Abstract
Introduction:
Nilotinib is a selective inhibitor of the BCR-ABL tyrosine kinase receptor and is used in the management of chronic myelogenous leukemia (CML). Nilotinib therapy at high doses is associated with elevated serum bilirubin levels. If the serum bilirubin level exceeds 3 times the upper limit of normal, the recommendation is to either adjust nilotinib dosage or temporarily discontinue the treatment. However, it is unclear whether hyperbilirubinemia indicates obvious liver histology damage.
Patient concerns:
A 24-year-old man with confirmed CML was treated with nilotinib therapy and developed hyperbilirubinemia after the treatment. Although the first remission of the hyperbilirubinemia was achieved after dose adjustment, the hematological parameters deteriorated. Thus, we initiated an antineoplastic therapy (at the standard dose) until complete remission of the CML was achieved. The pathogenic mechanism of hyperbilirubinemia may be related to the inhibition of uridine diphosphate-glucuronosyltransferase (UGT1A1) activity. Liver histological analysis revealed no significant liver damage. In addition, the patient had no family history of hyperbilirubinemia and liver disease.
Diagnosis:
The patient was admitted to our hospital under the diagnosis of hyperbilirubinemia, and histopathology by liver biopsy showed no obvious damage. We also detected a UGT1A1 mutation [ex1 c.686C > A (p.Pro229Gln)] in the patient and his mother.
Interventions:
When the nilotinib dose was decreased to 300 mg daily, the total bilirubin (TBIL) level decreased to 30 to 50 μmol/L for 1 month. However, because the Bcr-Abl/AblIS ratio did not correspond to the major molecular response (MMR; <0.1%), the nilotinib dose was readjusted to 400 mg daily. One week later, the TBIL and indirect bilirubin levels increased to 89 and 79 μmol/L, respectively. The levels of alanine transaminase and other liver functional indicators were normal.
Outcomes:
A Naranjo Adverse Drug Reaction (ADR) Probability Scale score of 13 indicates that hyperbilirubinemia is attributed to ADR caused by nilotinib rather than by drug-induced liver injury.
Conclusion:
Although reducing the nilotinib dose can alleviate the occurrence of hyperbilirubinemia, the effect of MMR is also reduced. Treatment of CML without dose adjustment or discontinuation of nilotinib therapy may be more advantageous.
Keywords: drug-induced liver injury, hyperbilirubinemia, nilotinib
1. Introduction
Nilotinib is a selective inhibitor of the BCR-ABL tyrosine kinase receptor [1] and is used to treat Philadelphia chromosome, which is associated with chronic myelogenous leukemia (CML).[2] Elevated serum aminotransferase levels are common in nilotinib therapy; however, only 4% to 9% of patients have been reported with levels 5 times higher than the upper limit of normal (ULN).[3] Nilotinib therapy at high doses is also associated with elevated serum bilirubin level.[4] If the alanine transaminase (ALT) or aspartate aminotransferase (AST) level increase significantly (i.e., if the ALT or AST level continues to be greater than 5 times the ULN, or the bilirubin level exceeds 3 times the ULN), the recommendation is to either adjust or temporarily discontinue the nilotinib dosage and initiate low-dose therapy.[5] We recently encountered a case of CML treated with nilotinib therapy, which led to hyperbilirubinemia. Although the first remission of the hyperbilirubinemia was achieved after dose adjustment, the hematological parameters deteriorated. Thus, we initiated an antineoplastic therapy (standard dose) until complete remission of the CML was achieved. The pathogenic mechanism of hyperbilirubinemia may be related to the inhibition of uridine diphosphate-glucuronosyltransferase (UGT1A1) activity. Liver histological analysis revealed no significant liver damage.
2. Ethics Statement
Ethics statement is not applicable for case reports according to the medical ethics committee of the Third Hospital of Zhenjiang Affiliated Jiangsu University, but informed consent was obtained from the patient for publication of this case report and the accompanying images. The study was conducted in accordance with the Declaration of Helsinki.
