Abstract
Immunosuppression can lead to hepatitis B virus (HBV) reactivation in hepatitis B core antigen antibodies (anti-HBc) positive patients, especially those undergoing chemotherapy, although there is limited data on solid organ recipients, especially lung transplantation. Our aim was to analyze the risk of HBV reactivation and the potential impact of anti-HBc-positive status (both donors and recipients) on prognosis in a lung, kidney, and liver transplantation cohort.
Retrospective analysis including data from all transplants in adults (2011–2012) in a tertiary hospital, with prospective HBV serology study to assess the risk of reactivation and its possible impact on survival.
In total, 392 transplant recipients were included (196 kidney, 113 lung, 83 liver). Pre-transplantation anti-HBc screening was more frequent in liver recipients (P < .001) and donors (P < .001) than in kidney or lung. Fifty-five (14%) recipients were anti-HBc-positive and were not undergoing antiviral prophylaxis. Three (5.4%) cases of HBV reactivation occurred: 2 in pre-transplant anti-HBc-positive recipients and 1 with prior unknown anti-HBc status. All were HBeAg+ with HBV deoxyribonucleic acid (DNA) >10E8 IU/mL and only mild fibrosis. Baseline recipient anti-HBc positive status was the only factor associated with HBV reactivation. No reactivation cases occurred in lung or kidney recipients of anti-HBc positive grafts. Survival was lower in lung transplants, especially in human immunodeficiency virus-infected patients and those with prior immunosuppression.
Anti-HBc positive status is a risk factor for HBV reactivation in solid organ recipients. Anti-HBc testing is highly recommended in solid-organ transplant recipients in order to identify those anti-HBc positive and therefore candidates for periodical hepatitis B surface antigen (HBsAg) and HBV DNA screening after transplant.
Keywords: hepatitis B core antigen antibodies, hepatitis B infection, hepatitis B reactivation, transplantation
1. Introduction
Hepatitis B virus (HBV) infection is a global health problem. Some 257 million people worldwide have chronic HBV infection, and roughly 2 billion show evidence of current or past HBV infection.[1] Despite the relatively low percentage of hepatitis B surface antigen (HBsAg) carriers in Western countries, up to 9% of the population test positive to hepatitis B core antigen antibodies (anti-HBc) and negative to HBsAg.[2]
The presence of anti-HBc positive alone is associated with a minimal risk of cirrhosis and hepatocellular carcinoma, and clearly greater survival.[3] However, immunosuppression may lead to HBV reactivation in these individuals. The importance of HBV reactivation lies in its clinical presentation, which ranges from transient acute hepatitis to chronic infection and acute liver failure, the latter associated with a high mortality rate.[4] Risk of reactivation varies according to HBV status and the type and duration of immunosuppressive therapy.[5,6]
In the transplantation setting, it is well recognized that the risk of HBV reactivation is very low in recipients receiving an anti-HBc-positive solid organ, with the exception of liver grafts.[3] Kidney, lung, and heart transplantation with anti-HBc-positive grafts has been proven safe in several studies,[7–10] with a estimated risk of reactivation around 0.3%.[11] HBsAg monitoring is advocated to identify the few cases of HBsAg seroreversion.[3] Liver transplantation from anti-HBc-positive donors is linked with a high risk of de novo HBV infection and therefore, lifelong prophylaxis with nucleos(t)ides analogs (NUC) is recommended.[3,12]
Although anti-HBc positivity has been associated with a high risk of HBV reactivation, especially in patients with hematological disease receiving rituximab-including chemotherapy regimens, its impact on solid organ recipients receiving immunosuppressive therapy has been scarcely explored. In fact, the international guidelines differ on the management of anti-HBc-positive solid-organ recipients. The European Association for the Study of the Liver (EASL)[3] recommends regular monitoring of both HBsAg and HBV deoxyribonucleic acid (DNA) without prophylaxis, whereas the American Association for the Study of Liver Diseases proposes limiting the duration of prophylaxis to 6 to 12 months after transplantation and during periods of intensified immunosuppression.[6]
The aim of this study was to analyze the risk of HBV reactivation and the potential impact of anti-HBc positive status (either donor or recipient) on survival in a real-world cohort of kidney, lung, and liver transplant recipients.
