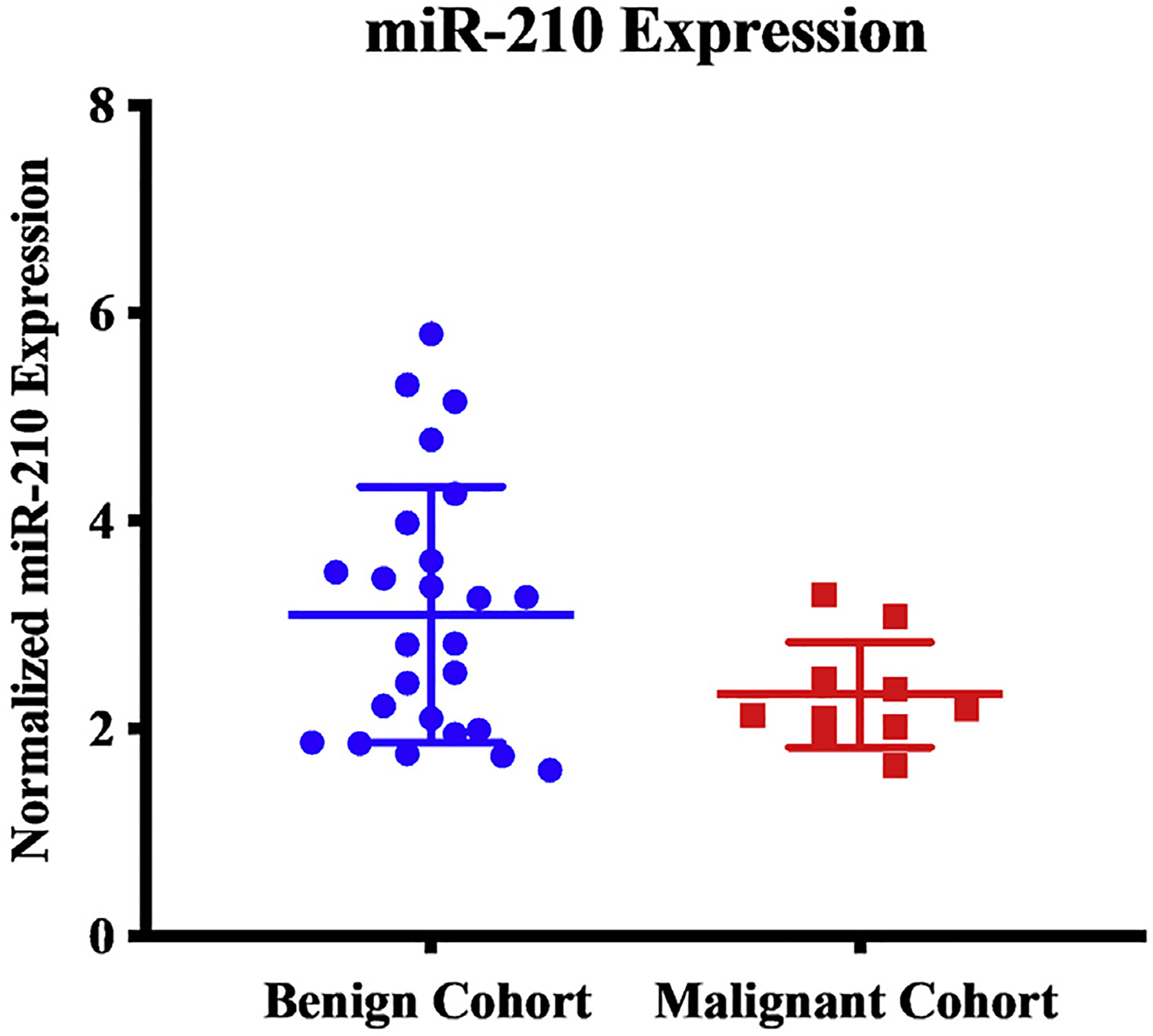
Patients with malignant pheochromocytomas and paragangliomas were found to have lower serum expression levels of miR-1210 than patients with benign pheochromocytomas and paragangliomas.
Table 2 –
Multivariable analysis to identify factors that are associated with metastatic/recurrent disease.
| Variable | Odds ratio | Confidence interval | P value |
|---|---|---|---|
| Tumor size | 1.294 | 0.932–1.796 | 0.124 |
| Serum miR-210 level | 0.413 | 0.134–1.274 | 0.124 |
Catecholamine production and miR-210 expression
Serum levels of dopamine, epinephrine, norepinephrine, fractionated metanephrines, fractionated normetanephrines, and urine-fractionated metanephrines were analyzed (Table 3). There was no association between levels of biochemical markers and serum miR-210 levels.
