Abstract
In this study, the National Health Insurance Research Database of Taiwan was used to examine the recurrence and death risk for stage 0 colorectal cancer patients. We examined stage 0 colorectal cancer patients to identify factors causing recurrence and death.
This is a retrospective study, and stage 0 colorectal cancer patients that are registered in the Taiwan Cancer Registry of the Health Promotion Administration in 2007 to 2012 were included. The database was linked to the National Health Insurance Research Database, and subjects were followed up until the end of 2016. The mean follow-up period was 69 months. Bivariate analysis methods (log-rank test) and Cox proportional hazards model were used to evaluate the risk of recurrence and death and demographic characteristics, economic factors, environmental factors, health factors, treatment and hospitals, and absence/presence of postoperative tests were used to examine related risk factors.
Our study showed that the 5-year recurrence rate and 5-year mortality rate for stage 0 colorectal cancer are 1.68% and 0.6%, respectively. For stage 0 colorectal cancer, age (61–74 years) is the only factor affecting recurrence in patients (hazard ratio (HR) = 2.44; 95% CI: 1.41–4.22), while age >75 years (HR = 4.35; 95% CI: 1.14–16.68) and Charlson Comorbidity Index >4 points (HR = 7.20, 95% CI: 2.60–19.94) can increase the risk of death. In contrast, patients who underwent one (HR = 0.27, 95% CI: 0.10–0.71) and two or more colonoscopies (HR = 0.26, 95% CI: 0.10–0.70) within 2 years after surgery can reduce the risk of death from stage 0 colorectal cancer. In addition, the risk of recurrence is higher in patients who underwent colonoscopic polypectomy (HR = 2.07, 95% CI: 0.98–4.33) and patients with rectal cancer (HR = 2.74, 95% CI: 0.96–7.83), but these differences are not statistically significant (P > .05).
From this study, we can see that age and comorbidity index increase the risk of recurrence and death for stage 0 colorectal cancer, while postoperative colonoscopy can decrease the risk of death.
Keywords: colorectal cancer, death risk, early colorectal cancer, recurrence risk, risk factors, stage 0
1. Introduction
The global incidence of colorectal cancer has been increasing year by year. In 2015, the incidence of colorectal cancer was ranked third in world cancer incidence. Every year, there are 1.65 million newly diagnosed colorectal cancer patients and 830,000 deaths.[1] The incidence of colorectal cancer is higher in developed countries. In the last 10 years, there are 140,000 newly diagnosed cases every year in the US.[2] In Taiwan, the number of new colorectal cancer cases and deaths is rapidly increasing every year. Currently, the incidence of colorectal cancer in Taiwan is ranked first amongst all cancers, and the age-standardized incidence for colorectal cancer (including stage 0) is 54.46 per 100,000 population[3]. The TMN system proposed by the American Joint Committee on Cancer (AJCC) is mainly used for colorectal cancer staging, in which tumor invasion depth, presence/absence of lymph node, or distal metastasis are used as criteria for staging.[4] Colorectal cancer treatment and prognosis is related to cancer staging. After appropriate surgical resection, the cure rate for stage 0 colorectal cancer is relatively high. This is because the clinicopathological definition for stage 0 colorectal cancer is lesions that are limited to the mucosal layer, and lymph node and distal metastases are absent. Therefore, this cancer does not tend to invade tissues, only colonoscopic resection is required, surgical resection of the intestine and lymph node dissection are not required to treat these lesions, and metastasis will not occur in theory.[5,6] However, there are occasionally some cases of stage 0 colorectal cancer with distal metastases after resection in clinical practice, and there are case reports of stage 0 patients with complicated distal metastasis in the literature.[7,8] As there are few case reports, there is no conclusion on what are the causes of recurrence or death in stage 0 colorectal cancer patients at present.
The incidence of colorectal cancer is associated with dietary habits,[9] exposure to external environmental factors, and genetics.[10,11] On the other hand, post-treatment survival rate is associated with many risk factors such as age, comorbidities, socioeconomic status, insurance, and periodic follow-up.[12–14] In theory, the survival rate of stage 0 colorectal cancer is extremely high, and recurrence or metastasis should be rare in theory. However, cases of stage 0 colorectal cancer recurrence or deaths can still be observed in clinical practice. A possible reason may be that medical institutions and governments do not pay close attention in the follow-up of stage 0 colorectal cancer patients, resulting in delay or lack of early detection of metastatic lesions, resulting in actual survival rate not reaching the expected theoretical levels.
