Supplemental Digital Content is available in the text
Keywords: cancer treatment, dental focal infection, perioperative oral management, risk factor
Abstract
Patients develop a number of oral complications during cancer treatments. Oral bacteria are associated with the onset of dental focal infections and the progression of oral mucositis. Dental focal infections are frequently associated with the systemic onset of bacteremia, sepsis, and pneumonia. The degeneration of oral function with these complications may become an obstacle to cancer treatments. Although comprehensive oral management, including oral care, the removal of dental focal infections, and improvements in oral function with dentures, is conducted for cancer patients in Japan, few studies have assessed its efficacy.
The aim of the present study was to investigate the incidence of dental/oral complications in cancer patients with perioperative oral managements (POMs) based on a large number of case series with a multicenter retrospective analysis.
The medical records of cancer patients with POMs were retrospectively reviewed and the incidence of oral complications and efficacy of oral management were investigated.
A total of 2744 cancer patients with POMs (1684 males and 1080 females, mean age 65.9 ± 13.0 years) were included and investigated in the present study. Among these patients, 2097 (76.4%) started POM before the initiation of cancer treatments, with 2130 (77.6%) receiving oral care only and 391 (14.2%) being subjected to invasive treatments, such as tooth extraction. The incidence of dental focal infections during the period of cancer treatments was 8.2%. The most frequent infection was acute periodontitis, including alveolar abscesses (112 patients, 4.1%). The incidence of grade 2 and 3 oral mucositis was 2.8%. Prolonged fever was observed in 113 patients (4.1%), with 7 having dental focal infections (6.2%). These incidence rates were lower than those reported previously.
Based on analyses of a large number of patients, the present results support the efficacy of oral management in cancer patients. However, further studies are needed to establish adequate oral management guidelines for cancer patients.
1. Introduction
Patients suffer from various oral complications in cancer treatments. Oral complications resulting from cancer treatments cause acute and late morbidities. Recent advances in cancer treatment have led to change the prevalence, nature, and severity of oral complications.[1] The rational for dental intervention prior to cancer treatment is based on increased incidence of intra-therapy complications and viridans sterptcoccal bacteremia in patient with poor oral health. [2–4] Especially, since patients performed chemotherapy were under immunosuppressed conditions, patients with chronic dental disease and poor oral hygiene were thought increase the risk for the development of acute odontogenic infections and potentially life-threatening systemic infections during periods of immunosuppression, which was thought to occur less frequency than other acute mucosal oral infections. [5,6] Therefore, in the National Institute of Health consensus statement on oral cancer therapy, dental foci was reported to one of the potential sources of systemic infections that needed to be eliminated or ameliorated before commencement of anticancer therapy. [7] In cancer patients, oral complications resulting from dental and oral disease sometimes interferes with successful cancer treatment. It has been reported that, in esophageal carcinoma patients, pre-operative oral care was an effective and easy method to prevent post-operative pneumonia in patients who are undergoing esophagectomy. [8,9] It has been elucidated that oral functional management prevents general complications during the perioperative period.[10] Since in 2012, perioperative oral managements (POMs) has been introduced by federal health insurance in Japan, and patients with cancer, cardiovascular diseases, and organ transplantation received dental and oral functional managements during perioperative period. The prevalence of oral and dental diseases may have possible impact on cancer treatments.
Although dental intervention protocol had been reported especially in hematopoietic malignancy,[11,12] the protocol of POMs for solid malignant tumor remains controversial. Additionally, the decision on the type of dental treatment performed was affected by the clinician's assessment of the clinical and radiographic condition of the pulpal and periodontal status of the tooth involved, the time available before cancer treatment initiation, and patient's immune status at the time of dental treatment.[13] Therefore, there is a lack of outcome-oriented trials to assess efficacy of a specific pre-cancer therapy dental protocol.[13] The aim of present study was to investigate the prevalence of dental/oral complications in the cancer patients who underwent POMs based on large number of case series with a multicenter retrospective analysis.