2. Patients and methods
2.1. Study population and design
This is a retrospective-prospective study carried out in a tertiary hospital in Spain. All lung, kidney, and liver transplantations performed in adults at least 18 years of age between January 2011 and December 2012 were included. The following variables were retrospectively collected from recipients: sex, date of birth, organ and date of transplantation, prior immunosuppressive therapy and type, body mass index (BMI), analytical data <6 months before the procedure, including alanine transferase (ALT) levels, anti-hepatitis C virus (HCV), human immunodeficiency virus (HIV), and HBV serology (anti-HBc, HBsAg, hepatitis B surface antibody [antiHBs], and HBV DNA in the case of positive HBsAg), immunosuppressive regimen, antiviral prophylaxis, last ALT value, post-transplantation HBV serology if available, history of organ rejection, and mortality. Donor variables: age, sex, BMI, HBV serology (anti-HBc, HBsAg, and anti-HBs if anti-HBc-positive). When evidence of HBV reactivation was detected during post-transplantation follow-up, data collected at that time point included ongoing immunosuppressive regimen, prior administration of rituximab, ALT level, HBV markers, and liver biopsy or elastography findings, if available.
Between October and December 2017 (5 or 6 years after transplantation) all living recipients with no HBV serology within the last 6 months were prospectively tested for HBsAg, anti-HBc, and HBV DNA in the case of presence of positive anti-HBc in either the donor or recipient. In patients who had died or were lost to follow-up, the last available HBV serology was used in the analysis.
HBV reactivation after transplantation was defined as a rise in HBV DNA compared with baseline or reappearance of HBsAg in patients previously testing negative (reverse seroconversion).[6] All recipients of an anti-HBc-positive liver graft received antiviral prophylaxis with NUC according to the international guidelines.[3,6] This study was conducted in accordance with the Declaration of Helsinski guidelines and the principles of Good Practice. Ethics committee was not requested since this is a retrospective study. Concerning the prospective its prospective part, periodical check-up of HBsAg and HBV DNA in anti-HBc positive recipients is recommended by the international guidelines for daily clinical practice.
2.2. Laboratory measurements
Serological markers for HBV (HBsAg, anti-HBc, and anti-HBs), HCV (anti-HCV), and HIV (anti-HIV) were analyzed by commercial enzyme immunoassays. The lower limit of quantification of anti-HBs was 10 mIU/mL and the upper, 500 mIU/mL. Serum HBV DNA was PCR-quantified with a COBAS 6800 HBV system (Roche Diagnostics, Mannheim, Germany): lower limit of quantification, 20 IU/mL and lower limit of detection, 10 IU/mL.
2.3. Statistical analysis
All statistical analyses were performed using IBM SPSS, 20 (SPSS Inc., Armonk, NY). Normally distributed quantitative variables were compared with the Student t test and expressed as the mean ± standard deviation (SD) or analyzed with the Mann–Whitney U test and expressed as the median and interquartile range (IQR) if non-normal distributed. Categorical variables were compared between groups using the chi-square or Fisher exact test, as appropriate. Variables showing statistical significance or with P < .10 in the univariate model were entered in a multivariate logistic regression model. The odds ratio (OR) and 95% confidence interval (CI) were calculated for independent predictive factors. Only patients with available data for all the variables were included in the multivariate analysis. A P-value < .05 was considered statistically significant.
3. Results
3.1. Baseline characteristics of the recipients and HBV screening prior to transplantation
A total of 392 solid organ transplants were carried out during the period of study: 196 kidney (50%), 113 lung (29%), and 83 liver (21%). Baseline characteristics of the recipients are summarized in Table 1. Overall, 248 (63%) were men and mean age was 54 years. There were no differences in the recipients’ characteristics by type of organ transplant except for the BMI, which was statistically lower in lung recipients than in kidney or liver (25 vs 26.4 vs 26.5 kg/m2, respectively; P < .001). Pre-procedure immunosuppressive therapy was more common in lung recipients (P < .001). Seven (2%) patients were HIV-infected, with a higher rate in liver transplantation than kidney or lung (6% vs 1% vs 0%, respectively; P = .004). The percentage of anti-HCV-positive patients was also higher in liver transplantation (P < .001). All recipients were screened for HBsAg prior to the transplant procedure (mandatory testing). Five (1.3%) were HBsAg-positive: 2 (2.4%) liver and 3 (1.6%) kidney recipients. All received antiviral therapy for HBV infection before and after transplantation. Anti-HBc antibodies were tested in 368 (94%) recipients (Fig. 1). Positive testing to anti-HBc was more common among men (18.6% vs 10.9%, P = .034) and liver recipients. Overall, 58 recipients were anti-HBc positive alone. Anti-HBs levels were determined in 44 (80%) of these patients and 33 (77%) showed values ≥10 mIU/mL (median 57 mIU/mL, IQR 22–338), with a higher percentage in lung and kidney recipients than liver (83% vs 86% vs 56%, P = .044).
Table 1.
Baseline characteristics of transplant recipients (N = 392).