3. Case report
A 24-year-old man was admitted to our hospital with a complaint of abdominal pain. His white blood cell count at admission was 361 × 109/L. CML was suspected and later confirmed on the basis of the bone marrow biopsy result and the expression of Philadelphia chromosome (Ph+) on the cytogenetic study. First, 400 mg of imatinib mesylate (IM; Glivec, Novartis, Basel, Switzerland) daily was prescribed. The dose was reduced by 1 tablet daily when the patient first complained of leg ache. A rapid decline was observed in the platelet count from 231 × 109/L to 54 × 109/L in 20 days, and the dose was reduced by 2 tablets when the platelet count declined to 34 × 109/L. After a month since the discontinuation of the nilotinib treatment, the Bcr-Abl/Abl ratio according to the international scale (IS) increased from 0.8% to 1%, and the nilotinib therapy (400 mg daily, twice a day; Tasigna; Novartis, Basel, Switzerland) was reinitiated.
The findings from the liver function test performed before treatment were as follows: ALT, 32 U/L (normal range, 4–40 U/L); AST, 25 U/L (4–40 U/L); total bilirubin (TBIL), 12.6 μmol/L (3.4–21.2 μmol/L); direct bilirubin, 6.4 μmol/L (0.8–5 μmol/L); albumin, 3.5 g/dL (3.5–5.5 g/dL); prothrombin time, 12 s (9–14 s), alkaline phosphatase (ALP), 46 U/L (35–105 U/L); and γ-glutamyl transpeptidase, 32 U/L (normal, 5–36 U/L; Fig. 1).
Figure 1.
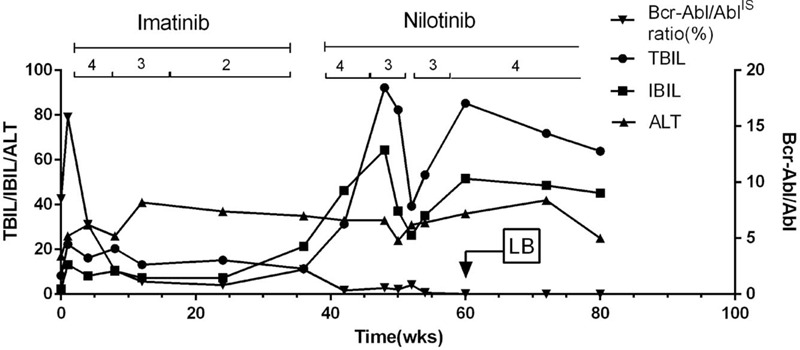
Course and evolution of the disease. 4 = 400 mg, 3 = 300 mg, 2 = 200 mg; LB, liver biopsy.
After 4 weeks of nilotinib treatment, the TBIL level increased to 91 μmol/L. When the nilotinib dose was decreased to 300 mg daily, the TBIL level decreased to 32 μmol/L, which was maintained at approximately 30 to 50 μmol/L for 1 month. However, because the Bcr-Abl/AblIS ratio did not correspond to the major molecular response (MMR, <0.1%), the nilotinib dose was readjusted to 400 mg daily. One week later, the TBIL and indirect bilirubin (IBIL) levels increased to 89 and 79 μmol/L, respectively. The levels of ALT and the other liver functional indicators were normal. Liver biopsy was performed, and the histopathology showed no obvious liver damage (Fig. 2). The Bcr-Abl/AblIS ratio was <0.01% after 4 months of 400-mg nilotinib treatment, but the TBIL and IBIL levels were 60 to 80 and 40 to 60 μmol/L, respectively (Fig. 1).
Figure 2.
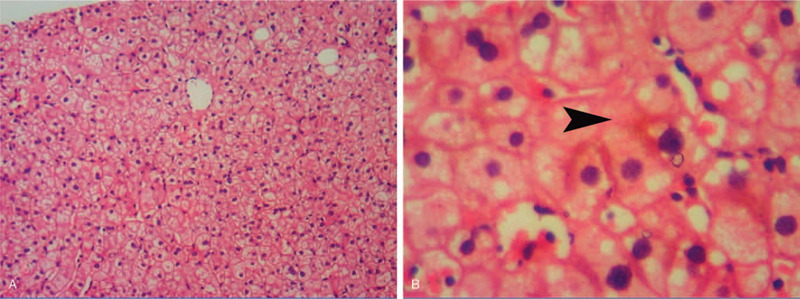
Microscopic features of the liver biopsy specimens. A: Normal hepatic lobule (hematoxylin and eosin staining, original magnification ×100). B: Bland cholestasis (hematoxylin and eosin staining, original magnification ×400).
