Supplemental Digital Content is available in the text
Keywords: bone, case-control studies, cohort studies, dementia, fracture, risk factors
Abstract
This study aimed to evaluate the risk of dementia after distal radius, hip, and spine fractures.
Data from the Korean National Health Insurance Service-National Sample Cohort were collected for the population ≥ 60 years of age from 2002 to 2013. A total of 10,387 individuals with dementia were matched for age, sex, income, region of residence, and history of hypertension, diabetes, and dyslipidemia with 41,548 individuals comprising the control group. Previous histories of distal radius, hip, and spine fractures were evaluated in both the dementia and control groups. Using ICD-10 codes, dementia (G30 and F00) and distal radius (S525), hip (S720, S721, and S722), and spine (S220 and S320) fractures were investigated. The crude and adjusted odds ratios (ORs) and 95% confidence intervals (CIs) of dementia in distal radius, hip, and spine fracture patients were analyzed using conditional logistic regression analyses. Subgroup analyses were conducted according to age, sex and region of residence.
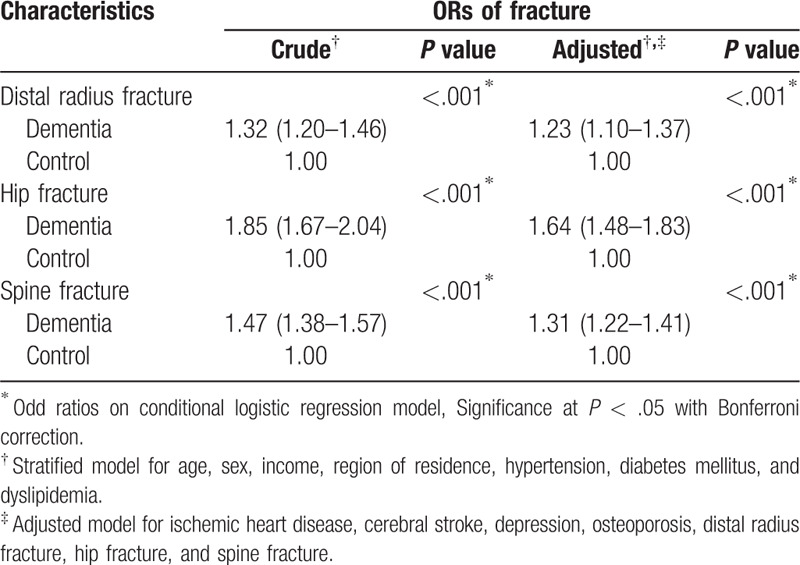
The adjusted ORs for dementia were higher in the distal radius, hip, and spine fracture group than in the non-fracture group (adjusted OR = 1.23, 95% CI = 1.10 –1.37, P < .001 for distal radius fracture; adjusted OR = 1.64, 95% CI = 1.48 – 1.83, P < .001 for hip fracture; adjusted OR = 1.31, 95% CI = 1.22 – 1.41, P < .001 for spine fracture). The results in subgroup analyses according to age, sex and region of residence were consistent.
Distal radius, hip, and spine fractures increase the risk of dementia.
