Abstract
Purpose
To evaluate clinical periodontal status and microbiologic pathogens in patients with chronic obstructive pulmonary disease (COPD) and periodontitis.
Patients and Methods
We conducted a case–control study of 60 periodontitis patients with COPD (case group) and 60 periodontitis patients with normal pulmonary function (control group). Their periodontal status and respiratory function were clinically examined. Real-time polymerase chain reaction assays were used to measure five dental pathogens and four respiratory pathogens in subgingival dental plaque. Spearman’s rank correlation coefficients (r2) were calculated to assess correlations of pathogens. Principal component analysis (PCA) was employed to assess the similarity of bacterial diversity between the two groups. Logistic regression was performed to examine the associations of periodontal variables and pathogens with COPD risk.
Results
COPD patients had fewer remaining teeth, higher plaque index (PLI), and more severe site percentages of clinical attachment level (CAL) than the controls. Although COPD patients tended to have relatively higher ranked means of Porphyromonas gingivalis, Tannerella forsythensis, Treponema denticola, and Haemophilus influenza than control participants, the differences were not significant. Some periodontal pathogens and respiratory pathogens were positively correlated with each other (r2 =0.29 to 0.47, all P < 0.05). The PCA graph showed that the distributions of pathogens were more dispersed but less discriminated in the COPD group than those in the control group. PLI (P = 0.045) and CAL ≥ 5mm site percentages (P = 0.01) were significantly associated with an increased risk of COPD, while pathogens were not associated with COPD.
Conclusion
Our results from this study do not indicate periodontal pathogens as potential predictors of COPD risk, despite significantly poor periodontal status associated with COPD.
Keywords: periodontal, COPD, bacteria, observational research
Introduction
Chronic Obstructive Pulmonary Disease (COPD) is one of the most common and costly respiratory diseases in the world. It is a leading cause of mortality globally.1 A recent epidemiological investigation in a large population revealed that the overall prevalence of COPD was 8.6% in China.2 The etiology of COPD is complex. Cigarette smoking, ambient air pollution, and parental history of respiratory diseases are some of the major risk factors for COPD. Inflammation induced by bacterial or viral infections is also an important contributor to the etiology of COPD. Respiratory pathogens, including Streptococcus pneumoniae (Sp), Klebsiella pneumoniae (Kp), Haemophilus influenza (Hi), and Pseudomonas aeruginosa (Pa), are common bacterial causes of COPD exacerbation.3–5
The association between periodontitis and COPD has been recognized over the past two decades. Scannapieco et al analyzed the US nationwide data from the National Health and Nutrition Examination Surveys I & NHANES III and first reported an association between poor oral health (including oral hygiene index and periodontal attachment loss) and COPD.6,7 A meta-analysis of 14 observational studies concluded that periodontal disease is an independent risk factor of COPD.8 A recent five-year prospective cohort study found a positive association of periodontitis severity with the risk of COPD.9 Another cohort study with 11,378 subjects also reported the positive association between oral health status and COPD.10 However, whether there is a cause-and-effect relationship remains unclear.
The exact mechanisms to explain the association of periodontal disease and COPD are still unknown. Aspiration of dental plaque and blood dissemination of inflammatory mediators from periodontal pockets may be two major pathways to worsen the COPD inflammatory status.11 Two studies suggested that respiratory pathogens from dental plaque serves as a reservoir of bacteria may cause pneumonia.12,13 A case–control study indicated that the median sputum antibody levels of two organisms periodontal pathogens [Fusobacterium nucleatum and Prevotella intermedia (Pi)] were significantly higher in patients with acute exacerbation of COPD (AE-COPD) than in controls.14 Tan’s study identified some lung pathogens (including Kp, Pa, and Sp) from dental plaque samples in patients with AE-COPD.15 A recent case–control study reported that the prevalence of dental pathogens and respiratory pathogens [Porphyromonas gingivalis (Pg), Kp, Pa, and Sp] in dental plaque was increased in participants with COPD.16 In this regard, respiratory pathogenic bacteria and their metabolites from dental plaque may promote airway inflammation in COPD patients.
Periodontitis is a chronic inflammatory disease caused by numerous periodontal pathogens. Pg, Tannerella forsythensis (Tf), and Treponema denticola (Td) constitute a bacterial complex named the “red complex”, which is strongly associated with advanced periodontal lesions.17 Pi is the most dominant periodontal pathogen commonly isolated from the periodontal abscess and increased in both COPD and periodontitis patients.18 Actinobacillus actinomycetemcomitans (Aa) is recognized as a major pathogenic factor of aggressive periodontitis.
However, there is limited research on periodontal pathogens and respiratory pathogens from dental plaque in COPD patients. We hypothesized that specific periodontal pathogens and respiratory pathogens may play a role in COPD pathogenesis. Therefore, we conducted a case–control study of periodontitis patients with and without COPD to investigate clinical periodontal variables and microbiologic levels of dental pathogens and respiratory pathogens from subgingival dental plaque.