We also detected a UGT1A1 mutation [ex1 c.686C > A (p.Pro229Gln)] in the patient and his mother (Fig. 3). On the basis of the data from a database (https://www.ncbi.nlm.nih.gov/clinvar/), the gene had a missense mutation that caused the pathogenesis, and both the patient and his mother were asymptomatic carriers due to the heterozygous mutation. This finding showed that the hyperbilirubinemia observed in the patient was induced by the nilotinib administration and suggested that nilotinib therapy may inhibit UGT1A1 activity, including bilirubin glucuronidation. To this end, the liver histology and function showed normal manifestations. Moreover, viral hepatitis (A–E), Epstein–Barr virus, and cytomegalovirus infections, and hereditary metabolic liver diseases were ruled out.
Figure 3.
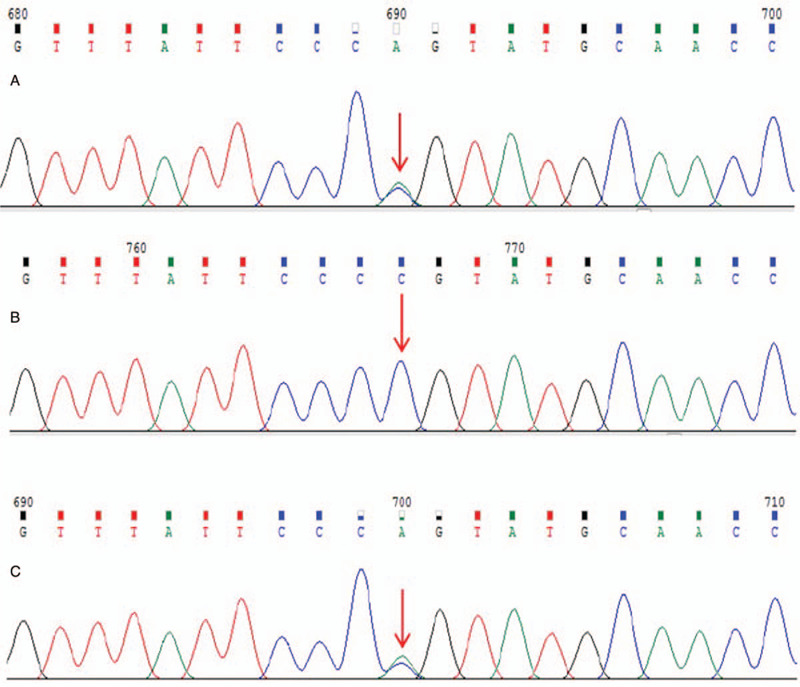
Subject: ex1 c.686C > A (p.Pro229Gln) Sanger sequencing map. A: Patient: had ex1 c.686C > A (p.Pro229Gln) gene mutation. B: Patient's father: had no ex1 c.686C > A (p.Pro229Gln) gene mutation. C: Patient's mother: had ex1 c.686C > A (p.Pro229Gln) gene mutation.
4. Discussion
Imatinib is a revolutionary drug used in the treatment of CML.[6] A single-drug therapy with imatinib can achieve complete cytogenetic responses and has attained an unprecedented overall survival rate.[7,8] However, one-third of the patients who used 400-mg IM did not achieve satisfactory outcomes.[9] Side effects such as progressive decrease in platelet count in patients restrict the use of IM in standard dose.[10,11] In 2007, nilotinib was approved in the United States for the treatment of Philadelphia chromosome-positive CML,[5] which is resistant or intolerant to previous treatments, including imatinib, thus opening up the possibility of using new treatment strategies for patients with CML.[12,13]
In a phase 1 dose-escalation study of nilotinib,[14] 119 patients with imatinib-resistant CML were enrolled and received different nilotinib doses (50, 100, 200, 400, 600, 800, and 1200 mg). The results showed that the high-dose groups (17%, 6/35, 600–1200 mg) had a higher incidence of severely elevated bilirubin level than the 400-mg dose group (9%, 3/32).
In a randomized open-label multicenter study,[15] nilotinib was shown to be a more potent inhibitor of BCR-ABL than imatinib. Moreover, the CCyR rates achieved in 12 months were significantly higher in the patients treated with nilotinib (300- or 400-mg dose) than in those treated with a similar imatinib dose. Of the patients in the 300- and 400-mg nilotinib dose groups, 53% (149/279) and 62% (171/277) had elevated TBIL levels, respectively, and 4% (10/279) and 8% (21/277) of the patients required dose adjustment and discontinuation of the nilotinib therapy, respectively.