Table 3 –
Preoperative biochemical data for the entire cohort (n = 35).
| Laboratory test | n† | Number with elevated levels, n (%) | Mean ± SD |
|---|---|---|---|
| Dopamine, serum (ULN = 46 pg/mL) | 32 | 9 (26) | 73 ± 187 |
| Epinephrine, serum (ULN = 83 pg/mL) | 30 | 7 (20) | 93 ± 174 |
| Norepinephrine, serum (ULN = 498 pg/mL) | 32 | 21 (60) | 2229 ± 3165 |
| Fractionated metanephrines, serum (ULN = 61 pg/mL) | 32 | 12 (34) | 202 ± 435 |
| Fractionated normetanephrines, serum (ULN = 112 pg/mL) | 31 | 27 (77) | 1708 ± 4256 |
| Fractionated metanephrine, urine (ULN = 261–400 mcg)* | 25 | 10 (29) | 1173 ± 2260 |
ULN = upper limit of normal; SD = standard deviation.
Range depending on patient’s blood pressure.
Number of patients with the available data.
Discussion
There are currently no reliable biomarkers to differentiate patients with benign and malignant PPGLs. MicroRNA-210 has been associated with PPGLs, SDHB mutation, and hypoxia.12–14 In this pilot study of a rare tumor, larger tumors and lower serum expression levels of miR-210 were found to be associated with malignant PPGLs on univariable analysis. Given the small sample size, neither variables were confirmed on multivariable analysis.
Approximately 10% of pheochromocytomas and 15%–35% of paragangliomas are malignant and carry a poor prognosis.16 As a result, there have been several attempts to distinguish between benign and malignant tumors at the time of diagnosis. Although there is currently no reliable method to predict malignancy, a few positive associations have been shown with increased age at time of diagnosis, SDHB mutation, extra-adrenal site of disease, persistent postoperative hypertension, and a tumor size greater than 5 cm.5 Our study confirmed on univariable analysis the correlation between a large tumor size and malignancy; however, this was not confirmed on multivariable analysis likely due to the small sample size. Overall, with minimal retrospective data on how clinical factors can predict future malignant PPGLs, it is difficult to select which patients would benefit from a more aggressive surveillance and treatment approach. This difficulty attests for the need of novel biomarkers to predict patients with malignant PPGLs.
Over the past decade, miRNA expression profiling has garnered increasing attention because of its prominent role in cancer biology. MicroRNAs have been found to facilitate tumor growth, invasion, angiogenesis, and immune evasion through the posttranscriptional regulation of various proteins.16 With the discovery that miRNA can be detected in different bodily fluids, the field has expanded to examine the potential use of miRNA as a biomarker to monitor clinical response and to predict survival or disease course.17 One benefit is that this monitoring could potentially be done through minimally invasive techniques (e.g., blood samples).16 Although the source of circulating miRNA remains unclear, there are two main hypotheses: (1) they are released on tumor cell death and lysis and (2) the tumor cells purposefully secrete miRNA in exosomes as a means of intracellular signaling.18
Some studies have profiled miRNA expression in patients with PPGLs, including miR-15a, miR-16, miR-483–5p, miR-183, miR-101, miR-139, miR-541, and miR-765.10,19,20 However, few studies have evaluated miR-210, and of those studies, all focused on miR-210 extracted from tumor and/or adrenal tissue, not serum. De Cubas et al analyzed tissue from PPGLs and normal adrenal glands and found that miR-210 was upregulated in VHL- and SDHB-related PPGLs.21 Tsang et al evaluated miR-210 expression in tumor samples from patients with PPGLs stratified by genetic mutation and concluded that miR-210 is overexpressed in PPGLs compared with normal adrenal tissue and in malignant tumors compared with benign. MicroRNA-210 expression was again found to be associated with those harboring germline mutations SDHB and VHL.14 Despite the characterization of tissue PPGL miRNA expression in multiple studies, miR-210 was only found to be differentially expressed in two studies. Finally, using The Cancer Genome Atlas, Fishbein et al found that overexpression of miR-210 in primary PPGL tissue was associated with SDHB and VHL germline mutations and more aggressive disease. This study defined aggressive disease by having distant metastatic events, positive local lymph nodes, or local recurrence.22 In our study, miR-210 was found to be under-expressed in the serum of patients with malignant disease compared with the patients with benign disease. The differing results may be explained by different rates of germline mutations, various microarray platforms, and varying tissue procurement procedures.