Patients and Methods
Study Population
From October 2018 to January 2020, we conducted a case–control study in which a total of 60 COPD patients and 60 controls with normal pulmonary function were recruited from the Stomatology Department and the Respiratory Department of Beijing Chao-Yang Hospital. All the participants had periodontitis. 654 control participants were selected from the clinical database of periodontitis and 60 were frequently matched with COPD patients in age, sex, body mass index, and smoking status.
All COPD cases were clinically diagnosed and confirmed by lung function examination for more than three years. Criteria used for the diagnosis of COPD and classification of severity were based on the Global Initiative for Chronic Obstructive Lung Disease (GOLD) spirometry guidelines.19 Trained and certified technicians conducted lung function measurements using spirometry. Physician-diagnosed COPD from stage II to IV (moderate to very severe), post-bronchodilator forced expiratory volume in first second/forced vital capacity (FEV1/FVC) ratio <0.7 and FEV1 < 80% of predicted value. Other inclusion criteria for cases and controls included: (1) aged 35 years and up with ≥ 15 teeth; and (2) Periodontitis from stage II to IV, criteria for diagnosing periodontitis stage were based on the new classification.20
Exclusion criteria for cases and controls: (1) having fever, worsening of respiratory symptoms, or medication change within the last month; (2) primary diagnosis of asthma; (3) history of lung volume reduction surgery, pneumonectomy, or lung transplantation; (4) history of any periodontal treatment in the last 6 months; (5) had received systemic antibiotic therapy within the previous 6 months.
Our study complied with the Declaration of Helsinki Ethical Principles for Medical Research Involving Human Subjects. The ethics committee of the Beijing Chao-Yang Hospital approved the study protocol (Number: 2018-Science-276). Each patient provided written informed consent before enrollment.
Periodontal Examination
Two trained dentists who were blinded to the patients’ COPD status conducted periodontal examinations independently. Clinical examination included measurement of pocket depth (PD), location of the cementoenamel junction (CEJ) to calculate clinical attachment level (CAL), bleeding index (BI), assessing of plaque index (PLI) and alveolar bone loss. PD and location of the CEJ were measured with a Williams periodontal probe at six sites for each tooth and recorded in millimeters. Gingival recession was recorded as a positive value if the free gingival margin occurred apical to the CEJ. CAL equals the distance from the CEJ to the pocket base (PD+ gingival recession = CAL). BI on probing was scored on a scale of 0 to 5 when any bleeding was visible.21 PLI for each tooth was determined after air drying on a scale of 0 to 3.22 Alveolar bone loss was examined by using full-mouth X-ray films. The examiner reliability was assessed of all the periodontal measurements with 20 patients. The correlations coefficients of agreement of PD and CAL were 0.90 and 0.94, respectively. The Kappa values of agreement of BI and PLI were 0.85 and 0.87, respectively.
Sample Collection
We obtained subgingival dental plaque samples from four first molar teeth: 16,26,36,46 of each participant. If a first molar was missing, an adjacent tooth was scored. After removal of supra-gingival plaque, subgingival plaque samples were collected with sterile Gracey curettes from the mesial side of each tooth. The entire plaque sample of each participant was placed in an Eppendorf tube. The dental plaque samples were washed twice by 200 μL of phosphate-buffered saline (0.1M, pH 7.4), centrifuged at 13,000 rpm for 5 minutes. The precipitates were stored at −20°C until required for analysis.
DNA Extraction
The dental plaque precipitate was processed for DNA extraction following the factory protocol (TIANamp bacteria DNA kit DP302, TIANGEN Biotech, Beijing, China). Briefly, the dental plaque precipitate was mixed with 180 μL of lysis buffer (20 mM Tris-HCl buffer, pH 8.0; 2 mM Na2-EDTA; 1.2% Triton; supplemented with 20 mg/mL lysozyme) and incubated for 40 mins at 37°C, then mixed with 20 μL of proteinase K and 220 μL of GB buffer, vortexed for 15 sec and incubated for 10 mins at 70°C. After anhydrous ethanol precipitation, the mixture was loaded on the column and was washed, and finally DNA was eluted with 50 μL of Tris-EDTA buffer (pH 8.0). The purified DNA samples from patients and controls were saved at −20°C for later real-time polymerase chain reaction (PCR) as templates.
Real-Time Polymerase PCR
Real-time quantitative PCR detections for five periodontal pathogens [Porphyromonas gingivalis (Pg), Actinobacillus actinomycetemcomitans (Aa), Prevotella intermedia (Pi), Tannerella forsythensis (Tf), and Treponema denticola (Td)] and four respiratory pathogens [Haemophilus influenza (Hi), Streptococcus pneumonia (Sp), Pseudomonas aeruginosa (Pa), and Klebsiella pneumonia (Kp)] and total common bacteria were performed in an ABI 7500 real-time PCR detection system (Applied Biosystems, Singapore).