2. Patients and methods
This study protocol was approved by the Committee on Medical Research of Shinshu University (♯3639). We published research plan and guaranteed opt-out opportunity by the homepage of each hospital.
In 2016, 2744 cancer patients who were underwent surgery, chemotherapy, radiotherapy, and the combination of these treatment modalities, and POMs at 1 of 7 university hospitals, including those of Nagasaki University, Kobe University, Nagoya City University, Nara Medical University, Wakayama Medical University, Osaka City University, and Shinshu University were included. In this study, patients with head and neck malignancy were excluded, since cancer treatment for these lesions affected directory on elucidation of oral status and functions. The medical records of these patients were reviewed retrospectively. The objective variable was occurrence of oral complications, including dental infections and oral mucositis, and prolonged fever in cancer treatment. The definitions of dental infections, oral mucositis, and fever of unknown origin were defined as below;
Dental infections: Painful caries of Grade 2, pulpitis, apical/marginal periodontitis, alveolar abscess with pain, etc.
Oral mucositis: Grading of oral mucositis according to Common Terminology Criteria of Adverse Events (CTCAE) ver.4.0.[14]
Fever of unknown origin: fever with ≥38.3°C which is unknown origin.
The predictor variables were defined as patient factors: age, sex, smoking and drinking habits, diabetes mellitus, the use of steroid or immunosuppressive agents, and white blood cell count (WBC) less than 1000/mm3; tumor treatment factors: site and treatment modalities; POMs intervention; POMs contents, start time, and completion. Patients who had not smoked for a year or longer were classified as not having a smoking habit.
In Japan, the Medical Insurance System established coverage for perioperative oral care in 2012. Basically, each patient who will be performed cancer treatment is recommended to be received oral care from a dentist and dental hygienist. POMs were generally started from the time the decision for hospitalization was made (Supplement 1). All patients received final oral cleaning by a dentist or dental hygienist the day before cancer treatment. Basically, cancer patients were receive POMs before staring the cancer therapy. However, due to the cancer treatment schedule, there were patients who could not started the POMs before the starting the cancer treatment. Therefore, patients who were referred for dental intervention prophylactically in cancer treatment or after treatment, were also received the POMs in this study.
The risk factors for dental infections, oral mucositis, fever of unknown origin, and efficacy of POMs on cancer treatment were statistically investigated with multivariate logistic regression. Statistical analyses were performed using JMP ver.13 (SAS Institute Inc., North Carolina, USA). P values <.05 were considered to be significant.
3. Results
Two thousand seven hundred and 44 cancer patients (1684 males and 1060 females) underwent POMs were investigated (Table 1). The characteristics of patients summarized in Table 1. Mean age was 65.9 ± 13.0 years-old. The most frequency site of cancer was 15.2% of lung, and 14.1% colon. Hematopoietic malignant tumor was seen in 12.2%. The smoking and drinking habits were seen in 38.1% and 17.0%, respectively. The users of steroid and/or immunosuppressive agents were 28.3%. Regarding the contents of cancer treatment, 75% of patients were performed the surgery, and 42.6% chemotherapy, and 14.2% radiation therapy, with overlapping. The start time of POMs was mainly pre-treatment in 76.4% of patients. The contents of POMs were only oral care in 77.6%, conservative treatment in 8.1%, and invasive treatment such as tooth extraction in 14.2%. The completion rate of POMs was 75.7%.
Table 1.
The characteristics of patients.