MicroRNA-210 has been shown to be increased under hypoxia, and as a result, it plays a role in cell survival under anaerobic conditions, cell cycle regulation, DNA damage and repair, and compromised mitochondrial function.23 Its expression has also been associated with the development of head and neck squamous cell carcinomas, pancreatic cancer, breast cancer, and lung cancer.13 Several studies have shown that miRNA expression levels in plasma can significantly differ from those in tumor tissue.23,24 Tanaka et al demonstrated that miR-92a expression levels were decreased in plasma of patients with leukemia despite its high expression in leukemic cells, suggesting that perhaps, the cancer cells both transcribe miR-92a and uptake it from the blood.25 Xu et al demonstrated that within the nervous system, secreted miRNAs are taken up by synaptosomes via an endocytic pathway.26 Our study demonstrated surprisingly lower expression levels of miR-210 in the serum of malignant population than benign population, and this seemingly paradoxical finding could either be because tumor tissue is actively absorbing miR-210 from the blood (perhaps through an endocytic pathway) or producing it and not releasing it into the vascular system.
Although this study demonstrated potential differences in miR-210 serum levels regarding tumor behavior, there are some limitations. Owing to the infrequent nature of PPGLs (2–8 per million people per year), the sample size was small but can serve as a robust pilot cohort for future studies. Furthermore, the different levels of miR-210 were not found to be significantly different on multivariable analysis, possibly explained by the limited sample size. In addition, only serum was available, thus not allowing a comparison of expression levels between serum and tumor tissue. A broader analysis by including tumor tissue and drawing serial serum samples could provide a more complete picture of the relationship between miR-210 expression levels and the malignant potential of PPGLs. By drawing serial serum samples and correlating those expression levels with tumor tissue, it could demonstrate that miR-210 is being taken up by the tumor and explain the discordance in previously cited studies. Finally, owing to the limited availability of tissue, germline and sporadic PPGLs are often grouped together for overall analysis. This is a limitation, again due to sample size, because patients with underlying germline mutations might have a different biomarker profile than those with sporadic PPGLs.
Conclusion
In conclusion, this study demonstrates that on univariable analysis, increased tumor size and lower serum miR-210 expression level are associated with malignant PPGLs. Further analyses of tumor tissue in parallel with serum miRNA expression levels and a larger sample size are needed to evaluate these results and their possible clinical significance for predicting which patients with PPGLs are at a higher risk to develop malignant disease. Because PPGLs are rare tumors, studies are often underpowered and the literature is limited in its ability to make progress in differentiating between malignant and benign disease. This pilot study serves as a guide by highlighting a potential biomarker (lower serum expression level of miR-210) and helping to focus future studies in determining what markers to prioritize during analysis.
Acknowledgment
This research was supported by the Intramural Research Program at the National Institutes of Health, Bethesda, MD, USA.
Footnotes
Disclosure
The authors have no conflicts of interest.
Presentation: This manuscript is being presented as a QuickShot presentation at the Academic Surgical Congress on February 5, 2019.
REFERENCES
- 1.Kiernan CM, Solorzano CC. Pheochromocytoma and paraganglioma: diagnosis, genetics, and treatment. Surg Oncol Clin N Am. 2016;25:19–38. [DOI] [PubMed] [Google Scholar]
- 2.Yeh MW, Livhits MJ, Duh QY. The adrenal glands In: Townsend CM Jr, Beauchamb RD, Evers BM, Mattox KL, eds. Sabiston Textbook of Surgery: The Biological Bases of Modern Surgical Practice. 20th ed Philadelphia, PA: Elsevier; 2016. [Google Scholar]
- 3.“Pheochromocytoma and paraganglioma treatment.” National Cancer Institute. 2018. Available at: www.cancer.gov/types/pheochromocytoma/hp/pheochromocytoma-treatment-pdq#_25_toc. Accessed February 8, 2018. [Google Scholar]
- 4.Lenders JWM, Pacak K, Walther MM, et al. Biochemical diagnosis of pheochromocytoma: which test is best? JAMA. 2002;287:1427–1434. [DOI] [PubMed] [Google Scholar]