The reactions for Pg, Aa, Pi, Tf, Td, Pa, and total common bacteria were performed with specific primers and probes synthesized by Invitrogen (Shanghai) CO. LTD. The forward primer, reverse primer, and probes sequences applied to each sample are shown in Table 1. The reaction system (25 μL in total) was prepared with the 2×SuperReal PreMix (Probe) according to the manufacturer’s instruction (FP206, Tiangen, China) as follows: 1μL forward primer (10 μM), 1μL reverse primer (10 μM), 0.5μL probe (10 μM), 2μL DNA template, 12.5μL 2×SuperReal PreMix, and 8μL H2O. PCR was performed with an initial denaturation step at 50°C for 2 min and 95°C for 3 min, followed by 40 cycles of incubation at 95°C for 15 s, 55 or 60°C (based on the Tm of primers) for 35s.
Table 1.
Sequences of Primers and Probes Used for Real-Time Quantitative PCR of Bacterial Pathogen Nucleic Acid Detection
| Bacterium Name | Primer Sequence (5ʹ→3ʹ) | Annealing Temperature (°C) |
|---|---|---|
| Pg | F: TTCGCTGTGGAAGCTTGACG R: ACTGACACTGAAGCACGAAG P: FAM- GCGTGGGTATCAAACAGGAT-TAMRA |
60.0 |
| Aa | F: TTACGCACATGTCCGAGCAAG R: AGCCATGGTGTTGATTCAGCGT P: FAM- AATCGTCGCTTGTTGAGGCG-TAMRA |
60.0 |
| Pi | F: AGATATCATGACGAACTCCGATTGC R: CGAGCTTAAGCGTCAGTAACACT P: FAM -CCGTACGCTGCCTTC-TAMRA |
60.0 |
| Tf | F: TGGTCCGTGTCTCAGTACC R: TTGCGGATGGGCATGCGTA P: FAM- CCATTAGGTAGTTGGTGAGG-TAMRA |
60.0 |
| Td | F: CCGAATGTGCTCATTTACATAAAGGT R: GATACCCATCGTTGCCTTGGT P: FAM- ATCTCAGTCCCAATGTGTCC-TAMRA |
60.0 |
| Pa | F: TTGGCACCTTCACCGGAAG R: ATGGAAATGCTGAAATTCGG P: FAM- TTGGAGGAGCAACCCACAGC-TAMRA |
60.0 |
| Total | F: GGTAAGGTTCTTCGCGTTGC R: GATTAGATACCCTGGTAGTCCA P: FAM-AACCACATGCTCCACC-MGB |
55.0 |
Abbreviations: PCR, polymerase chain reaction; F, forward primer; R, reverse primer; P, probe; Pg, Porphyromonas gingivalis; Aa, Actinobacillus actinomycetemcomitans; Pi, Prevotella intermedia; Tf, Tannerella forsythensis; Td, Treponema denticola; Pa, Pseudomonas aeruginosa.
Sp, Hi, and Kp were detected by real-time PCR using commercial kits (JB20103, JB20104, JB20105; BioPerfectus technologies, Jiangsu, China).
Statistical Analysis
SPSS statistical package (SPSS 26.0) was used for data analyses. Chi-squared test was used to compare characteristics between cases and controls for categorical variables. The distributions of continuous variables were examined using the Kolmogorov–Smirnov normality test. An independent sample t-test was used to compare characteristics between cases and controls for normally distributed continuous variables. We transformed the bacterial numbers to their natural logarithm since they were exponential. Because the natural logarithms of bacterial species were abnormally distributed, we used Wilcoxon signed rank-sum test to compare them. Spearman’s rank correlation coefficients were calculated to assess the correlations of periodontal and respiratory pathogens. Principal component analysis (PCA) was applied to visualize similarities or differences in the bacterial diversity between COPD cases and controls. R (R Core Team, 2019, version 3.6.1) and Package gglpot2 (Springer-Verlag, 2016, version 3.2.0) were used for PCA statistical testing and graph generation. Logistic regression was performed to calculate the odds ratio (OR) and 95% confidence interval (CI) for evaluating the associations of pathogens and clinical periodontal variables with risk of COPD with adjustment for age, gender, body mass index, and smoking status.
Results
Demographic Characteristics
Table 2 shows the basic demographic characteristics of the study population. The mean age of the COPD group was 63.1±10.1 years; 81.7% were male; the mean age of the control group was 60.0±9.4 years; 63.3% were male. COPD patients and control participants were frequently matched in age, sex, body mass index, smoking status, smoking pack-years, smoking cessation time, residence place, marriage status, and living status (all P > 0.05).
Table 2.