1. Introduction
Osteoporotic or fragility fractures are defined as any fractures that result from low trauma, such as falls.[1] Fragility fractures commonly involve the distal radius, hip, and spine.[2] These fractures are prevalent in the elderly and account for considerable socioeconomic burdens. The prevalence of fragility fractures is 20.5% in women ≥65 years old in the United States (US),[3] and the annual incidence of distal radius fracture for men aged ≥65 is approximately 160 per 100,000 persons in the US.[4] In Korea, the annual incidence of distal radius fractures is 246 to 278 per 100,000 persons[5] and of hip fractures is approximately 1500 in women and 5000 in men.[6] The incidence of spine fracture in Korea is estimated to be 526 to 544 per 100,000 persons in men and 1.58 to 1.60 per 100,000 persons in women; these values are higher than the worldwide incidence, which ranged from 48 to 1083 per 100,000 persons.[7] Moreover, there has been an increase in fragility fractures in recent years because of the aging population, urban lifestyle, and osteoporosis.[8,9] In addition to osteoporosis, several risk factors for such fractures have been reported, including balance function, physical activity, and general health status.[10]
Dementia has been suggested to be related to fragility fractures. Indeed, several previous studies have demonstrated an increased risk of hip fractures in dementia patients.[11–14] However, the association between dementia and other types of fractures, such as distal radius and spine fractures, has rarely been considered in previous studies. Furthermore, only a few studies have evaluated the risk of dementia after fractures.[15] In one population-based study, an increased risk of dementia in patients with previous histories of fracture was reported,[15] with a hazard ratio of 1.38 for dementia in a fracture group compared with a nonfracture group (95% CI = 1.32–1.45). However, that study included a population ≥ 20 years old and did not differentiate vascular dementia or specify the types of fractures. Additionally, possible confounders of age, sex, region of residence, and past medical histories were not matched between the fracture and nonfracture groups in the previous study.
The running hypothesis of the present study was that fracture may increase the risk of neurodegenerative dementia in the elderly population. Because the incidence of dementia is rare and often related to vascular causes in young adults, the present study excluded this population and included those ≥ 60 years old. The dementia and control groups were matched and adjusted for demographic factors and past medical histories. Moreover, the types of fractures were specified and analyzed for their relationship to dementia.
2. Patients and methods
2.1. Study population and data collection
The ethics committee of Hallym University (2017-I102) approved the use of these data. The requirement for written informed consent was waived by the institutional review board. All methods were performed in accordance with the guidelines and regulations of the ethics committee of Hallym University.
This national cohort study relied on data from the Korean National Health Insurance Service-National Sample Cohort (NHIS-NSC). To prevent nonsampling errors, the NHIS selects samples directly from the entire population database. The details of the methods used to perform these procedures are provided by the National Health Insurance Sharing Service [16].
2.2. Participants selection
Of 1,025,340 participants with 114,369,638 medical claim codes, we included participants who were diagnosed with dementia (n = 13,102). Dementia was defined as a diagnosis of Alzheimer's disease (G30) or dementia in Alzheimer's disease (F00). For the accuracy of the diagnosis, we only selected participants who were treated ≥2 times. The description of the diagnosis of dementia is provided in the supplementary material (see Supplementary File S1).
The history of fracture was evaluated using ICD-10 codes. Distal radius fracture was defined as a fracture of the lower end of the radius (S525). Hip fracture was defined as a fracture of the head and neck of the femur (S720), a pertrochanteric fracture (S721), or a subtrochanteric fracture of the femur (S722). Spine fracture was defined as a fracture of a thoracic vertebra (S220) or a fracture of a lumbar vertebra (S320).
The dementia participants were matched 1:4 with participants (control group) who were never diagnosed with dementia from 2002 through 2013 among this cohort. The control group was selected from the overall population (n = 1,012,238). The matching was processed for age, group, sex, income group, region of residence, and history of hypertension, diabetes mellitus, and dyslipidemia. To prevent selection bias when choosing the matched participants, the control group participants were sorted using a random number order, and they were then selected from top to bottom. It was assumed that the matched control participants were evaluated at the same time as each matched dementia participant (index date = date of dementia diagnosis). Therefore, patients in the control group who died before the index date of the matched dementia participant were excluded. Dementia participants for whom we could not identify enough matched participants were excluded (n = 1,118). Furthermore, we excluded participants under 60 years old (n = 1,597). Finally, the 1:4 matching resulted in the inclusion of 10,387 dementia participants and 41,548 control participants (Fig. 1). However, the subjects were not matched for ischemic heart disease, cerebral stroke, depression, or osteoporosis because strict matching based on these characteristics increased the drop-out rate of subjects due to a lack of control participants.
Figure 1.