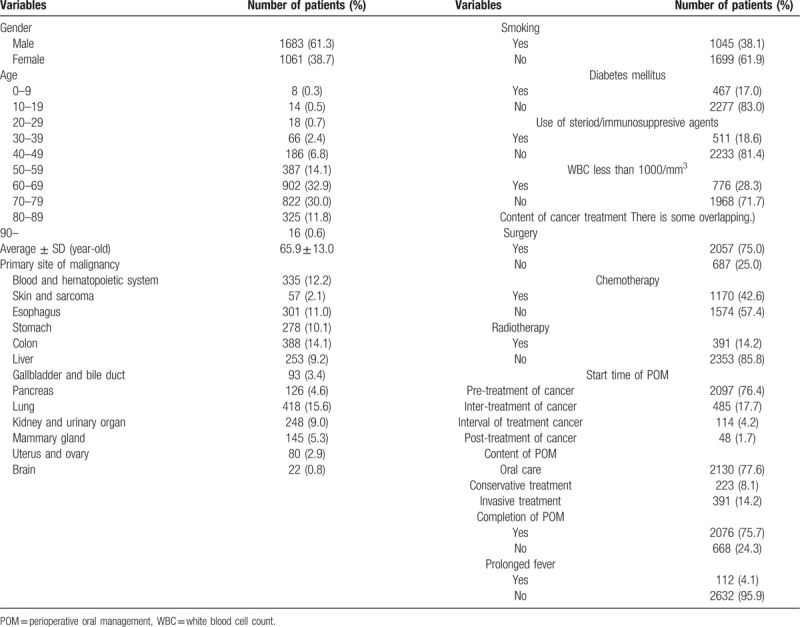
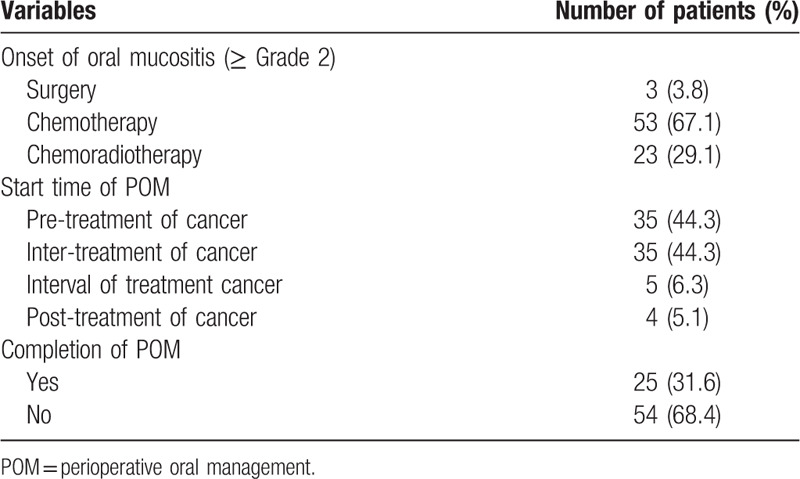
The prevalence of dental infections during cancer treatment was 8.2% (224/2744 cases) (Table 2). Acute apical periodontitis and alveolar abscess were most frequently occurred in 49.6%. Acute marginal periodontitis was 20.1%. Dental caries and pulpitis were 10.7% and 4.0%, respectively. Oral candidiasis was occurred in 6.7%. Among these dental infections, 56.7% were occurred in chemotherapy and 25.9% in perioperative period. The prevalence oral mucositis was occurred in 6.2%. Sever oral mucositis was occurred in 79 patients (2.8%) (Table 3). Among them, although, regarding the start time of POMs, 44.3% of patients was started at pre-treatment, the completion rate of POMs was 31.6%. In sever oral mucositis patients, 96.2% was occurred in chemotherapy/chemoradiotherapy. The prevalence of prolonged fever in cancer treatment was 4.1% (112/2,744 cases) and mainly in chemotherapy/chemoradiotherapy. Among 112 cases, prolonged fever might be thought the association with dental focal infections was 6.2% (7/112 cases), retrospectively. These 7 cases were all conversion from chronic periodontitis to acute during chemotherapy. Three cases of 7 cases were postponed cancer therapy. During cancer treatment, the prevalence of total oral adverse events, including oral infections and oral mucositis, was 15.2% (417/2744 cases) with overlapping. The effects of oral adverse events on cancer treatment schedule were postponement in 0.7% and discontinuation in 0.4%.
Table 2.
The characteristics of dental infections.