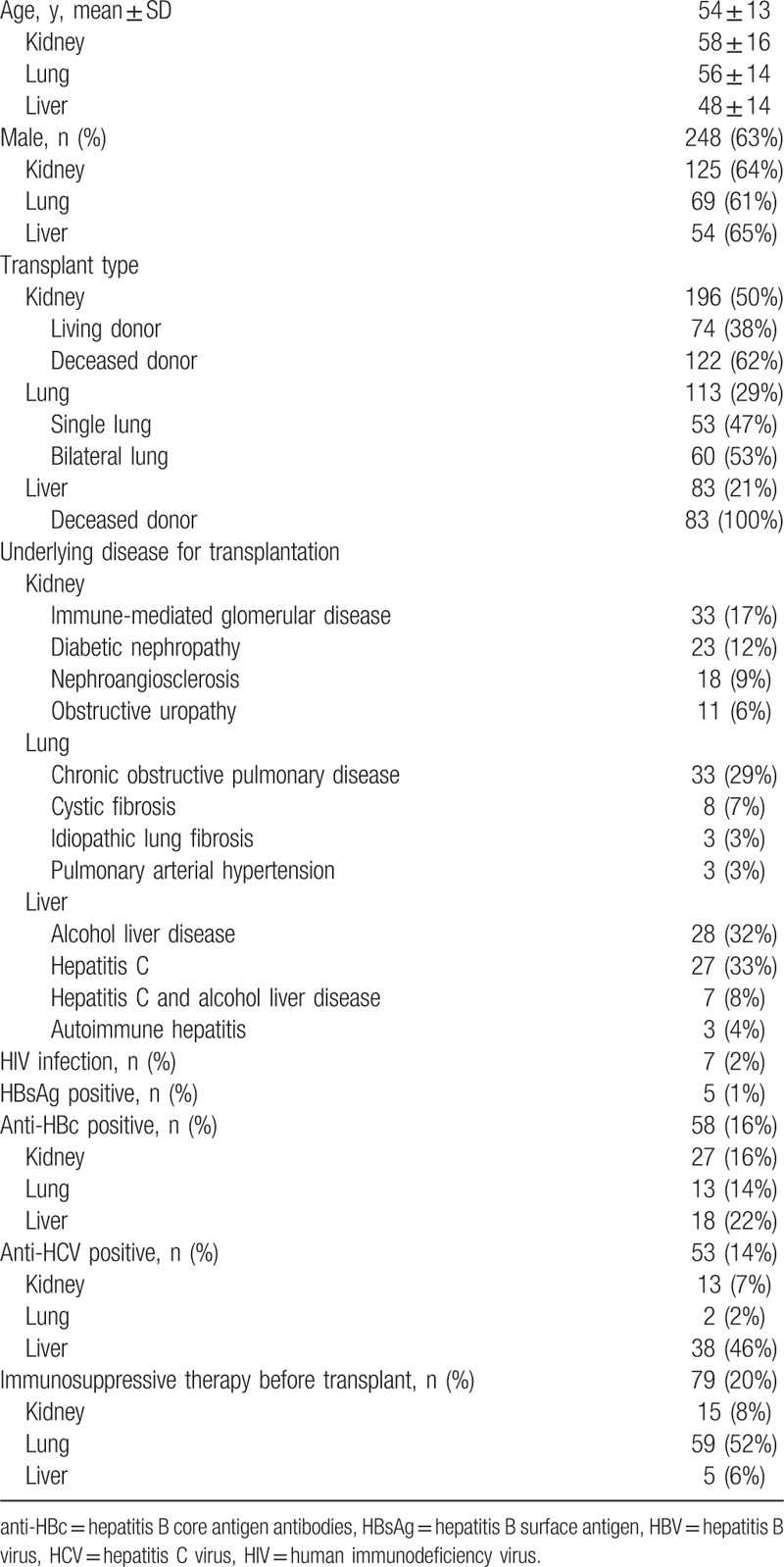
Figure 1.
Percentage of recipients and donors screened in each graft type and percentage testing anti-HBc positive. anti-HBc = hepatitis B core antigen antibodies.
3.2. Characteristics of the donors and HBV screening prior to transplantation
As to the donors, 173 (51%) were men (data available from 340 cases), mean age was 55 years, and mean ALT value was 48 IU/mL. Donor age was significantly younger in lung transplants than in liver or kidney (49 vs 56 vs 58 years, respectively; P < .001). There were no differences in ALT levels according to the transplanted organ (P = .82). At the time of the study, HBsAg-positive donors were excluded from organ donation in Spain so no donor tested positive. Screening for anti-HBc was carried out more often in liver graft donors (Fig. 1).