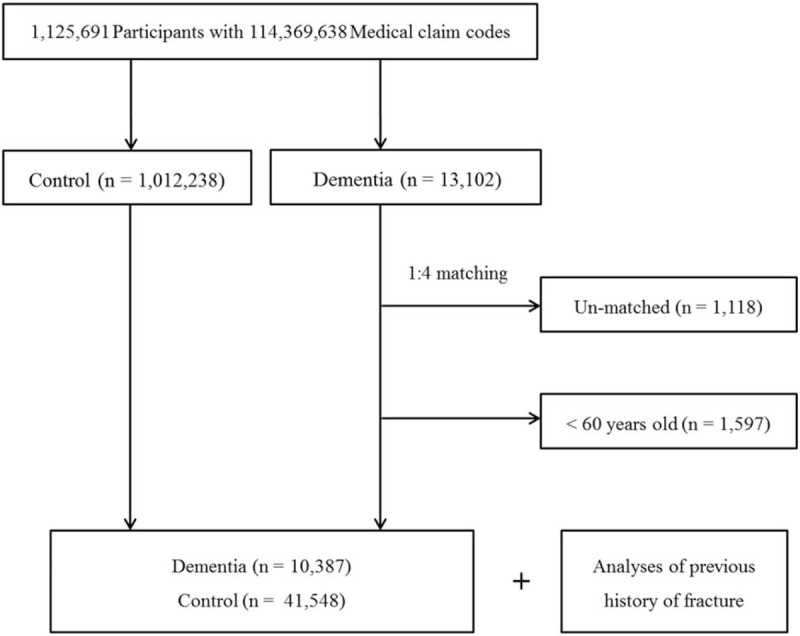
A schematic illustration of the participant selection process that was used in the present study. Of a total of 1,025,340 participants, 10,387 dementia participants were matched with 41,548 control participants for age, group, sex, income group, region of residence, and history of hypertension, diabetes mellitus, and dyslipidemia.
2.3. Variables
The age groups were classified using 5-year intervals: 60–64, 65–69, 70–74, 75–79, 80–84, and 85+ years old. A total of 6 age groups were designated. The income groups were initially divided into 41 classes (one health aid class, 20 self-employment health insurance classes, and 20 employment health insurance classes). These groups were recategorized into 11 classes (class 1 [lowest income]−11 [highest income]). Regions of residence were divided into 16 areas according to administrative districts. These regions were regrouped into urban (Seoul, Busan, Daegu, Incheon, Gwangju, Daejeon, and Ulsan) and rural (Gyeonggi, Gangwon, Chungcheongbuk, Chungcheongnam, Jeollabuk, Jeollanam, Gyeongsangbuk, Gyeongsangnam, and Jeju) areas.
The past medical histories of the participants were evaluated using ICD-10 codes. For the accuracy of diagnosis, hypertension (I10 and I15), diabetes (E10-E14), and dyslipidemia (E78) were reported if the participants were treated ≥ 2 times. Ischemic heart disease (I24 and I25) and stroke (I60-I66) were reported if the participants were treated ≥1 time. Depression was defined as the use of ICD-10 codes F31 (bipolar affective disorder) through F39 (unspecified mood disorder) by a psychiatrist ≥2 times. Osteoporosis was defined using ICD-10 codes M80 (osteoporosis with pathological fracture), M81 (osteoporosis without pathological fracture), and M82 (osteoporosis in diseases classified elsewhere) in participants who were treated ≥2 times or in those who were diagnosed based on bone density testing using X-ray or computed tomography (CT) (Claim codes: E7001-E7004, HC341-HC345).
2.4. Statistical analyses
The chi-square test was employed to compare differences in general characteristics between the dementia and control groups.
To analyze the odds ratio (OR) of dementia for each fracture type, a conditional logistic regression model was applied. In this analysis, crude (simple) and adjusted models were used (ischemic heart disease, cerebral stroke, depression, osteoporosis, distal radius fracture, hip fracture, and spine fracture), and 95% confidence intervals (CI) were calculated. In these analyses, age, sex, income, region of residence, dementia, hypertension, diabetes mellitus, and dyslipidemia were stratified.