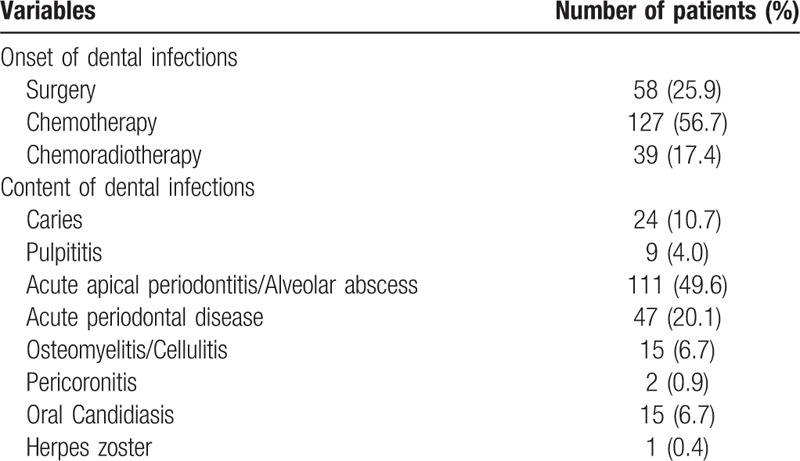
Table 3.
The characteristics of severe oral mucositis (≥Grade 2).
A multivariate analysis was performed to investigate the effects of POMs on the onset oral infections and process of cancer treatment. The onset of dental infections in cancer treatment was associated significantly with age (<66 vs ≧66, risk ratio(RR); 0.681, 95% confidential interval (CI); 0.495–0.936, P < .05), chemotherapy (no vs yes, RR:3.107, 95%CI:2.030–4.757, P < .0001), use of steroid/immunosuppressive agents (no vs yes, RR:1.721, 95%CI:1.173–2.527, P < .01), pre-treatment of POMs (no vs yes, RR:0.425, 95%CI:0.308–0.586, P < .0001), contents of POMs (oral care vs conservative treatment, RR:0.354, 95%CI:0.220–0.570, P < .0001, oral care vs invasive treatment, RR:0.091, 95%CI:0.063–0.131, P < .0001, conservative treatment vs invasive treatment, RR:0.258, 95%CI:0.160–0.417, P < .0001), and the completion of POMs (no vs yes, RR:0.573, 95%CI:0.406–0.809, P < .001) (Table 4). The onset of prolonged fever in cancer treatment was associated significantly with WBC less than 1000/mm3 (no vs yes, RR: 2.628, 95%CI: 1.725–4.000, P < .001) and use of steroid/immunosuppressive agents (no vs yes, RR:3.346, 95%CI:2.072–5.403, P < .001) (Table 5). Perioperative period was significantly reduced the risk (no vs yes, RR: 0.222, 95%CI: 0.131–0.376, P < .001). The completion of POMs had the tendency of risk reduction (no vs yes, RR: 0.651, 95%CI: 0.413–1.027, P = .065). Cancer treatment schedule was significantly affected by chemotherapy (no vs yes, RR: 4.679, 95%CI: 1.393–15.716, P < .05), radiotherapy (no vs yes, RR: 3.534, 95%CI:1.301–9.600, P < .05), and dental infections (no vs yes, RR:9.557, 95%CI:4.396–20.778, P < .001). The pre-treatment of POMs reduced the risk significantly (no vs yes, RR: 0.403, 95%CI: 0.189–0.860, P < .05) (Table 6).
Table 4.
A multivariate analysis of onset of dental infection.