Increased serum bilirubin level induced by nilotinib administration mostly causes indirect hyperbilirubinemia, as was observed in the present study, and is not associated with elevated serum enzyme levels or symptoms. No cases of nilotinib-induced liver failure have been reported.[4,16] However, the existing literature recommends dose adjustment and discontinuation to reduce TBIL levels elevated to > 3 times the ULN.
In this case, the elevated TBIL level obviously improved through dose adjustment at the beginning of the TBIL level elevation, which also affected the response of the Bcr-Abl/AblIS ratio. Hua et al [17] reported that a 44-year-old man with CML developed metabolic acidosis within 10 hours of nilotinib administration, which rapidly progressed to multiorgan and hepatic failure (bilirubin, 4.4 mg/dL; ALT, 1031 U/L). Furthermore, the autopsy showed massive liver necrosis.
UGT1A1 is a key enzyme in bilirubin metabolism. Its activity can lead to indirect hyperbilirubinemia, which is associated with many hereditary hyperbilirubinemic conditions such as Gilbert or Crigler-Najjar syndrome.[18] Although Gilbert syndrome is often described as a benign laboratory finding, it may alter drug metabolism by decreasing the ability to conjugate drugs.[19] Nilotinib-induced hyperbilirubinemia has been reported to be mainly related to the decrease in UGT1A1 activity [20–23] (Table 1).
Table 1.
Nilotinib-induced hyperbilirubinemia associated with UGT1A1 activity.
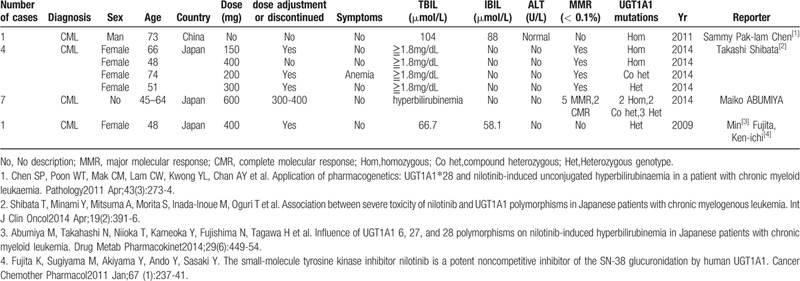
Singer et al [24] reported that patients with CML who have high-risk mutations in UGT1A1 have an elevated risk of nilotinib-induced hyperbilirubinemia than patients with wild-type UGT1A1. Therefore, in their study, they recommended a reduced initial nilotinib dose for these patients to reduce the risk of hyperbilirubinemia. However, although reducing the nilotinib dose can alleviate the occurrence of hyperbilirubinemia, the effect of the MMR is also reduced without considering that high bilirubin level itself can cause slight damage to the human body.
Whether nilotinib-induced hyperbilirubinemia is a drug-induced liver injury (DILI) remains to be elucidated. An international DILI Expert Working Group of clinicians and scientists developed uniform clinical chemistry criteria for DILI as follows [25]:
-
(1)
ALT level of ≥5 times the ULN
-
(2)
ALP levels of ≥2 times the ULN (particularly with accompanying elevations in 5′-nucleotidase or γ-glutamyl transpeptidase concentration in the absence of a known bone pathology underlying the increase in ALP level)
-
(3)
ALT level of ≥3 times the ULN
-
(4)
TBIL levels of ≥2 times the ULN. On the basis of these criteria, nilotinib-induced hyperbilirubinemia is not a DILI.
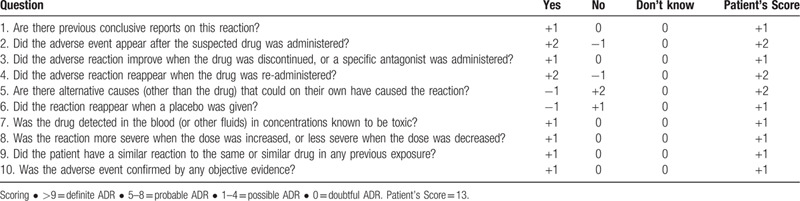
To assess the probability of adverse drug reactions (ADR), we used the Naranjo ADR Probability Scale [26] (Table 2). A score of 13 indicates that hyperbilirubinemia is attributed to ADR caused by nilotinib rather than to DILI.