For subgroup analysis, we divided the participants by age (<70 years old and ≥70 years old), sex (male and female), and region of residence (urban and rural).
Two-tailed analyses were conducted, and P values less than .05 were considered to indicate significance. Bonferroni correction was used for multiple comparisons. The results were analyzed using SPSS v. 21.0 (IBM, Armonk, NY).
3. Results
The rates of distal radius, hip, and spine fractures were higher in the dementia group than in the control group (all P < .001, Table 1). In the dementia group and the control group, the incidence of distal radius fracture was 5.1% (531/10,387) and 3.9% (1,630/41,548), respectively; the incidence of hip fracture was 5.7% (591/10,387) and 3.2% (1,327/41,548), respectively; and the incidence of spine fracture was 14.0% (1,452/10,387) and 10.0% (4168/41,548), respectively. Age, sex, income levels, region of residence, and past medical history of hypertension, diabetes mellitus, and dyslipidemia were matched between the dementia and control groups. Overall, past medical histories of ischemic heart disease, cerebral stroke, depression, and osteoporosis were higher in the dementia group than in the control group (all P < .001).
Table 1.
General characteristics of participants.
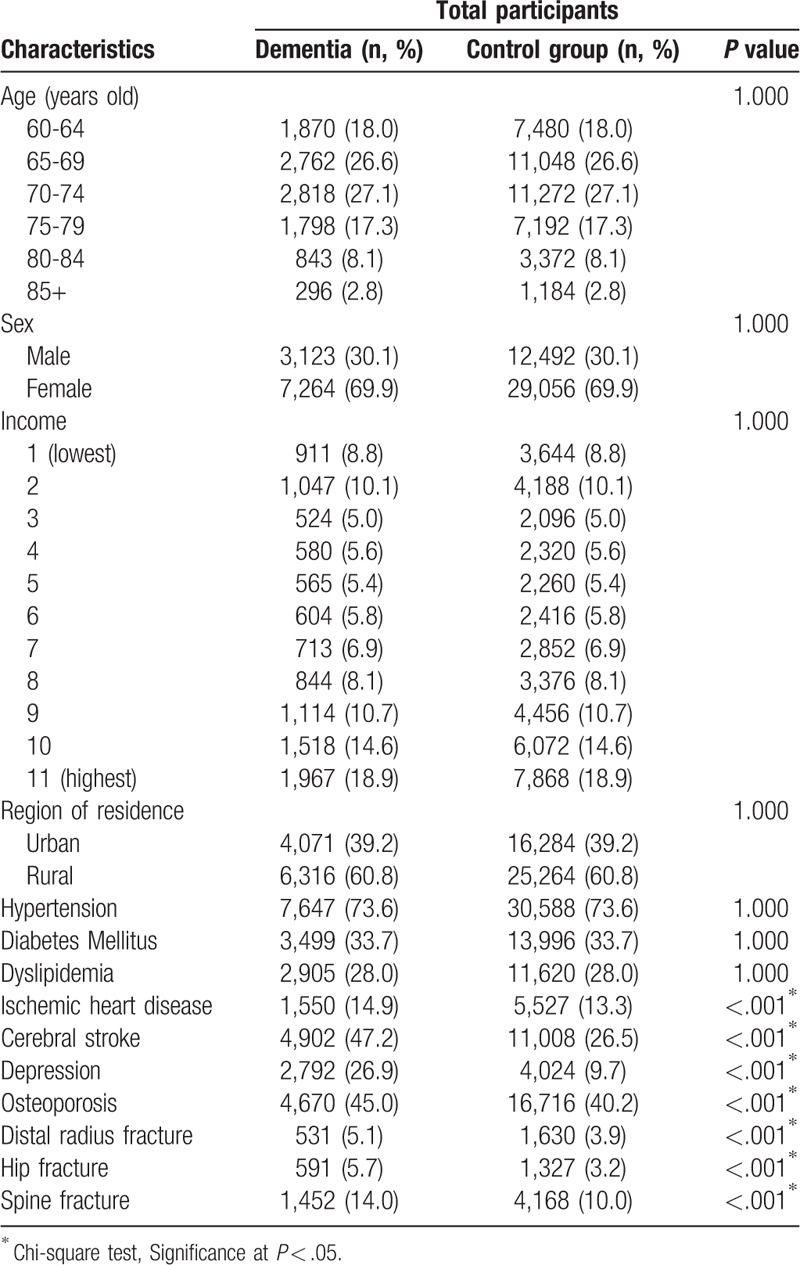
The ORs of dementia were higher in the distal radius, hip, and spine fracture group than in the non-fracture group (adjusted OR = 1.23, 95% CI = 1.10–1.37, P < .001 for distal radius fracture; adjusted OR = 1.64, 95% CI = 1.48–1.83, P < .001 for hip fracture; adjusted OR = 1.31, 95% CI = 1.22–1.41, P < .001 for spine fracture) (Table 2).