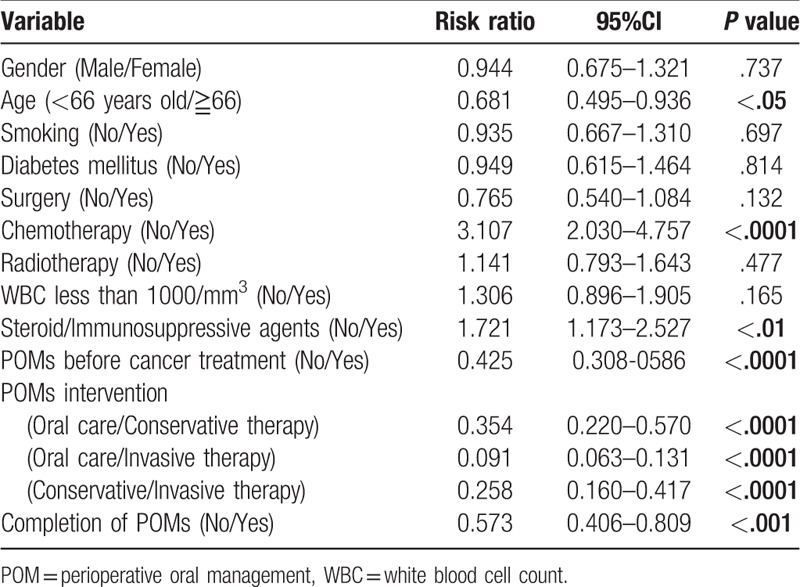
Table 5.
A multivariate analysis of onset of prolonged fever.
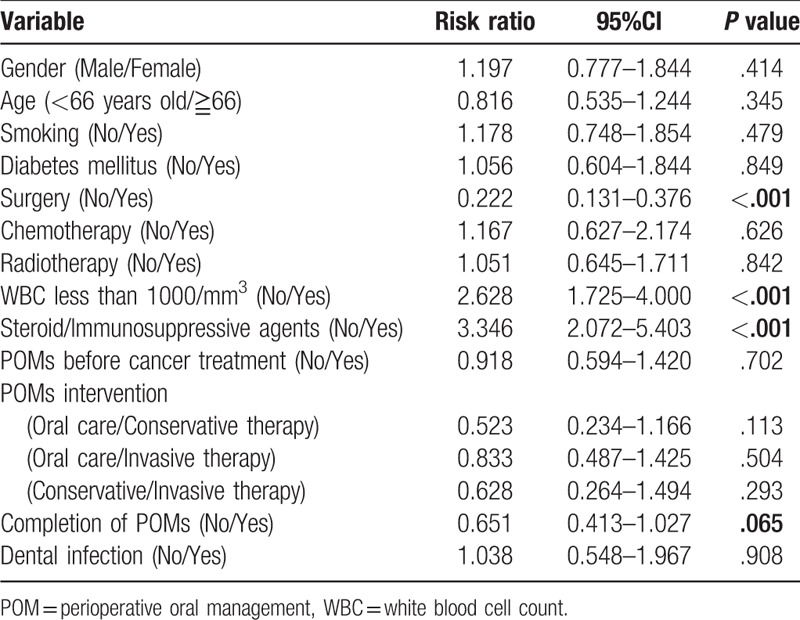
Table 6.
A multivariate analysis of variables affecting cancer treatment schedule.
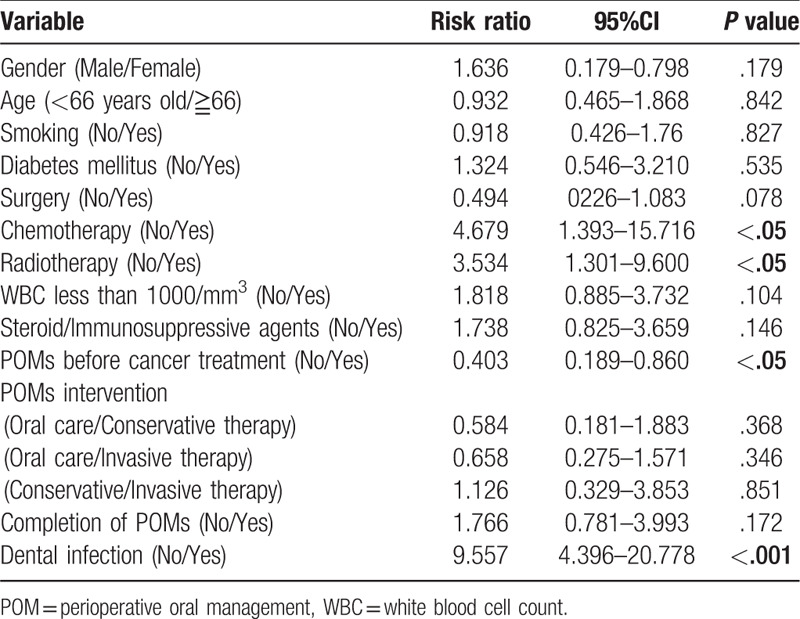
4. Discussion
In this study, the incidence of dental/oral complications in cancer patients with perioperative oral management was investigate based on a large number of case series with a multicenter retrospective analysis. The intervention rate of POMs before the initiation of cancer therapy was 76.4%. The incidence of dental focal infections during the period of cancer treatments was 8.2%. A multivariate analysis revealed that the significant risk factors for dental infections in patients undergoing cancer treatment were age, chemotherapy, use of steroid/immunosuppressive agents, pre-treatment of POMs, contents of POMs, and the completion of POMs. Cancer treatment schedule was significantly affected by chemotherapy, radiotherapy, and dental infections.