- 5.Feng F, Zhu Y, Wang X, et al. Predictive factors for malignant pheochromocytoma: analysis of 136 patients. J Urol. 2011;185:1583–1590. [DOI] [PubMed] [Google Scholar]
- 6.Ellis RJ, Patel D, Prodanov T, et al. Response after surgical resection of metastatic pheochromocytoma and paraganglioma: can postoperative biochemical remission be predicted? J Am Coll Surg. 2013;217:489–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plouin PF, Chatellier G, Fofol I, Corvol P. Tumor recurrence and hypertension persistence after successful pheochromocytoma operation. Hypertension. 1997;29:1133–1139. [DOI] [PubMed] [Google Scholar]
- 8.Ruan K, Fang X, Ouyang G. MicroRNAs: novel regulators in the hallmarks of human cancer. Cancer Lett. 2009;285:116–126. [DOI] [PubMed] [Google Scholar]
- 9.Mishra S, Yadav T, Rani V. Exploring miRNA based approaches in cancer diagnostics and therapeutics. Crit Rev Oncol Hematol. 2016;98:12–23. [DOI] [PubMed] [Google Scholar]
- 10.Patterson EE, Holloway AK, Weng J, Fojo T, Kebebew E. MicroRNA profiling of adrenocortical tumors reveals miR-483 as a marker of malignancy. Cancer. 2011;117:1630–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patterson E, Webb R, Weisbrod A, et al. The microRNA expression changes associated with malignancy and SDHB mutation in pheochromocytoma. Endocr Relat Cancer. 2012;19:157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang X, Le QT, Giaccia AJ. MiR-210dmicromanager of the hypoxia pathway. Trends Mol Med. 2010;16:230–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang X, Zuo J. Emerging roles of m16iR-210 and other noncoding RNAs in the hypoxic response. Acta Biochim Biophys Sin (Shanghai). 2014;46:220–232. [DOI] [PubMed] [Google Scholar]
- 14.Tsang VHM, Dwight T, Benn DE, et al. Overexpression of miR-210 is associated with SDH-related pheochromocytomas, paragangliomas, and gastrointestinal stromal tumours. Endocr Relat Cancer. 2014;21:415–426. [DOI] [PubMed] [Google Scholar]
- 15.Song J, Bai Z, Han W, et al. Identification of suitable reference genes for qPCR analysis of serum microRNA in gastric cancer patients. Dig Dis Sci. 2012;57:897–904. [DOI] [PubMed] [Google Scholar]
- 16.Harari A, Inabnet 3rd WB. Malignant pheochromocytoma: a review. Am J Surg. 2011;201:700–708. [DOI] [PubMed] [Google Scholar]
- 17.Hayes J, Peruzzi PP, Lawler S. MicroRNAs in cancer: biomarkers, functions and therapy. Trends Mol Med. 2014;20:460–469. [DOI] [PubMed] [Google Scholar]
- 18.Ohshima K, Inoue K, Fujiwara A, et al. Let-7 microRNA family is selectively secreted into the extracellular environment via exosomes in a metastatic gastric cancer cell line. PLoS One. 2010;5:e13247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meyer-Rochow GY, Jackson NE, Conaglen JV, et al. MicroRNA profiling of benign and malignant pheochromocytomas identifies novel diagnostic and therapeutic targets. Endocr Relat Cancer. 2010;17:835–846. [DOI] [PubMed] [Google Scholar]
- 20.Tombol Z, Eder K, Kovacs A, et al. MicroRNA expression profiling in benign (sporadic and hereditary) and recurring adrenal pheochromocytomas. Mod Pathol. 2010;23:1583–1595. [DOI] [PubMed] [Google Scholar]
- 21.de Cubas AA, Leandro-Garcia LJ, Schiavi F, et al. Integrative analysis of miRNA and mRNA expression profiles in pheochromocytoma and paraganglioma identifies genotype-specific markers and potentially regulated pathways. Endocr Relat Cancer. 2013;20:477–493. [DOI] [PubMed] [Google Scholar]
- 22.Fishbein L, Leshchiner I, Walter V, et al. Comprehensive molecular characterization of pheochromocytoma and paragantlioma. Cancer Cell. 2017;31:181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pigati L, Yaddanapudi SC, Iyengar R, et al. Selective release of microRNA species from normal and malignant mammary epithelial cells. PLoS One. 2010;5:e13515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zedan AH, Hansen TF, Assenholt J, Pleckaitis M, Madsen JS, Osther PJS. microRNA expression in tumour tissue and plasma in patients with newly diagnosed metastatic prostate cancer. Tumour Biol. 2018;40, 1010428318775864. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka M, Oikawa K, Takanashi M, et al. Down regulation of miR-92 in human plasma is a novel marker for acu8te leukemia patients. PLoS One. 2009;4:35532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu J, Chen Q, Zen K, Zhang C, Zhang Q. Synaptosomes secrete and uptake functionally active microRNAs via exocytosis and endocytosis pathways. J Neurochem. 2013;124:15–25. [DOI] [PubMed] [Google Scholar]