Table 2.
Naranjo Adverse Drug Reaction Probability Scale: Items and score.
In conclusion, nilotinib is a selective tyrosine kinase receptor inhibitor used in the treatment of CML. Hyperbilirubinemia is a common complication of nilotinib therapy, whose pathogenic mechanism may be related to the inhibition of UGT1A1 activity. In IBIL, hyperbilirubinemia (jaundice) has a less severe effect on patients. Considering that no significant liver damage was observed in the liver histological examination in the present case, treatment of CML without dose adjustment or discontinuation of nilotinib therapy may be more advantageous.
Author contributions
Investigation: Yun Ye and Xingbei Zhou
Supervision: Youwen Tan
Writing – original draft: Youwen Tan and Yun Ye
Writing – review & editing: Youwen Tan
Footnotes
Abbreviations: ALT = alanine transaminase, AST = aspartate aminotransferase, CCyR = complete cytogenetic response, CML = chronic myelogenous leukemia, MMR = major molecular response, TBIL = total bilirubin, UGT1A1 = uridine diphosphate-glucuronosyltransferase, ULN = upper limit of normal.
How to cite this article: Tan Y, Ye Y, Zhou X. Nilotinib-induced liver injury: A case report. Medicine. 2020;99:36(e22061).
YT and YY contributed equally to this work.
This work was supported by the Medical Project of the Health Department of Jiangsu Province (H2018021; http://www.jswst.gov.cn/).
The authors have no conflicts of interest to disclose
All data generated or analyzed during this study are included in this published article [and its supplementary information files]
References
- [1].Kantarjian HM, Giles F, Gattermann N, et al. Nilotinib (formerly AMN107), a highly selective BCR-ABL tyrosine kinase inhibitor, is effective in patients with Philadelphia chromosome-positive chronic myelogenous leukemia in chronic phase following imatinib resistance and intolerance. Blood 2007;110:3540–6.. [DOI] [PubMed] [Google Scholar]
- [2].le Coutre P, Ottmann OG, Giles F, et al. Nilotinib (formerly AMN107), a highly selective BCR-ABL tyrosine kinase inhibitor, is active in patients with imatinib-resistant or -intolerant accelerated-phase chronic myelogenous leukemia. Blood 2008;111:1834–9.. [DOI] [PubMed] [Google Scholar]
- [3].Kantarjian HM, Hochhaus A, Saglio G, et al. Nilotinib versus imatinib for the treatment of patients with newly diagnosed chronic phase, Philadelphia chromosome-positive, chronic myeloid leukaemia: 24-month minimum follow-up of the phase 3 randomised ENESTnd trial. Lancet Oncol 2011;12:841–51.. [DOI] [PubMed] [Google Scholar]
- [4].Breccia M, Alimena G. Nilotinib therapy in chronic myelogenous leukemia: the strength of high selectivity on BCR/ABL. Curr Drug Targets 2009;10:530–6.. [DOI] [PubMed] [Google Scholar]
- [5].Deremer DL, Ustun C, Natarajan K. Nilotinib: a second-generation tyrosine kinase inhibitor for the treatment of chronic myelogenous leukemia. ClinTher 2008;30:1956–75.. [DOI] [PubMed] [Google Scholar]
- [6].Uz B, Dolasik I. Comment: management of de novo chronic myelogenous leukemia and imatinib-induced acute rhabdomyolysis with the second-generation tyrosine kinase inhibitor dasatinib. Ann Pharmacother 2016;50:334–5.. [DOI] [PubMed] [Google Scholar]
- [7].Rossari F, Minutolo F, Orciuolo E. Past, present, and future of Bcr-Abl inhibitors: from chemical development to clinical efficacy. J Hematology Oncol 2018;11:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Balabanov S, Braig M, Brummendorf TH. Current aspects in resistance against tyrosine kinase inhibitors in chronic myelogenous leukemia. Drug Discov Today Technol 2014;11:89–99.. [DOI] [PubMed] [Google Scholar]