In a systematic review of dental disease during cancer therapy,[16] the weighted overall prevalence of dental caries was 28.1% and the overall DMFT for patients who were post-antineoplastic therapy was 9.19. The weighted prevalence of dental infections/abscess during chemotherapy was reported 5.8%.[13] In this study, the prevalence of dental infections was 8.2% and relatively high compared with previous report.[13] Among dental infections, acute inflammation including marginal and apical periodontitis, and alveolar abscess was most frequency seen in 69.7%. The conversion rate of previously diagnosed chronic disease to acute inter-therapy inflammation was reported 4%, and 10% of previous diagnosed severe chronic periodontitis was converted to acute periodontitis.[16] Although pre-existing oral conditions were not investigated in this study, conversion from chronic to acute lesion might be easily occurred in patients who were preformed cancer therapy, especially in immunosuppressive conditions. In a multivariate analysis of risk factor of dental infections in cancer patients, age <66 years-old, receiving chemotherapy, use of steroid/immunosuppressive agents, and contents and completion of POMs was detected as significant risk factors. Especially, among the contents of POMs, invasive treatment reduced the risk for the onset of dental infection significantly. On the other hand, oncologic treatment outcomes were reported to be unaffected by the presence of chronic dental disease or acute exacerbations of these disease states regardless of severity.[16] However, based on our results, it might be more beneficial for younger patients who would be performed chemotherapy and used steroid/immunosuppressive agents to intervene with POMs form pre-treatment and to complete POMs.
To the best of our knowledge, there have been no reports to investigate the association between dental infection and prolonged fever in cancer treatment based on relatively large number of patients. In this report, the incidence of prolonged fever in cancer treatment was 4.1%, and 6.2% of them was thought to be associated with dental infections. Additionally, the effects of oral adverse events on cancer treatment schedule were postponement in 0.7% and discontinuation in 0.4%. Despite the low prevalence of dental infections, these infections have been reported to cause bloodstream bacteremia and become potentially life-threatening in immunosuppressed individuals [13] A multivariate analysis in this study revealed that prolonged fever in cancer treatment was associated significantly with WBC less than 1000/mm3and use of steroid/immunosuppressive agents. The completion of POMs had the tendency of risk reduction. Additionally, cancer treatment schedule was significantly affected by chemotherapy, radiotherapy, and dental infections. The pre-treatment of POMs might reduce the risk significantly. Therefore, it was suggested that patients who would be expected the myelosuppressed/immunosuppressed conditions in cancer treatment might be needed to intervene POMs at pre-treatment. Due to the low consciousness of dental infections in health care providers, dental infections might be overlooked as the cause of the prolonged fever. Although it remains unclear how Porphyromonas gingivalis, an important periodontal pathogen in periodontitis, translocates from periodontal pocket to the subendothelial lining of the major elastic arteries, it may be facilitated by bacteremic seeding, followed by direct adherence to and invasion into the initial lining of the artery or alternatively, the bacteria may invade cells such as neutrophils or monocytes, which then actively transport the organism to the subendothelial space in a “piggy-back” fashion.[17] Microbial translocation was reported to contribute to febrile episodes in adults with chemotherapy-induced neutropenia.[18] The further investigation will be needed to clarify the association between the precise mechanism of microbial translocation of oral bacteria, occult dental infections and fever of unknown origin, and to clarify the precise mechanism of microbial translocation of oral bacteria based on chemokine and chemotaxis.