Table 2.
Crude and adjusted odd ratio (95% confidence interval) of distal radius/hip/spine fracture for dementia.
In the subgroup analysis according to age, the participants ≥70 years old in the dementia group showed a high adjusted OR for dementia in the distal radius fracture group (Table 3). The adjusted ORs for dementia were high in both the subgroups of participants < 70 years old and ≥ 70 years old in the hip and spine fracture groups. According to sex, the female subgroup in the distal radius fracture group demonstrated a high adjusted OR for dementia (Table 4). The adjusted ORs for dementia were high in both the male and female subgroups in the hip and spine fracture group. According to region of residence, both urban and rural subgroups in the distal radius fracture, hip fracture, and spine fracture groups exhibited a high adjusted OR for dementia (Supplemental table S1).
Table 3.
Crude and adjusted odd ratio (95% confidence interval) of distal radius/hip/spine fracture for dementia in subgroup analysis according to age.
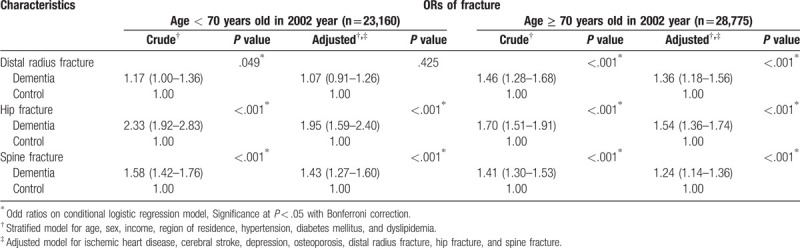
Table 4.
Crude and adjusted odd ratio (95% confidence interval) of distal radius/hip/spine fracture for dementia in subgroup analysis according to sex.
4. Discussion
In the present study, the adjusted ORs for dementia were higher in the distal radius, hip, and spine fracture group than in the control group, with consistent results in subgroup analyses according to age and sex. Although several previous studies have reported an increased risk of fractures in dementia patients, only a few retrospective studies have evaluated the history of fracture as a risk factor for dementia.[15] Several plausible factors, including predisposition to balance problems, inflammatory responses accompanying fractures, and treatment-related complications, may influence the high risk of dementia in fracture patients.
Impaired balance function in fracture patients might mediate the risk of dementia. Previous studies have reported an association between vestibular asymmetry and distal radius and hip fractures.[17–19] In addition to vestibular asymmetry, other sensory impairments related to balance function, including proprioceptive controls, were found to be decreased in patients with distal radius fracture compared with patients in a physical activity-matched control group.[20] In addition, decreased balance function has been suggested to be related to dementia.[21–23] Static posturography results are associated with mild cognitive impairment, as measured using a mini-mental state examination test, in men ≥60 years old.[21] Additionally, patients with mild cognitive impairment to mild-to-moderate Alzheimer's disease display decreased static postural control abilities compared with cognitively healthy participants,[22] and a meta-analysis demonstrated that quantitative gait parameters, including velocity, stride length, and stride time, are decreased in patients with mild cognitive impairment.[23] Therefore, impaired balance function in patients with mild cognitive impairment might manifest as fragility fractures before the onset of dementia.