There are very few studies in the world on the risk of death in early colorectal cancer compared with colorectal cancer at other stages. The aim of this study was to use national data to examine the incidence ratio of recurrence and death in stage 0 colorectal cancer, factors related to recurrence, and risk of cancer death.
2. Material and methods
2.1. Data sources and study population
This study employed a retrospective cohort design, and the subjects were newly diagnosed, stage 0 colorectal cancer patients from 2007 to 2012. These patients were followed up until 2016, and each study subject was followed up for at least 4 years. The exclusion criteria were patients who previously suffered from stage 0 and above colorectal cancer, colorectal cancer patients aged below 20 years, and patients who suffered from other cancers.
The data source for this study was the National Cancer Registry, Causes of Death File, and National Health Insurance Research Database (NHIRD). In order to understand comorbidity severity, medical data was reviewed from January 1st, 2005 onwards.
2.2. Variable definitions and explanations
The variables in this study included gender, age, monthly salary, urbanization of residence area, tumor site, Charlson Comorbidity Index (CCI), treatment method, hospital level and attributes, number of colonoscopies within 2 years after surgery, and number of colon cancer index tests.
For environmental factors, urbanization of residence area was used as an indicator divided into 7 levels, of which the highest was level 1, and the lowest was level 7. The indicator of urbanization of residence areas published by Liu et al in 2006 was employed. This indicator used the 2000 Taiwan census data to investigate the development stratification of 359 townships in Taiwan. The urbanization level of residential areas was derived from several factors, including population density, population ratio of people with college or above educational levels, population ratio of older people over 65, population ratio of people being agriculture workers, and the number of physicians per 100,000 people.[15] The monthly salary was divided into 4 groups: ≤18,780, 18,781–28,800, 28,801–45,800, and ≥45,801 NTD.
Tumor site was divided into 3 groups, namely right colon, left colon, and rectum. The right colon included the cecum (ICD-03 code: C18.0), ascending colon (C18.2), hepatic flexure colon (C18.3), and transverse colon (C18.4). The left colon included the splenic flexure colon (C18.5), descending colon (C18.6), sigmoid colon (C18.7), and rectosigmoid junction (C19.9). The code for rectum is C20.9.
The CCI was used for comorbidity severity, as it is currently the most commonly used comorbidity measurement marker. In this study, the method published by Deyo et al in 1992 was employed, which used ICD-9-CM diagnosis codes to define the original CCI category.[16] In this study, the primary and secondary diagnoses registered in health insurance data in the recent 2 years before a definite diagnosis of stage 0 colorectal cancer were included in CCI calculations.
The treatments used included colonoscopic polypectomy, traditional open surgery, and laparoscopic surgery. For the level of hospitals, hospitals were classified as medical centers, metropolitan hospitals, and district hospitals. In addition, hospitals were further classified as public and private hospitals for hospital attributes. Postoperative follow-up was obtained from the health insurance database to find out if the patients had undergone a colonoscopy test and carcinoembryonic antigen (CEA) test within 2 years after treatment. Patients were divided into 3 groups based on the number of tests (0, 1, and 2 or more).
2.3. Outcome measures
The National Cancer Registry was linked to the Causes of Death File to find out if the patient had died during the follow-up period, the survival duration, and whether the cause of death was due to colorectal cancer. In addition, National Cancer Registry data on whether recurrence occurred during follow-up or new colorectal cancer occurred were used to determine recurrence and recurrence time.
2.4. Statistical analysis
This is a retrospective cohort study, in which the number and percentage of stage 0 colorectal cancer patients with recurrence or death were calculated.
With regards to whether recurrence or death occurred during the observation period, the log-rank test (for bivariate analysis) and Cox proportional hazards model were used to examine the risk of recurrence or death and factors affecting these risks. The Kaplan-Meier curve was plotted as a survival curve for stage 0 colorectal cancer patients. The SAS 9.4 statistical software (SAS Institute Inc., Cary, NC) was used for secondary database processing and statistical analysis. All tests were carried out with a significance level of α = 0.05. This study has been approved by the research ethics committee of China Medical University hospital (IRB No. CMUH107-REC3-080).