3.3. Recipients’ mortality rates and associated factors
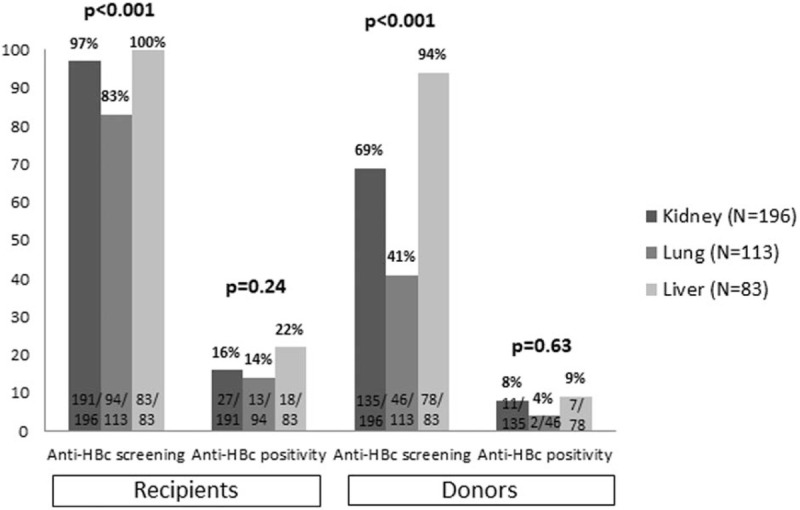
The overall survival rate was 73%, with a higher percentage in kidney than liver or lung transplant (83% vs 68% vs 59%, P < .001) (Fig. 2). Mean survival time was 80 months (95% CI 77–84) in kidney transplantation, 67 months (95% CI 60–75) in liver, and 63 months (95% CI 57–70) in lung (P < 0.001). Table 2 summarizes the factors associated with lower survival. Factors associated with lower survival included variables related to host (age >55 years, BMI < 30 kg/m2, HIV infection, positive anti-HBc, or positive anti-HCV), to donor (positive anti-HBc), transplanted organ and clinical evolution (graft rejection and ALT levels). However, on multivariate analysis, HIV infection and previous immunosuppressive therapy were the only factors associated with higher mortality (Table 3). Five of the 7 (71%) HIV-infected patients undergoing transplantation died during follow-up: 4 liver transplant recipients (1 due to liver failure, 1 Pneumocystis jirovecii pneumonia, 1 lymphoma complicated with gastrointestinal bleeding, and 1 laryngeal neoplasm) and 1 kidney recipient due to retroperitoneal bleeding.
Figure 2.
Mortality by type of transplanted solid organ.
Table 2.
Univariate analysis of factors associated with mortality.
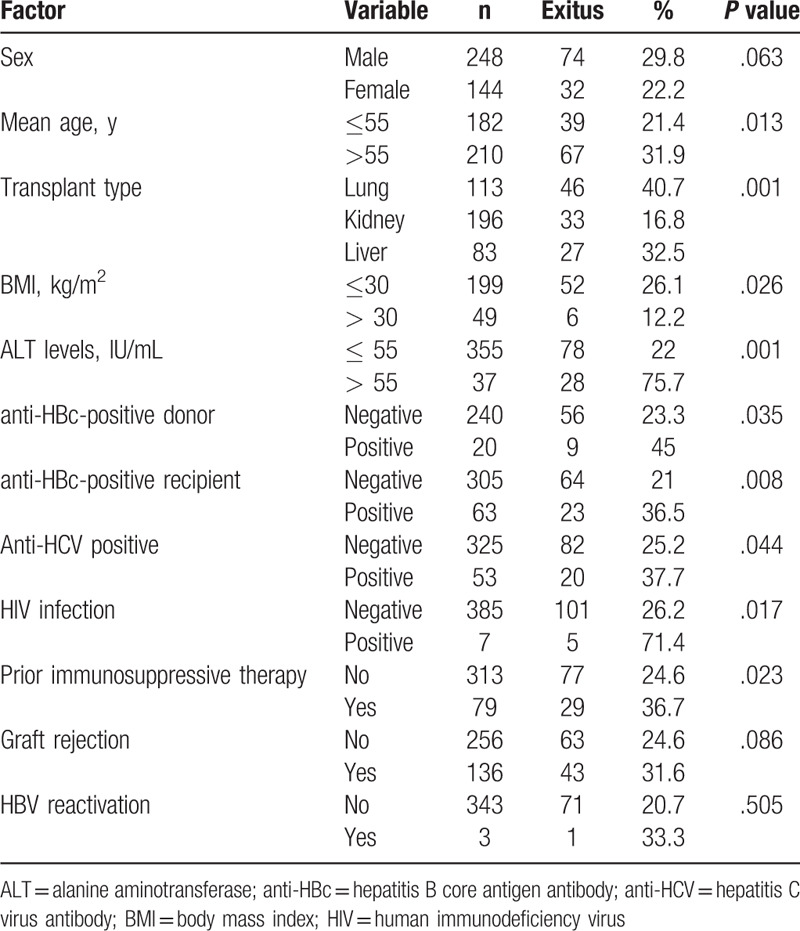
Table 3.