Demographic Characteristics in COPD Case and Control Groups
| Characteristics | COPD Group (n=60) | Control Group (n=60) | P-value |
|---|---|---|---|
| Age (y) (Mean±SD) | 63.1±10.1 | 60.0±9.4 | 0.97 |
| BMI (kg/m2) (Mean±SD) | 25.7±4.2 | 24.9±3.3 | 0.57 |
| Gender [n (%)] | 0.07 | ||
| Male | 49 (81.7) | 38 (63.3) | |
| Female | 11 (18.3) | 22 (36.7) | |
| Smoking status [n (%)] | 0.88 | ||
| Non-smoker | 14 (23.3) | 17 (28.3) | |
| Former smoker | 30 (50.0) | 29 (48.3) | |
| Current smoker | 16 (26.7) | 14 (23.3) | |
| Smoking pack-years (Mean±SD) | 8300±4000 | 6200±3200 | 0.21 |
| Quit smoking time (y) (Mean±SD) | 9.6±5.3 | 10.7±5.9 | 0.38 |
| Place of residence [n (%)] | 0.54 | ||
| Urban | 53 (88.3) | 55 (91.6) | |
| Rural | 7 (11.7) | 5 (8.3) | |
| Marriage status [n (%)] | 0.73 | ||
| Single | 5 (8.3) | 4 (6.7) | |
| Married | 55 (91.6) | 56 (93.3) | |
| Living status [n (%)] | 0.70 | ||
| Living alone | 4 (6.7) | 3 (5.0) | |
| Living with family | 56 (93.3) | 57 (95.0) |
Notes: P value obtained from Student’s t-test for continuous variables and Chi-squared test for categorical variables.
Abbreviations: COPD, chronic obstructive pulmonary disease; BMI, body mass index.
Lung Function and Periodontal Health Status
Table 3 shows the lung function and clinical periodontal indexes of the study population. COPD patients had much worse lung function compared with the participants of the control group (FEV1% of predicted: 61.0±19.9 vs 94.6±7.6; FEV1/FVC: 0.53±0.11 vs 0.77±0.04, all P < 0.0001).
Table 3.
Lung Function and Periodontal Indexes in COPD Case and Control Groups
| Clinical Parameters | COPD Group (n=60) | Control Group (n=60) | P-value |
|---|---|---|---|
| Lung function (Mean±SD) | |||
| FEV1% of predicted | 61.0±19.9 | 94.6±7.6 | <0.0001* |
| FEV1/FVC | 0.53±0.11 | 0.77±0.04 | <0.0001* |
| Periodontal index (Mean±SD) | |||
| Number of remaining teeth | 23.8±5.2 | 26.0±3.6 | 0.03* |
| Probing depth (PD) (mm) | 3.29±0.55 | 3.20±0.57 | 0.51 |
| Clinical attachment level (CAL) (mm) | 5.15±1.04 | 4.93±0.77 | 0.35 |
| Bleeding index (BI) | 2.45±0.54 | 2.42±0.62 | 0.86 |
| Plaque index (PLI) | 2.39±0.64 | 1.98±0.51 | 0.02* |
| Alveolar bone loss | 1.73±0.56 | 1.68±0.52 | 0.72 |
| Severe site percentages | |||
| PD ≥ 5mm site percentages | 13.0±5.5 | 12.7±6.3 | 0.77 |
| CAL ≥ 5mm site percentages | 30.3±15.5 | 19.1±10.4 | 0.02* |
Notes: P value obtained from Student’s t-test. *P<0.05 statistically significant.
Abbreviations: COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in one second; FVC, forced vital capacity.
COPD patients had fewer remaining teeth (23.8±5.2 vs.26.0±3.6, P = 0.03), higher PLI (2.39±0.64 vs 1.98±0.51, P = 0.02), and more severe site percentages of CAL (CAL ≥ 5mm site: 30.3±15.5 vs 19.1±10.4, P = 0.02) than the controls with normal pulmonary function. There were no significant differences in other periodontal parameters (PD, CAL, BI, alveolar bone loss, and PD ≥ 5mm site percentages) between COPD patients and controls.