3. Results
3.1. Characteristics of study patients
A total of 4808 stage 0 colorectal cancer patients were included in this study. Most of these patients were male (n = 2983, 62.04%); in the age group of 61 to 74 years (n = 2089, 43.45%), followed by the ≤60 years age group (n = 1878, 39.06%). Most patients had a monthly salary of 18,781 to 28,800 NTD (39.37%), followed by less than 18,780 NTD (24.17%). Most patients lived in places with level 2 of urbanization (n = 1519, 31.59%), followed by level 1 (n = 1401, 29.14%). Most patients had a CCI score of 0–1 points (n = 3032, 63.69%), and there were 528 patients (10.98%) with 4 or more points. For tumor site, most patients had left colon tumors (n = 2433, 50.60%), while right colon tumors were seen less frequently (n = 987, 20.53%). Colonoscopic polypectomy (n = 3801, 79.06%) was mostly used in the treatment of stage 0 colorectal cancer. Most patients underwent ≥2 colonoscopies within 2 years after surgery (n = 2056, 42.76%) and 1148 (23.88%) patients did not undergo colonoscopy at all. Most patients did not undergo CEA testing within 2 years after surgery (n = 3141, 65.33%).
3.2. Recurrence and survival rates
Among the 4808 patients in this study (Table 1), recurrence occurred in 84 patients. The mean follow-up period for recurrence was 69.07 ± 24.70 months, and the recurrence rate was 1.81 per 1000 person-years. During the follow-up period, 748 stage 0 patients died, of which 31 died due to colorectal cancer. The mean follow-up period for death was 69.84 ± 24.20 months, and mortality rate due to colorectal cancer was 0.66 per 1000 person-years.
Table 1.
Characteristics of study patients.
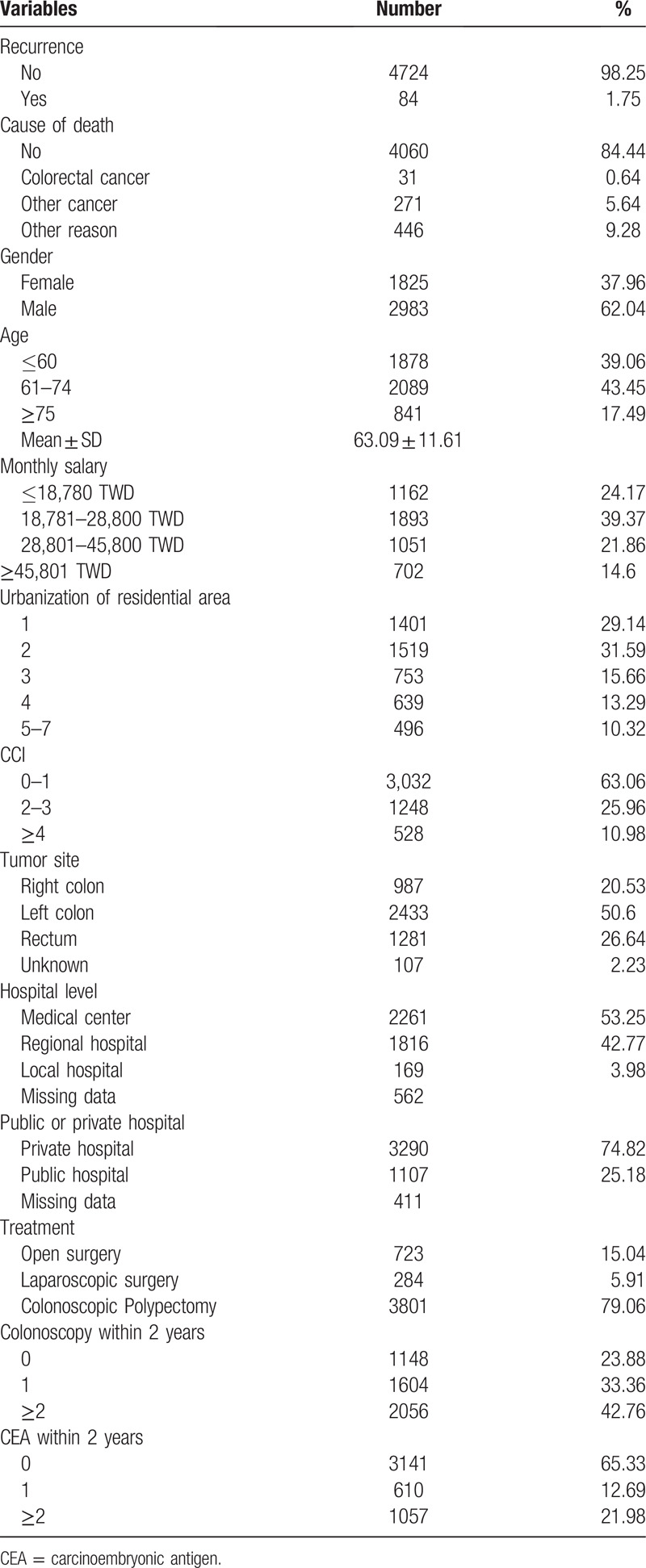
We calculated the 1-, 3-, and 5-year recurrence rates which were 0.57%, 1.18%, 1.68%, respectively. The 1-, 3-, and 5-year cancer specific mortality rates were 0.13%, 0.3%, 0.6%, respectively (Table 2). Figure 1 shows the Kaplan-Meier curve for recurrence and death due to colorectal cancer for stage 0 patients.