Multivariate analysis of factors associated with mortality.
3.4. HBV reactivation
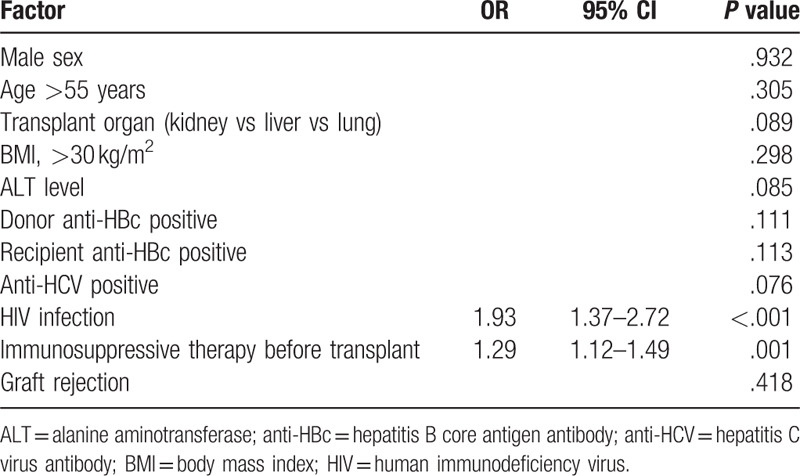
The risk of HBV reactivation was calculated according to the pre-transplantation HBV status of recipients (N = 392): 305 (78%) were anti-HBc negative, 58 (15%) HBsAg negative/anti-HBc positive, 5 (1.2%) HBsAg positive, and 24 (6%) unknown. Concerning the 305 anti-HBc-negative recipients, prospective data on HBsAg status were collected in 290, and there were no cases of HBV infection (Fig. 3). In the 24 patients without anti-HBc data before transplantation, prospective HBV reactivation screening was available in 6, and 1 of them showed HBV reactivation (HBeAg positive, HBV DNA >10E8 IU/mL, and anti-HBc IgM negative).
Figure 3.
Percentage of recipients who underwent prospective HBsAg screening and percentage testing positive according to their pre-transplant anti-HBc status. ∗anti-HBc positive recipients without antiviral prophylaxis. anti-HBc = hepatitis B core antigen antibodies, HBsAg = hepatitis B surface antigen.
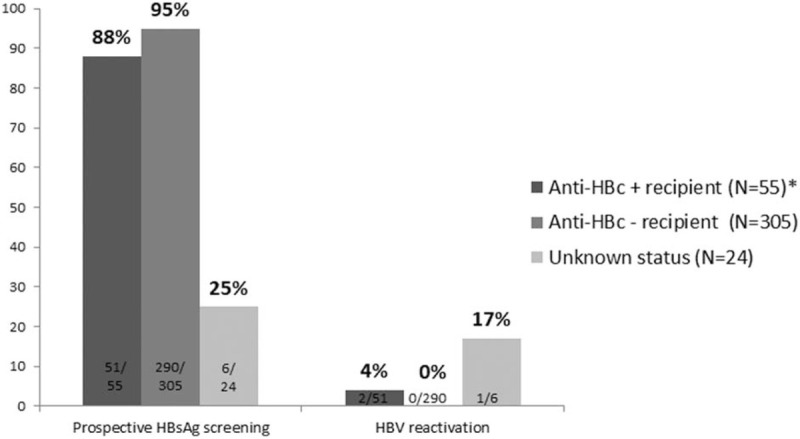
Overall, 58 recipients were anti-HBc positive before transplantation. However, 3 underwent antiviral prophylaxis (2 HIV-infected patients treated with a nucleotide analogue-containing antiretroviral regimen and 1 patient given lamivudine after receiving an anti-HBc-positive liver graft). Therefore, the risk of HBV reactivation at 5 years after transplantation was assessable in 55 anti-HBc-positive recipients without prophylaxis. HBV reactivation screening could be performed in 51 (93%) of these patients, and 2 (4%) cases of reactivation occurred (Fig. 4).
Figure 4.
Percentage of anti-HBc positive recipients who underwent post-transplantation HBsAg screening and percentage testing positive according to the transplanted solid organ. anti-HBc = hepatitis B core antigen antibodies, HBsAg = hepatitis B surface antigen.