Periodontal Pathogen and Respiratory Pathogen from Subgingival Dental Plaque
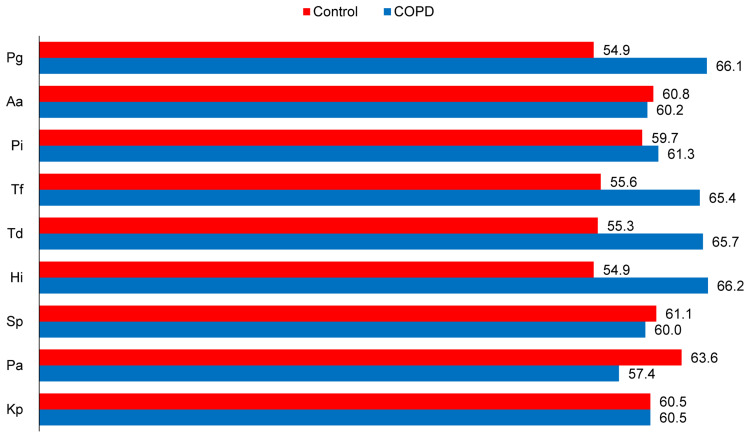
Figure 1 presents the ranked mean of log-transformed values (natural logarithms) of five periodontal pathogens and four respiratory pathogens from subgingival dental plaque in the COPD group and control group. There were no significant differences in the ranked means of the nine pathogens (Pg, Aa, Pi, Tf, Td, Hi, Sp, Pa, and Kp) between COPD patients and controls (all P > 0.05), although COPD patients tended to have higher ranked means of Pg, Tf, Td, and Hi than controls.
Figure 1.
Mean ranks of natural log-transformed values of each bacterial species between COPD case and control groups.
Note: Since the log-transformed values of each bacterial species were abnormally distributed, Wilcoxon signed rank sum test was used to compare the median difference between case and control groups (n=60 in each group, all P >0.05).
Abbreviations: COPD, chronic obstructive pulmonary disease; Pg, Porphyromonas gingivalis; Aa, Actinobacillus actinomycetemcomitans; Pi, Prevotella intermedia; Tf, Tannerella forsythensis; Td, Treponema denticola; Pa, Pseudomonas aeruginosa; Sp, Streptococcus pneumonia; Hi, Haemophilus influenza; Kp, Klebsiella pneumonia.
Table 4 presents the Spearman correlation coefficients between five periodontal pathogens and four respiratory pathogens in control participants. Pg was positively correlated with Hi; Pi was positively correlated with Tf, Td, and Hi, Tf was positively correlated with Hi and Kp; Hi was positively correlated with Pa; Sp was positively correlated with Pa. Their Spearman correlation coefficients were weak or moderate, ranging from 0.29 to 0.47, respectively (all P < 0.05).
Table 4.
Spearman Correlation Coefficients for Correlations Between Periodontal and Lung Pathogens Among Controls
| Periodontal Pathogens | Lung Pathogens | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pg | Aa | Pi | Tf | Td | Hi | Sp | Pa | Kp | |
| Pg | 0.11 | 0.13 | 0.23 | 0.14 | 0.29* | 0.03 | −0.13 | 0.03 | |
| Aa | −0.02 | 0.18 | 0.18 | 0.20 | 0.23 | 0.11 | −0.12 | ||
| Pi | 0.32* | 0.33** | 0.45** | 0.21 | 0.24 | 0.14 | |||
| Tf | 0.12 | 0.41** | 0.10 | 0.06 | 0.29* | ||||
| Td | 0.24 | 0.25 | 0.15 | 0.15 | |||||
| Hi | 0.13 | 0.32* | 0.22 | ||||||
| Sp | 0.47** | 0.13 | |||||||
| Pa | 0.13 | ||||||||
| Kp | |||||||||
Notes: The bold values indicate the Spearman correlation coefficients between pathogens were statistically significant; **P <0.01 statistically significant; *P <0.05 statistically significant.
Abbreviations: Pg, Porphyromonas gingivalis; Aa, Actinobacillus actinomycetemcomitans; Pi, Prevotella intermedia; Tf, Tannerella forsythensis; Td, Treponema denticola; Pa, Pseudomonas aeruginosa; Sp, Streptococcus pneumonia; Hi, Haemophilus influenza; Kp, Klebsiella pneumonia.
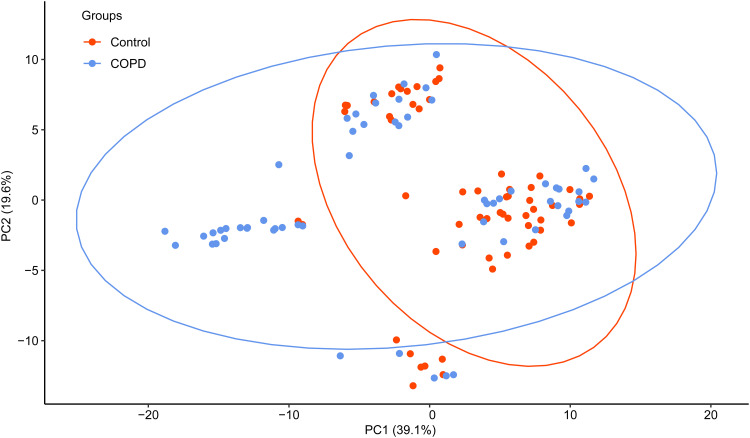
Figure 2 shows the principal component analysis (PCA) graph of bacterial diversity of five periodontal pathogens and four respiratory pathogens. Pathogens in the COPD group distributed more dispersedly than the control group, but the graph revealed no significant discrimination of these pathogens between COPD and control groups.