Moreover, the inflammatory response and reactive oxidative stress during the fracture-healing process may increase the risk of dementia. Several inflammatory factors, including TNF-α and interleukin-6 (IL-6), are increased following fractures,[24] and a recent meta-analysis demonstrated that inflammatory markers, including C-reactive protein and IL-6, are associated with dementia.[25] In an animal study, erythrocyte levels of reactive oxygen species (ROS), such as malondialdehyde, were found to be elevated during the fracture-healing process.[26] These excessive ROS may cause oxidative brain injury and increase the risk of dementia.[27] Although no previous study has described the direct impact of ROS during the fracture-healing process on neuroinflammation and dementia, two prospective cohort studies reported the simultaneous elevation of inflammatory markers in both the cerebrospinal fluid and peripheral blood following fracture[28,29].
Complications following fractures or fracture surgery, including decreased physical activity and postoperative delirium, might increase the risk of dementia. A cohort study of a population ≥65 years old observed a functional decline in mobility and activities of daily living in participants after fracture during an 8-year follow-up period.[30] A retrospective study reported that approximately 31.8% (43/135) of hip fracture patients with postoperative delirium were diagnosed with dementia;[31] in that study, postoperative delirium increased by 15.6 times the risk of new-onset dementia within 3 years after surgery (95% CI = 2.6–91.6).[31]
Furthermore, several previous studies have demonstrated an increased risk of fractures in dementia patients.[11–14] The risk of hip fracture was 2.57 times higher in Alzheimer's disease patients than in a control group (95% CI = 2.32–2.84).[11] Another study described a 1.92 times higher risk of hip fracture in dementia patients, including those with Alzheimer's disease and unspecified dementia (95% CI = 1.48–2.49).[12] Moreover, the risk of hip fracture is high in patients with dementia and chronic diseases, such as those with chronic kidney disease on dialysis.[13]
The present study is based on nationwide representative data, the validity of which was verified in a previous study.[32] Because NHIS data include all Korean citizens, without exception, there were no missing participants. The control group was randomly selected and matched for age, sex, income, region of residence, and medical history of hypertension, diabetes, and dyslipidemia. Income and region of residence were included because they are crucial factors determining the availability of medical care. According to region of residence, both urban and rural subgroups demonstrated increased ORs for dementia in fracture groups. A previous study reported a high prevalence of osteoporotic fractures in postmenopausal women due to low socioeconomic status and poor nutritional status, with low bone marrow density and vitamin D, in a rural population[33]. Nonetheless, sedentary behavior, which might be higher in the urban population, might increase the risk of osteoporosis and fracture in this group[34].
Several limitations of our study should be noted. The dementia group in this study was selected based on ICD-10 codes and a history of ≥ 2 instances of treatment for the condition (see Supplemental File S2). The prevalence of dementia assumed in this study was comparable to that reported by the central dementia center of Korea (see Supplemental File S2). However, there might have been potential misdiagnoses of dementia due to the lack of information regarding actual clinical records, and the data from the NHIS-NSC do not include the severity of dementia or each fracture. In addition, although our analysis was adjusted for several potentially confounding factors, others were not considered, such as body mass index, smoking status, and alcohol consumption.
In conclusion, a history of a distal radius, hip, or spine fracture is associated with an increased risk of dementia in patients ≥60 years of age.
Author contributions
Conceptualization: Hyo Geun Choi.
Formal analysis: Hyo Geun Choi.
Writing – original draft: So Young Kim, Hyo Geun Choi.
Writing – review & editing: So Young Kim, Joon Kyu Lee, Jae-Sung Lim, Bumjung Park, Hyo Geun Choi.
Hyo Geun Choi orcid: 0000-0003-1655-9549.
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: CIs = confidence intervals, HIRA = health insurance review & assessment, NHIS-NSC = Korean National Health Insurance Service-National Sample Cohort, ORs = odds ratios.