Table 2.
Recurrence and CRC specific mortality rate in patients with stage 0 colorectal cancer.
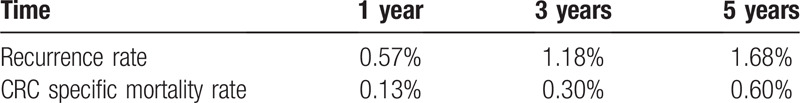
Figure 1.
Kaplan-Meier curves of cancer recurrence and death in patients with stage 0 colorectal cancer.
3.3. Recurrence and survival-related risk factors
The log rank test was used for bivariate analysis of the risk factors for recurrence in stage 0 colorectal cancer patients (Table 3). From the table, we can see that age is the only variable that significantly affected recurrence (P < .05) as patients aged 61 to 74 years had the highest recurrence ratio, which was 2.39% during the follow-up period. In comparison, the ratio of patients of < 60 years with recurrence during the follow-up period was 1.01%. When the multivariate Cox proportional hazard model was used to examine factors affecting recurrence in stage 0 colorectal cancer patients, age was found to be a significant risk factor (P < .05): Compared with patients aged ≦60 years, the hazards ratio (HR) for patients aged 61 to 74 years was 2.44 (95% CI: 1.41–4.22), which was significantly higher. The HR for recurrence risk in patients aged ≧75 years was 2.04 (95% CI: 0.99–4.19). Although the recurrence risk was observed to be higher for patients who underwent colonoscopic polypectomy, this was not statistically significant (HR = 2.07, 95% CI: 0.98–4.33, P = .06).
Table 3.
Relative risk and factors associated with colorectal cancer recurrence in patients with stage 0.
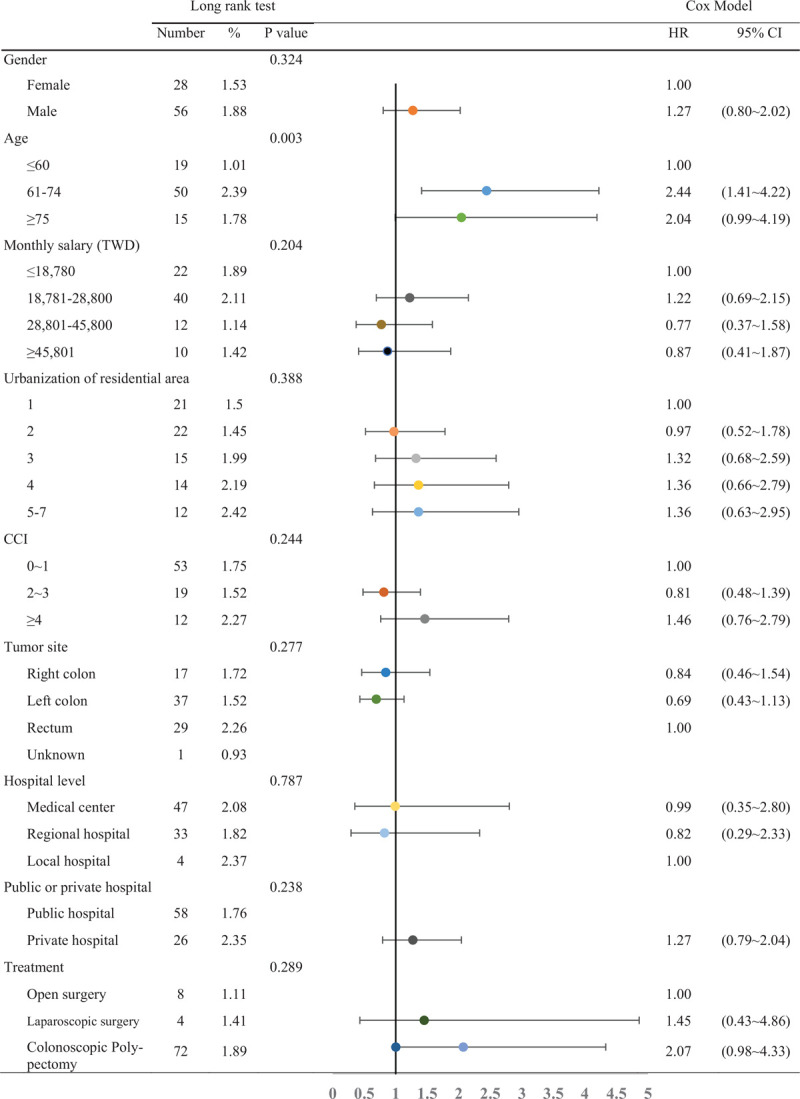
The log-rank test was employed as bivariate analysis to examine the risk factors for death due to stage 0 colorectal cancer (Table 4), and it was found that age, CCI, tumor site, treatment method, and the number of subsequent colonoscopies during follow-up affected death from stage 0 colorectal cancer (P < .05). In patients aged >75 years, the mortality rate during the follow-up period was 1.66%, while the mortality rate for patients aged < 60 years was only 0.21%. The mortality rate during the follow-up