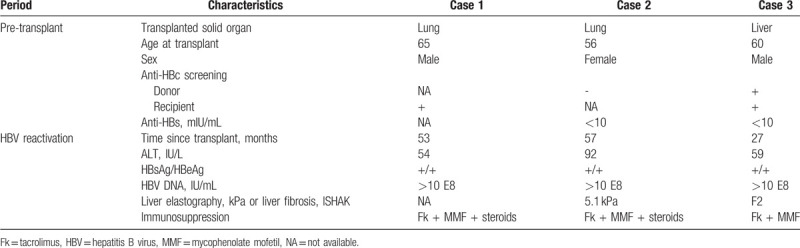
Characteristics of the 3 patients experiencing HBV reactivation are summarized in Table 4. All had detectable HBV DNA (>10E8 IU/mL), HBsAg tested positive (reverse seroconversion), HBeAg was also positive, and ALT values were ≤2 times upper limit of normality. Liver fibrosis was assessed in 2 patients, one by elastography and the other by biopsy, and was found to be mild in both cases. The 2 latter initiated therapy with NUC with later normalization of ALT values but persistence of HBsAg. The other patient died from multiorgan failure before the beginning of NUC. None of these patients had undergone treatment with rituximab. It should be noted that 1 patient with HBV reactivation was an anti-HBc-positive liver recipient who also received an anti-HBc-positive graft. He was initially treated with lamivudine, but discontinued treatment by personal decision. Hence, de novo HBV infection rather than reactivation cannot be definitely ruled out. The single factor associated with HBV reactivation on univariate analysis was anti-HBc-positive status of the recipients (P = .003).
Table 4.
Characteristics of the 3 cases of post-transplantation HBV reactivation.
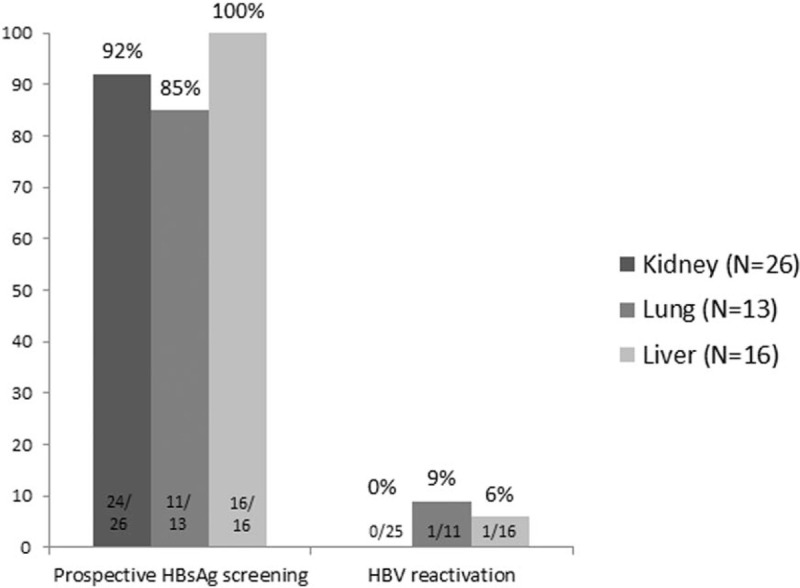
Focusing on the donors’ role, among the 309 patients who received a lung or kidney transplant, anti-HBc screening was available in 181 (59%) donors (Fig. 1), and anti-HBc was positive in 13 (7%). Following transplantation, HBsAg and HBV DNA testing could be performed in 11 of the 13 recipients of grafts from anti-HBc-positive donors and there were no cases of HBV infection. Seven of 78 (9%) liver grafts were from an anti-HBc-positive donor, and reverse seroconversion to HBsAg positive was observed in the single recipient who had stopped antiviral prophylaxis, although, as was mentioned above, this patient was also anti-HBc positive.
4. Discussion
The risk of HBV reactivation in anti-HBc-positive patients has been extensively investigated in patients with hematological malignancies diseases and in liver transplant recipients,[13–15] but there is little related real-world information in kidney recipients and,[16–18] scarce in lung transplantation.[19] Prospective HBsAg screening detected 3 cases of HBV reactivation in almost 400 solid organ recipients and identified previous anti-HBc-positive status as the only associated risk factor.
Two of the 3 patients with HBV reactivation had tested anti-HBc positive before the procedure and in the third patient, preoperative anti-HBc status was unknown, though analytical data at the time of HBV infection diagnosed was suggestive of HBV reactivation at the light of HBeAg positivity, anti-HBc IgM negativity and very high HBV DNA levels, all of them typical findings of HBV reactivation instead of acute hepatitis B.