The strong point of this study was a study to investigate the incidence of dental infections and efficacy of POMs on the cancer treatment based on relatively large number of patients with multicenter retrospective analysis. Moreover, it was important to clarify the target which might be needed POMs at pre-treatment. The limitation of this study was based on retrospective study. Therefore, there was no configuration of control group in this study. However, in ethical concerns, it is difficult to conduct prospective randomized control study of evaluation of eradicating all oral infections prior to patients undergoing cancer therapy comparing with no dental treatment, because POMs have been covered by the Japanese medical insurance system since 2012, and most Japanese patients now receive POMs before cancer treatment. The ability to rule out invasive fungal and viral infections such as aspergillosis and cytomegalovirus, is varied widely based on available diagnostic tools, also different diagnostic approach could affect the incidence of prolonged fever in cancer patients. Altogether there are several ignored confounders that may affect obtained results. Additionally, the contents of anti-cancer agents were not investigated with the retrospective nature of this study, the types of anti-cancer drugs also might affect obtained results. As the 7 hospitals do not have a unified oral care protocol, it is not clear which of the procedures was effective in prevention of oral infections during cancer treatment. The protocol and intention of dental intervention for cancer patients remains controversial. Although in hematopoietic malignancy patients, some protocols of dental intervention have been reported to be beneficial,[11,12] to establish the guideline of POMs for cancer patient will be needed for standardization of dental intervention.
In conclusion, the intervention ratio of pre-treatment POMs was 76.4%. The completion rate of POMs was 75.7%. The incidence of dental infections was 8.2% during cancer treatment. The significant risk factors for dental infections in patients undergoing cancer treatment were age, chemotherapy, use of steroid/immunosuppressive agents, pre-treatment of POMs, contents of POMs, and the completion of POMs. The effects of oral adverse events on cancer treatment schedule were postponement in 0.7% and discontinuation in 0.4%. Cancer treatment schedule was significantly affected by chemotherapy, radiotherapy, and dental infections. The pre-treatment of POMs reduced the risk of oral complications significantly.
Author contributions
Conceptualization: Shin-Ichi Yamada, Masahiro Umeda, Hiroshi Kurita.
Data curation: Shin-Ichi Yamada, Sakiko Soutome, Takumi Hasegawa, Itaru Tojyo, Hirokazu Nakahara, Mao Kawakami, Marina Hirose, Shigeyuki Fujita, Takahide Komori, Tadaaki Kirita, Yasuyuki Shibuya, Masahiro Umeda.
Formal analysis: Shin-Ichi Yamada.
Investigation: Shin-Ichi Yamada.
Writing – original draft: Shin-Ichi Yamada.
Writing – review & editing: Shin-Ichi Yamada, Hiroshi Kurita.
Supplementary Material
Footnotes
Abbreviations: CI = 95% confidential interval, CTCAE = Common Terminology Criteria of Adverse Events, POMs = perioperative oral managements, RR = risk ratio, WBC = white blood cell count.
How to cite this article: Yamada Si, Soutome S, Hasegawa T, Tojyo I, Nakahara H, Kawakami M, Hirose M, Fujita S, Komori T, Kirita T, Shibuya Y, Umeda M, Kurita H. A multicenter retrospective investigation on the efficacy of perioperative oral management in cancer patients. Medicine. 2020;99:10(e19129).
This study was approved by the Committee on Medical Research of Shinshu University (No. 3639).