Figure 2.
Principal component analysis of bacterial diversity in dental plaque in COPD case and control subjects.
Note: COPD case and control subjects are colored in blue and red, respectively (n=60 in each group).
Abbreviation: COPD, chronic obstructive pulmonary disease.
Multivariate-Adjusted Analyses of Pathogen and Periodontal Variables with COPD
We used logistic regression analyses to identify the variables as potential predictors of COPD risk. Table 5 presents the adjusted ORs and 95% CIs of COPD in relation to Pg and periodontal variables. After adjusted for age, gender, body mass index, and smoking status in logistic regression models, PLI (P = 0.045) and CAL≥5mm site percentages (P = 0.01) were significantly associated with COPD, whereas Pg (P = 0.09) and other pathogens were not significantly associated with COPD (data not shown except Pg).
Table 5.
Multivariate-Adjusted ORs and 95% CIs of COPD Risk in Relation to Three Periodontal Variables
| Periodontal Variables | OR | 95% CI | P-value* |
|---|---|---|---|
| Pg | 0.91 | 0.82–1.02 | 0.09 |
| Plaque index (PLI) | 3.55 | 1.03–12.2 | 0.045* |
| CAL≥5mm site percentages | 1.05 | 1.01–1.10 | 0.01* |
Notes: The logistic regression analyses were adjusted for age, gender, body mass index and smoking status. *P <0.05 statistically significant.
Abbreviations: OR, odd ratio; CI, confidence interval; COPD, chronic obstructive pulmonary disease; Pg, Porphyromonas gingivalis; CAL, clinical attachment level.
Discussion
In this case–control study of 60 COPD patients and 60 controls, we found that COPD patients appeared to have poor periodontal status as reflected by fewer remaining teeth, higher plaque index and more severe site percentages of CAL than controls with normal pulmonary function. These findings are consistent with our former study23 and a five years follow-up cohort study.9 A recent descriptive study suggested that the prevalence of periodontal disease was higher among those with COPD compared to non-COPD controls.24 Another case–control study reported that oral health status was associated with self-reported respiratory health.25 These results confirm the association between periodontitis and COPD. Our data shows although COPD patients tended to have relatively higher ranked means of Pg, Tf, Td, and Hi than controls, the differences did not reach statistical significance. Surprisingly, all the five dental pathogens and four respiratory pathogens from subgingival dental plaque in the study showed no significant differences between the two groups. In contrast, other studies have suggested that COPD patients have more prevalence of Pg and an increased abundance of Pi.16,18 These inconsistent results might have been produced by study heterogeneity due to differences in study design, statistical handling, or patient populations. While the oral cavity is a reservoir of COPD pathogens and the method to take an oral sample is simple, the number of respiratory pathogens in dental plaque should be lower than in sputum and bronchial lavage fluid.
We found some positive associations between periodontal and respiratory pathogens, such as Pi were positively but moderately correlated with Td and Hi. There are over 500 bacteria species living in the microenvironment of the oral cavity. Interactions between periodontal and respiratory pathogens may lead to further inflammation. A famous bacterial complex named the “red complex” which composed of Pg, Tf, and Td has been strongly associated with severe periodontal lesions and causes alveolar bone resorption. Previous research also detected some lung pathogens from dental plaque samples in patients with AE-COPD.15 It’s natural to find a positive association between periodontal and respiratory pathogens.
The PCA graph of five periodontal pathogens and four respiratory pathogens showed their poor capabilities to discriminate correctly between COPD cases and controls. The distribution of pathogens in the COPD group was more dispersed than the control group. This may be due to the fact that our samples were obtained from subgingival plaque in which the periodontal pathogens showed higher aggregation than respiratory pathogens.
Although the amount of Pg and other periodontal pathogens failed to predict COPD risk, periodontal measures including PLI and CAL≥5mm site percentages were robustly and significantly associated with COPD. The data confirmed that periodontal disease is an independent risk factor of COPD. The findings are in agreement with our previous study, which reported a positive association between periodontal indexes and lower quality of life in COPD patients.26 A recent review also supported that periodontitis and COPD could be pathologically associated and reduction in dental plaque biofilms appears to reduce the risk for AE-COPD.27,28 Further studies should be conducted to reveal the shared pathophysiological mechanisms underlying the association of periodontal disease and COPD.
The present study is one of the few studies to specifically evaluate the microbiologic pathogens in COPD and periodontitis patients. Although no positive results were obtained, our study did a useful exploration to reveal the correlation between COPD and clinical periodontal variables. Nonetheless, several limitations merit consideration. First, this study had a relatively small number of patients in each group. Second, the COPD patients in our study were all in stable status, the dominant bacteria in COPD exacerbations (such as Sp, Kp, Pa) may decrease than exacerbated COPD. Third, the respiratory microbiologic pathogen samples from dental plaque may less representative than those samples from sputum or bronchial lavage on COPD. Finally, due to observational study nature, we cannot completely rule out the possibilities of false negative results and residual confounding.