The role of anti-HBc positivity in recipients of non-liver solid organs has been mainly investigated in the kidney transplant setting, likely because of the classical high prevalence of anti-HBc in patients previously exposed to hemodialysis.[20] The 2 largest studies in this line were carried out in Asia (Korea and China) and included up to 1200 kidney transplant patients. The reported HBV reactivation rates ranged from 1.2% to 5.6%.[16,19] Research performed in Europe with smaller cohorts has reported similar reactivation rates.[17,21] In lung transplant, previous studies have proved the safety of anti-HBc-positive grafts but, to our knowledge, there were no specific data on the potential role of anti-HBc positivity on prognosis and risk of HBV reactivation except for a study presented in abstract form.[19]
Baseline anti-HBs level >100 mIU/mL has been pointed out as a protective factor in a cohort of kidney transplant recipients.[17] More recently, a retrospective study in Korea reported that the post-transplantation HBV infection rate was higher in anti-HBc-positive patients and undetectable anti-HBs than in those with detectable anti-HBs (5.6% vs 1.2%, P < .001).[22] In our study, anti-HBs titers prior to transplantation were available in 2 of the 3 cases of HBV reactivation, and in both patients they were <10 mIU/mL.
One concept derived from the present study is the low concern about anti-HBc positivity in recipients of solid organs other than liver, as evidenced by the lower percentage of screening performed in kidney and lung recipients. The same was true for donor screening, likely because it is well established that use of an anti-HBc-positive graft is not linked with de novo HBV infection in non-liver solid transplantation.[7,23]
Our study has the limitation of its retrospective nature, in which data were missing for some patients, such as the pretransplantation anti-HBc status in 24 patients. Nonetheless, this fact is a useful indication of real-world clinical practice regarding HBV screening prior to transplantation. Prospective screening for HBV reactivation was carried out 5 to 6 years after transplantation; hence, systematic risk assessment for this event during intensified immunosuppression, especially in the immediate post-transplantation period, was not performed. However, the main point of the study (anti-HBc screening in recipients) was available in 94% of the patients included. Finally, de novo HBV infection cannot be definitely excluded in one of the patients with reactivation, as he received an anti-HBc-positive liver graft and abandoned lamivudine antiviral prophylaxis. An advantage of the study is that it provides data on lung transplantation, a setting lacking information about the potential risk of HBV reactivation in anti-HBc-positive recipients except for an study only published in abstract form including 33 anti-HBc-positive lung transplant,[19] without no data on specific incidence of HBV reactivation in this setting. Taking into account the results of our study, but also the limitations, data from a prospective study with periodical HBsAg determinations would be interesting to address the impact of anti-HBc positivity of recipients of non-liver transplantations.
In conclusion, we report data from almost 400 solid organ transplant patients with prospective HBsAg screening for HBV reactivation. Three cases of reactivation were documented, and recipient prior anti-HBc-positive status was the only factor associated with this event. This finding highlights the importance of pre-transplant anti-HBc screening of recipients in order to identify patients who will benefit from post transplant periodical testing for HBsAg and HBV DNA to rule out HBV reactivation.
Acknowledgments
English language support was provided by Celine Cavallo.
Author contributions
None.
Footnotes
Abbreviations: ALT = alanine transferase, anti-HBc = hepatitis B core antigen antibodies, anti-HBs = hepatitis B surface antibody, BMI = body mass index, CI = confidence interval, DNA = deoxyribonucleic acid, HBsAg = hepatitis B surface antigen, HBV = hepatitis B virus, HCV = hepatitis C virus, HIV = human immunodeficiency virus, IQR = interquartile range, NUC = nucleos(t)ides analogs, OR = odds ratio, SD = standard deviation.
How to cite this article: Álvarez-López P, Riveiro-Barciela M, Oleas-Vega D, Flores-Cortes C, Román A, Perelló M, Berastegui C, Castells L, Esteban R, Buti M. Anti-HBc impacts on the risk of Hepatitis B reactivation but not on survival of solid-organ transplant recipients. Medicine. 2020;99:9(e19407).
This work has not funding.
Source of funding: None declared.
The authors have no conflicts of interest to disclose.