How to cite this article: Kim SY, Lee JK, Lim JS, Park B, Choi HG. Increased risk of dementia after distal radius, hip, and spine fractures. Medicine. 2020;99:10(e19048).
The authors have no conflicts of interest to declare. This work was supported in part by research grants (NRF-2018-R1D1A1A02085328 and NRF-2017R1C1B1007696) from the National Research Foundation (NRF) of Korea.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Cummings SR, Melton LJ. Epidemiology and outcomes of osteoporotic fractures. Lancet 2002;359:1761–7. [DOI] [PubMed] [Google Scholar]
- [2].Center JR, Bliuc D, Nguyen TV, et al. Risk of subsequent fracture after low-trauma fracture in men and women. JAMA 2007;297:387–94. [DOI] [PubMed] [Google Scholar]
- [3].Rodrigues AM, Eusebio M, Santos MJ, et al. The burden and undertreatment of fragility fractures among senior women. Arch Osteoporos 2018;13:22. [DOI] [PubMed] [Google Scholar]
- [4].Wright NC, Hooker ER, Nielson CM, et al. The epidemiology of wrist fractures in older men: the Osteoporotic Fractures in Men (MrOS) study. Osteoporos Int 2018;29:859–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Jo YH, Lee BG, Kim HS, et al. Incidence and seasonal variation of distal radius fractures in Korea: a population-based study. J Korean Med Sci V 33 2018;e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lee YK, Kim JW, Lee MH, et al. Trend in the age-adjusted incidence of hip fractures in south korea: systematic review. Clin Orthop Surg 2017;9:420–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ballane G, Cauley JA, Luckey MM, et al. Worldwide prevalence and incidence of osteoporotic vertebral fractures. Osteoporos Int 2017;28:1531–42. [DOI] [PubMed] [Google Scholar]
- [8].Koo OT, Tan DM, Chong AK. Distal radius fractures: an epidemiological review. Orthop Surg 2013;5:209–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Porrino JA, Jr, Maloney E, Scherer K, et al. Fracture of the distal radius: epidemiology and premanagement radiographic characterization. AJR Am J Roentgenol 2014;203:551–9. [DOI] [PubMed] [Google Scholar]
- [10].Dewan N, MacDermid JC, Grewal R, et al. Risk factors predicting subsequent falls and osteoporotic fractures at 4 years after distal radius fracture-a prospective cohort study. Arch Osteoporos 2018;13:32. [DOI] [PubMed] [Google Scholar]
- [11].Tolppanen AM, Lavikainen P, Soininen H, et al. Incident hip fractures among community dwelling persons with Alzheimer's disease in a Finnish nationwide register-based cohort. PloS One 2013;8:e59124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wang HK, Hung CM, Lin SH, et al. Increased risk of hip fractures in patients with dementia: a nationwide population-based study. BMC Neurol 2014;14:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Maravic M, Ostertag A, Urena P, et al. Dementia is a major risk factor for hip fractures in patients with chronic kidney disease. Osteoporos Int 2016;27:1665–9. [DOI] [PubMed] [Google Scholar]
- [14].Huang SW, Lin JW, Liou TH, et al. Cohort study evaluating the risk of hip fracture among patients with dementia in Taiwan. Int J Geriatr Psychiatry 2015;30:695–701. [DOI] [PubMed] [Google Scholar]
- [15].Tsai CH, Chuang CS, Hung CH, et al. Fracture as an independent risk factor of dementia: a nationwide population-based cohort study. Medicine 2014;93:e188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].http://nhiss.nhis.or.kr/. [Google Scholar]
- [17].Kristinsdottir EK, Nordell E, Jarnlo GB, et al. Observation of vestibular asymmetry in a majority of patients over 50 years with fall-related wrist fractures. Acta Otolaryngol V 121 2001;481–5. [PubMed] [Google Scholar]