The authors have no conflicts of interests to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Epstein JB, Thariat J, Bensadoun RJ, et al. Oral complications of cancer and cancer therapy: from cancer treatment to survivorship. CA Cancer J Clin 2012;62:400–22. [DOI] [PubMed] [Google Scholar]
- [2].Peterson DE, Overholser CD. Increased morbidity associated with oral infection in patients with acute nonlymphocytic leukemia. Oral Surg Oral Med Oral Pathol 1981;51:390–3. [DOI] [PubMed] [Google Scholar]
- [3].Kennedy HF, Morrison D, Tomlinson D, et al. Gingivitis and toothbrushes: potential roles in viridans streptococcal bacteraemia. J Infect 2003;46:67–70. [DOI] [PubMed] [Google Scholar]
- [4].Peterson DE, Doerr W, Hovan A, et al. Osteoradionecrosis in cancer patients: the evidence base for treatment-dependent frequency, current management strategies, and future studies. Support Care Cancer 2010;18:1089–98. [DOI] [PubMed] [Google Scholar]
- [5].Greenberg MS. Prechemotherapy dental treatment to prevent bacteremia. NCI Monogr 1990;9:49–50. [PubMed] [Google Scholar]
- [6].Peterson DE. Pretreatment strategies for infection prevention in chemotherapy patients. NCI Monogr 1990;9:61–71. [PubMed] [Google Scholar]
- [7].Oral complications of cancer therapies: diagnosis, prevention, and treatment. The National Institutes of Health Consensus Development Conference Statement. Oncology (Williston Park). 1991;5:64, 69-76. [PubMed] [Google Scholar]
- [8].Soutome S, Yanamoto S, Funahara M, et al. Preventive effect on post-operative pneumonia of oral health care among patients who undergo esophageal resection: a multi-center retrospective study. Surg Infect (Larchmt) 2016;17:479–84. [DOI] [PubMed] [Google Scholar]
- [9].Soutome S, Yanamoto S, Funahara M, et al. Effect of perioperative oral care on prevention of postoperative pneumonia associated with esophageal cancer surgery: a multicenter case-control study with propensity score matching analysis. Medicine (Baltimore) 2017;96:e7436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Crall JJ. Oral health policy development since the Surgeon General's Report on Oral Health. Acad Pediatr 2009;9:476–82. [DOI] [PubMed] [Google Scholar]
- [11].Yamagata K, Onizawa K, Yanagawa T, et al. A prospective study to evaluate a new dental management protocol before hematopoietic stem cell transplantation. Bone Marrow Transplant 2006;38:237–42. [DOI] [PubMed] [Google Scholar]
- [12].Tsuji K, Shibuya Y, Akashi M, et al. Prospective study of dental intervention for hematopoietic malignancy. J Dent Res 2015;94:289–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hong CH, Napeñas JJ, Hodgson BD, et al. A systematic review of dental disease in patients undergoing cancer therapy. Support Care Cancer 2010;18:1007–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Common Terminology Criteria for Adverse Events (CTCAE) v4.0. Available at: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40 Accessed June 26, 2019. [PubMed] [Google Scholar]
- [15].Axelsson P, Lindhe J. Effect of fluoride on gingivitis and dental caries in a preventive program based on plaque control. Community Dent Oral Epidemiol 1975;3:156–60. [DOI] [PubMed] [Google Scholar]
- [16].Toljanic JA, Bedard JF, Larson RA, et al. A prospective pilot study to evaluate a new dental assessment and treatment paradigm for patients scheduled to undergo intensive chemotherapy for cancer. Cancer 1999;85:1843–8. [PubMed] [Google Scholar]
- [17].Suwatanapongched P, Surarit R, Srisatjaluk R, et al. Translocation of Porphyromonas gingivalis infected monocytes and associated cellular responses. Asian Pac J Allergy Immunol 2010;28:192–9. [PubMed] [Google Scholar]
- [18].Wong M, Barqasho B, Ohrmalm L, et al. Microbial translocation contribute to febrile episodes in adults with chemotherapy-induced neutropenia. PLoS One 2013;8:e68056. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