References
- [1].WHO. Hepatitis B. 2018. [Google Scholar]
- [2].Salleras L, Dominguez A, Bruguera M, et al. Declining prevalence of hepatitis B virus infection in Catalonia (Spain) 12 years after the introduction of universal vaccination. Vaccine 2007;25:8726–31. [DOI] [PubMed] [Google Scholar]
- [3].European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis, B., virus, infection. J Hepatol 2017;67:370–98. [DOI] [PubMed] [Google Scholar]
- [4].Lau GK, Lee CK, Liang R. Hepatitis B virus infection and bone marrow transplantation. Crit Rev Oncol/Hematol 1999;31:71–6. [DOI] [PubMed] [Google Scholar]
- [5].Perrillo RP, Gish R, Falck-Ytter YT. American Gastroenterological Association Institute technical review on prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology 2015;148:221.e3–44.e3. [DOI] [PubMed] [Google Scholar]
- [6].Terrault NA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018;67:1560–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Dhillon GS, Levitt J, Mallidi H, et al. Impact of hepatitis B core antibody positive donors in lung and heart-lung transplantation: an analysis of the United Network For Organ Sharing Database. Transplantation 2009;88:842–6. [DOI] [PubMed] [Google Scholar]
- [8].Hartwig MG, Patel V, Palmer SM, et al. Hepatitis B core antibody positive donors as a safe and effective therapeutic option to increase available organs for lung transplantation. Transplantation 2005;80:320–5. [DOI] [PubMed] [Google Scholar]
- [9].Madayag RM, Johnson LB, Bartlett ST, et al. Use of renal allografts from donors positive for hepatitis B core antibody confers minimal risk for subsequent development of clinical hepatitis B virus disease. Transplantation 1997;64:1781–6. [DOI] [PubMed] [Google Scholar]
- [10].Veroux M, Puliatti C, Gagliano M, et al. Use of hepatitis B core antibody-positive donor kidneys in hepatitis B surface antibody-positive and -negative recipients. Transplant Proc 2005;37:2574–5. [DOI] [PubMed] [Google Scholar]
- [11].Mahboobi N, Tabatabaei SV, Blum HE, et al. Renal grafts from anti-hepatitis B core-positive donors: a quantitative review of the literature. Transplant Infect Dis 2012;14:445–51. [DOI] [PubMed] [Google Scholar]
- [12].Cholongitas E, Papatheodoridis GV, Burroughs AK. Liver grafts from anti-hepatitis B core positive donors: a systematic review. J Hepatol 2010;52:272–9. [DOI] [PubMed] [Google Scholar]
- [13].Barcena R, Moraleda G, Moreno J, et al. Prevention of de novo HBV infection by the presence of anti-HBs in transplanted patients receiving core antibody-positive livers. World J Gastroenterol 2006;12:2070–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Huang H, Li X, Zhu J, et al. Entecavir vs lamivudine for prevention of hepatitis B virus reactivation among patients with untreated diffuse large B-cell lymphoma receiving R-CHOP chemotherapy: a randomized clinical trial. JAMA 2014;312:2521–30. [DOI] [PubMed] [Google Scholar]
- [15].Loomba R, Liang TJ. Hepatitis B reactivation associated with immune suppressive and biological modifier therapies: current concepts, management strategies, and future directions. Gastroenterology 2017;152:1297–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chen GD, Gu JL, Qiu J, et al. Outcomes and risk factors for hepatitis B virus (HBV) reactivation after kidney transplantation in occult HBV carriers. Transpl Infect Dis 2013;15:300–5. [DOI] [PubMed] [Google Scholar]
- [17].Kanaan N, Kabamba B, Marechal C, et al. Significant rate of hepatitis B reactivation following kidney transplantation in patients with resolved infection. J Clin Virol 2012;55:233–8. [DOI] [PubMed] [Google Scholar]
- [18].Lee J, Park JY, Huh KH, et al. Rituximab and hepatitis B reactivation in HBsAg-negative/anti-HBc-positive kidney transplant recipients. Nephrol Dial Transplant 2017;32:906. [DOI] [PubMed] [Google Scholar]
- [19].Gonzalez A, Forouton F, Humar A, et al. Hepatitis b reactivation among non-liver solid organ transplant recipients. Am J Transplant 2016;13: suppl: Abstract# C290. [Google Scholar]
- [20].Fontenele AM, Filho NS, Ferreira AS. Occult hepatitis B in patients on hemodialysis: a review. Ann Hepatol 2013;12:527–31. [PubMed] [Google Scholar]
- [21].Berger A, Preiser W, Kachel HG, et al. HBV reactivation after kidney transplantation. J Clin Virol 2005;32:162–5. [DOI] [PubMed] [Google Scholar]
- [22].Jeon JW, Kim SM, Cho H, et al. Presence of hepatitis B surface antibody in addition to hepatitis B core antibody confers protection against hepatitis B virus infection in hepatitis B surface antigen-negative patients undergoing kidney transplantation. Transplantation 2018;102:1717–23. [DOI] [PubMed] [Google Scholar]
- [23].De Feo TM, Grossi P, Poli F, et al. Kidney transplantation from anti-HBc+ donors: results from a retrospective Italian study. Transplantation 2006;81:76–80. [DOI] [PubMed] [Google Scholar]