Conclusion
The findings from our case–control study suggest that periodontal pathogens may not be potential predictors of COPD risk, although COPD patients appeared to have poor periodontal health status. Our results do not support the clinical usefulness of microbiological profiles from periodontal plaque in predicting COPD risk. With current rapid development in the field of microbiota, further studies are required to clarify the role of pathogens from dental plaque in the etiology and progression of COPD.
Acknowledgment
This study was supported by the National Natural Science Foundation of China (No. 81500854 and No. 81870763).
Abbreviations
COPD, chronic obstructive pulmonary disease; Pg, Porphyromonas gingivalis; Aa, Actinobacillus actinomycetemcomitans; Pi, Prevotella intermedia; Tf, Tannerella forsythensis; Td, Treponema denticola; Pa, Pseudomonas aeruginosa; Sp, Streptococcus pneumonia; Hi, Haemophilus influenza; Kp, Klebsiella pneumonia; AECOPD, acute exacerbation of chronic obstructive pulmonary disease; GOLD, global initiative for chronic obstructive lung disease; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; PD, probing depth; CEJ, cementoenamel junction; CAL, clinical attachment level; BI, bleeding index; PLI, plaque index; PCR, polymerase chain reaction; PCA, principal component analysis; OR, odds ratio; CI, confidence interval; BMI, body mass index.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Vogelmeier CF, Román-Rodríguez M, Singh D, Han MK, Rodríguez-Roisin R, Ferguson GT. Goals of COPD treatment: focus on symptoms and exacerbations. Respir Med. 2020;166:105938. doi: 10.1016/j.rmed.2020.105938 [DOI] [PubMed] [Google Scholar]
- 2.Wang C, Xu J, Yang L, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet. 2018;391(10131):1706–1717. doi: 10.1016/S0140-6736(18)30841-9 [DOI] [PubMed] [Google Scholar]
- 3.Kawamatawong T, Apiwattanaporn A, Siricharoonwong W. Serum inflammatory biomarkers and clinical outcomes of COPD exacerbation caused by different pathogens. Int J Chron Obstruct Pulmon Dis. 2017;12:1625–1630. doi: 10.2147/COPD.S132132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu D, Hou C, Li Y, et al. Analysis of the bacterial community in chronic obstructive pulmonary disease sputum samples by denaturing gradient gel electrophoresis and real-time PCR. BMC Pulm Med. 2014;14:179. doi: 10.1186/1471-2466-14-179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Eldere J, Slack MP, Ladhani S, Cripps AW. Non-typeable Haemophilus influenzae, an under-recognised pathogen. Lancet Infect Dis. 2014;14(12):1281–1292. doi: 10.1016/S1473-3099(14)70734-0 [DOI] [PubMed] [Google Scholar]
- 6.Scannapieco FA, Ho AW. Potential associations between chronic respiratory disease and periodontal disease: analysis of national health and nutrition examination survey III. J Periodontol. 2001;72(1):50–56. doi: 10.1902/jop.2001.72.1.50 [DOI] [PubMed] [Google Scholar]
- 7.Scannapieco FA, Papandonatos GD, Dunford RG. Associations between oral conditions and respiratory disease in a national sample survey population. Ann Periodontol. 1998;3(1):251–256. doi: 10.1902/annals.1998.3.1.251 [DOI] [PubMed] [Google Scholar]
- 8.Zeng XT, Tu ML, Liu DY, Zheng D, Zhang J, Leng W. Periodontal disease and risk of chronic obstructive pulmonary disease: a meta-analysis of observational studies. PLoS One. 2012;7(10):e46508. doi: 10.1371/journal.pone.0046508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takeuchi K, Matsumoto K, Furuta M, et al. Periodontitis is associated with chronic obstructive pulmonary disease. J Dent Res. 2019;98(5):534–540. doi: 10.1177/0022034519833630 [DOI] [PubMed] [Google Scholar]
- 10.Barros SP, Suruki R, Loewy ZG, Beck JD, Offenbacher S, de Torres JP. A cohort study of the impact of tooth loss and periodontal disease on respiratory events among COPD subjects: modulatory role of systemic biomarkers of inflammation. PLoS One. 2013;8(8):e68592. doi: 10.1371/journal.pone.0068592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Linden GJ, Herzberg MC; Working group 4 of joint EFP/AAP workshop. Periodontitis and systemic diseases: a record of discussions of working group 4 of the joint EFP/AAP workshop on periodontitis and systemic diseases. J Clin Periodontol. 2013;40(Suppl 14):S20–S23. doi: 10.1111/jcpe.12091 [DOI] [PubMed] [Google Scholar]