period for rectal cancer was 1.25%, while the mortality rate for right colon cancer during the follow-up period was only 0.33%. In patients who underwent traditional open surgery, the mortality rate for colon cancer was 1.94%, while the mortality rate for patients who underwent colonoscopic polypectomy was 0.45%, and no deaths occurred in patients who underwent laparoscopic surgery. The mortality rate due to colon cancer for patients who did not undergo colonoscopy follow-up 2 years after treatment was 1.57%, while the mortality rate for those who underwent 2 or more colonoscopies was only 0.34%.
Table 4.
Relative risk and factors associated with colorectal cancer death in patients with stage 0.
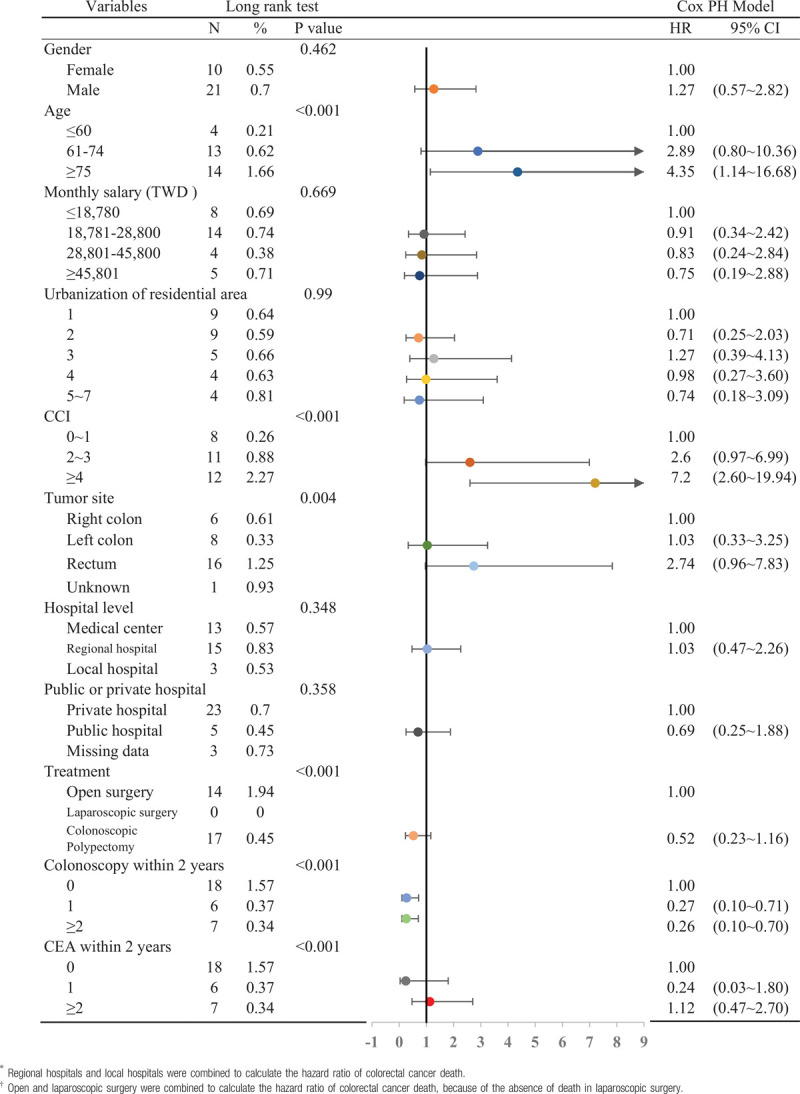
Finally, the Cox proportional hazard model was used to examine factors affecting the risk of death in stage 0 colorectal cancer patients (Table 4). Results showed that patients aged >75 years had a significantly higher risk of death than those aged < 60 years (HR: 4.35, 95% CI: 1.14–16.68). Compared with patients with a CCI score of 0–1 point, patients with a CCI score of ≥4 had an HR of 7.20 (95% CI: 2.60–19.94). In addition, compared with patients who did not undergo colonoscopy examination within 2 years after surgery, those who underwent one (HR: 0.27, 95% CI: 0.10–0.71) or two or more colonoscopies (HR: 0.26, 95% CI: 0.10–0.70) had a significantly lower risk of dying from colorectal cancer. In bivariate analysis, rectal cancer increased the mortality rate of stage 0 colorectal cancer. However, in the Cox model, although the risk of death was slightly higher for rectal cancer compared with right colon cancer (HR: 2.74, 95% CI: 0.96–7.83), this difference was not statistically significant (P = .06).
4. Discussion