- [18].Ekvall Hansson E, Dahlberg LE, Magnusson M. Vestibular rehabilitation affects vestibular asymmetry among patients with fall-related wrist fractures - a randomized controlled trial. Gerontology 2015;61:310–8. [DOI] [PubMed] [Google Scholar]
- [19].Kristinsdottir EK, Jarnlo GB, Magnusson M. Asymmetric vestibular function in the elderly might be a significant contributor to hip fractures. Scand J Rehabil Med 2000;32:56–60. [DOI] [PubMed] [Google Scholar]
- [20].Baldursdottir B, Petersen H, Jonsson PV, et al. Sensory impairments and wrist fractures: A case-control study. J Rehabil Med 2018;50:209–15. [DOI] [PubMed] [Google Scholar]
- [21].Goto S, Sasaki A, Takahashi I, et al. Relationship between cognitive function and balance in a community-dwelling population in Japan. Acta Otolaryngol 2017;1–4. [DOI] [PubMed] [Google Scholar]
- [22].Deschamps T, Beauchet O, Annweiler C, et al. Postural control and cognitive decline in older adults: position versus velocity implicit motor strategy. Gait Posture 2014;39:628–30. [DOI] [PubMed] [Google Scholar]
- [23].Bahureksa L, Najafi B, Saleh A, et al. The impact of mild cognitive impairment on gait and balance: a systematic review and meta-analysis of studies using instrumented assessment. Gerontology 2017;63:67–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Yu MD, Su BH, Zhang XX. Morphologic and molecular alteration during tibia fracture healing in rat. Eur Rev Med Pharmacol Sci V 22 2018;1233–40. [DOI] [PubMed] [Google Scholar]
- [25].Darweesh SKL, Wolters FJ, Ikram MA, et al. Inflammatory markers and the risk of dementia and Alzheimer's disease: A meta-analysis. Alzheimers Dement 2018;14:1233–40. [DOI] [PubMed] [Google Scholar]
- [26].Yeler H, Tahtabas F, Candan F. Investigation of oxidative stress during fracture healing in the rats. Cell Biochem Funct V 23 2005;137–9. [DOI] [PubMed] [Google Scholar]
- [27].Mecocci P, Boccardi V, Cecchetti R, et al. A long journey into aging, brain aging, and Alzheimer's disease following the oxidative stress tracks. J Alzheimers Dis 2018;62:1319–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hall RJ, Watne LO, Idland AV, et al. Cerebrospinal fluid levels of neopterin are elevated in delirium after hip fracture. J Neuroinflammation 2016;13:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Neerland BE, Hall RJ, Seljeflot I, et al. Associations between delirium and preoperative cerebrospinal fluid C-Reactive protein, Interleukin-6, and Interleukin-6 receptor in individuals with acute hip fracture. J Am Geriatr Soc 2016;64:1456–63. [DOI] [PubMed] [Google Scholar]
- [30].Piirtola M, Lopponen M, Vahlberg T, et al. Fractures as an independent predictor of functional decline in older people: a population-based study with an 8-year follow-up. Gerontology 2012;58:296–304. [DOI] [PubMed] [Google Scholar]
- [31].Olofsson B, Persson M, Bellelli G, et al. Development of dementia in patients with femoral neck fracture who experience postoperative delirium-A three-year follow-up study. Int J Geriatric Psychiatry 2018;33:623–32. [DOI] [PubMed] [Google Scholar]
- [32].Lee J, Lee JS, Park SH, et al. Cohort Profile: The National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int J Epidemiol 2017;46:e15. [DOI] [PubMed] [Google Scholar]
- [33].Gomez-de-Tejada Romero MJ, Navarro Rodriguez MD, Saavedra Santana P, et al. Prevalence of osteoporosis, vertebral fractures and hypovitaminosis D in postmenopausal women living in a rural environment. Maturitas 2014;77:282–6. [DOI] [PubMed] [Google Scholar]
- [34].Braun SI, Kim Y, Jetton AE, et al. Sedentary behavior, physical activity, and bone health in postmenopausal women. J Aging Phys Act 2017;25:173–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