- 12.Didilescu AC, Skaug N, Marica C, Didilescu C. Respiratory pathogens in dental plaque of hospitalized patients with chronic lung diseases. Clin Oral Investig. 2005;9(3):141–147. doi: 10.1007/s00784-005-0315-6 [DOI] [PubMed] [Google Scholar]
- 13.El-Solh AA, Pietrantoni C, Bhat A, et al. Colonization of dental plaques: a reservoir of respiratory pathogens for hospital-acquired pneumonia in institutionalized elders. Chest. 2004;126(5):1575–1582. doi: 10.1378/chest.126.5.1575 [DOI] [PubMed] [Google Scholar]
- 14.Brook I, Frazier EH. Immune response to Fusobacterium nucleatum and Prevotella intermedia in the sputum of patients with acute exacerbation of chronic bronchitis. Chest. 2003;124(3):832–833. doi: 10.1378/chest.124.3.832 [DOI] [PubMed] [Google Scholar]
- 15.Tan L, Wang H, Li C, Pan Y. 16S rDNA-based metagenomic analysis of dental plaque and lung bacteria in patients with severe acute exacerbations of chronic obstructive pulmonary disease. J Periodontal Res. 2014;49(6):760–769. doi: 10.1111/jre.12159 [DOI] [PubMed] [Google Scholar]
- 16.Tan L, Tang X, Pan C, Wang H, Pan Y. Relationship among clinical periodontal, microbiologic parameters and lung function in participants with chronic obstructive pulmonary disease. J Periodontol. 2019;90(2):134–140. doi: 10.1002/JPER.17-0705 [DOI] [PubMed] [Google Scholar]
- 17.Mysak J, Podzimek S, Sommerova P, et al. Porphyromonas gingivalis: major periodontopathic pathogen overview. J Immunol Res. 2014;2014:476068. doi: 10.1155/2014/476068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu X, Chen J, Xu M, et al. 16S rDNA analysis of periodontal plaque in chronic obstructive pulmonary disease and periodontitis patients. J Oral Microbiol. 2017;9(1):1324725. doi: 10.1080/20002297.2017.1324725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176(6):532–555. doi: 10.1164/rccm.200703-456SO [DOI] [PubMed] [Google Scholar]
- 20.Tonetti MS, Greenwell H, Kornman KS. Staging and grading of periodontitis: framework and proposal of a new classification and case definition. J Clin Periodontol. 2018;45(Suppl 20):S149–S161. doi: 10.1111/jcpe.12945 [DOI] [PubMed] [Google Scholar]
- 21.Mazza JE, Newman MG, Sims TN. Clinical and antimicrobial effect of stannous fluoride on periodontitis. J Clin Periodontol. 1981;8(3):203–212. doi: 10.1111/j.1600-051x.1981.tb02031.x [DOI] [PubMed] [Google Scholar]
- 22.Silness J, Löe H. Periodontal disease in pregnancy II. Correlation between oral hygiene and periodontal condition. Acta Odontol Scand. 1964;22:121–135. doi: 10.3109/00016356408993968 [DOI] [PubMed] [Google Scholar]
- 23.Wang Z, Zhou X, Zhang J, et al. Periodontal health, oral health behaviours, and chronic obstructive pulmonary disease. J Clin Periodontol. 2009;36(9):750–755. doi: 10.1111/j.1600-051X.2009.01448.x [DOI] [PubMed] [Google Scholar]
- 24.Lopez-de-Andrés A, Vazquez-Vazquez L, Martinez-Huedo MA, et al. Is COPD associated with periodontal disease? A population-based study in Spain. Int J Chron Obstruct Pulmon Dis. 2018;13:3435–3445. doi: 10.2147/COPD.S174898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baldomero AK, Siddiqui M, Lo CY, et al. The relationship between oral health and COPD exacerbations. Int J Chron Obstruct Pulmon Dis. 2019;14:881–892. doi: 10.2147/COPD.S194991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou X, Wang Z, Song Y, Zhang J, Wang C. Periodontal health and quality of life in patients with chronic obstructive pulmonary disease. Respir Med. 2011;105(1):67–73. doi: 10.1016/j.rmed.2010.06.017 [DOI] [PubMed] [Google Scholar]
- 27.Hobbins S, Chapple IL, Sapey E, Stockley RA. Is periodontitis a comorbidity of COPD or can associations be explained by shared risk factors/behaviors? Int J Chron Obstruct Pulmon Dis. 2017;12:1339–1349. doi: 10.2147/COPD.S127802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sabharwal A, Gomes-Filho IS, Stellrecht E, Scannapieco FA. Role of periodontal therapy in management of common complex systemic diseases and conditions: an update. Periodontol 2000. 2018;78(1):212‐226. doi: 10.1111/prd.12226 [DOI] [PubMed] [Google Scholar]