- [9].Fava C, Rege-Cambrin G, Saglio G. The choice of first-line chronic myelogenous leukemia treatment. Ann Hematol 2015;94: Suppl 2: S123–31.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Santos FP, Kantarjian H, Quintas-Cardama A, et al. Evolution of therapies for chronic myelogenous leukemia. Cancer J 2011;17:465–76.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Martinez Pascual C, Valdes Mas M, de la Pena Moral JM, et al. Fulminating hepatitis for imatinib in a patient with chronic myeloid leukaemia. Medicina clinica 2011;137:329–30.. [DOI] [PubMed] [Google Scholar]
- [12].Jarkowski A, Sweeney RP. Nilotinib: a new tyrosine kinase inhibitor for the treatment of chronic myelogenous leukemia. Pharmacotherapy 2008;28:1374–82.. [DOI] [PubMed] [Google Scholar]
- [13].Usuki K, Tojo A, Maeda Y, et al. Efficacy and safety of nilotinib in Japanese patients with imatinib-resistant or -intolerant Ph+ CML or relapsed/refractory Ph+ ALL: a 36-month analysis of a phase I and II study. Int J Hematol 2012;95:409–19.. [DOI] [PubMed] [Google Scholar]
- [14].Kantarjian H, Giles F, Wunderle L, et al. Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL. N Engl J Med 2006;354:2542–51.. [DOI] [PubMed] [Google Scholar]
- [15].Saglio G, Kim DW, Issaragrisil S, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N EnglJ Med 2010;362:2251–9.. [DOI] [PubMed] [Google Scholar]
- [16].Koren-Michowitz M, le Coutre P, Duyster J, et al. Activity and tolerability of nilotinib: a retrospective multicenter analysis of chronic myeloid leukemia patients who are imatinib resistant or intolerant. Cancer 2010;116:4564–72.. [DOI] [PubMed] [Google Scholar]
- [17].Hua J, Iwaki Y, Inoue M, et al. Tumor lysis syndrome soon after treatment with hydroxyurea followed by nilotinib in 2 patients with chronic-phase chronic myelogenous leukemia. Int J Hematol 2013;98:243–6.. [DOI] [PubMed] [Google Scholar]
- [18].Ha VH, Jupp J, Tsang RY. Oncology drug dosing in gilbert syndrome associated with UGT1A1: a summary of the literature. Pharmacotherapy 2017;37:956–72.. [DOI] [PubMed] [Google Scholar]
- [19].Takano M, Sugiyama T. UGT1A1 polymorphisms in cancer: impact on irinotecan treatment. Pharmacogenomics PersMed 2017;10:61–8.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Fujita K, Sugiyama M, Akiyama Y, et al. The small-molecule tyrosine kinase inhibitor nilotinib is a potent noncompetitive inhibitor of the SN-38 glucuronidation by human UGT1A1. Cancer Chemother Pharmacol 2011;67:237–41.. [DOI] [PubMed] [Google Scholar]
- [21].Chen SP, Poon WT, Mak CM, et al. Application of pharmacogenetics: UGT1A1∗28 and nilotinib-induced unconjugated hyperbilirubinaemia in a patient with chronic myeloid leukaemia. Pathology 2011;43:273–4.. [DOI] [PubMed] [Google Scholar]
- [22].Shibata T, Minami Y, Mitsuma A, et al. Association between severe toxicity of nilotinib and UGT1A1 polymorphisms in Japanese patients with chronic myelogenous leukemia. Int J Clin Oncol 2014;19:391–6.. [DOI] [PubMed] [Google Scholar]
- [23].Abumiya M, Takahashi N, Niioka T, et al. Influence of UGT1A1 6, 27, and 28 polymorphisms on nilotinib-induced hyperbilirubinemia in Japanese patients with chronic myeloid leukemia. Drug Metab Pharmacokinet 2014;29:449–54.. [DOI] [PubMed] [Google Scholar]
- [24].Singer JB, Shou Y, Giles F, et al. UGT1A1 promoter polymorphism increases risk of nilotinib-induced hyperbilirubinemia. Leukemia 2007;21:2311–5.. [DOI] [PubMed] [Google Scholar]
- [25].Aithal GP, Watkins PB, Andrade RJ, et al. Case definition and phenotype standardization in drug-induced liver injury. Clin Pharmacol Ther 2011;89:806–15.. [DOI] [PubMed] [Google Scholar]
- [26].Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981;30:239–45.. [DOI] [PubMed] [Google Scholar]