The global incidence of colorectal cancer is increasing in recent decades. Colorectal cancer (CRC) is the third most commonly diagnosed malignancy and the fourth leading cause of cancer-related deaths worldwide.[1] The incidence of colorectal cancer in Asia is also showing a rapid increase. In recent decades, the incidence of colorectal cancer in Asian countries has increased by 2 to 4 times.[17] It may be caused by westernization of diet, aging population, smoking, and lack of exercise.[18] In Taiwan, as in other Asian countries, the incidence of colorectal cancer has increased year by year. It is now the first leading cancer in Taiwan. The age-standardized incidence for colorectal cancer (including stage 0) is 54.46 per 100,000 people.[3] The incidence of young-onset CRC significantly increased in both men and women, and this trend is similar to that observed in Western countries.[19] Many risk factors may be related to the increase of incidence of colorectal cancer in Taiwan, such as westernized dietary lifestyle, smoking, alcohol consumption, etc. However, we could not obtain this information from the database and could not include them in the study.
As there is no invasion past the mucosal layer in stage 0, and lymph reflux occurs in the matrix region that is deep in the mucosal layer, stage 0 colorectal cancer will not result in lymph node metastasis in theory, and intestinal resection and lymph node dissection are not required when treating stage 0 colorectal cancer. A small-scale study in the same hospital in South Korea pointed out that a total of 64 stage 0 patients underwent colonoscopic resection, and no recurrence or metastasis occurred.[6] John Hopkins Hospital in the USA also examined 15 stage 0 poorly differentiated colorectal patients who underwent lesion resection, and pathological section reports did not find lesions associated with the primary tumor or invasion.[5] Clinical patient statistics from a single medical center in Taiwan also analyzed 118 stage 0 patients who underwent surgical resection, and pathological tests did not find any lymph node metastasis, and no recurrence occurred in these patients subsequently. This means that recurrence or metastasis should not occur in theory, even if surgical resection was not carried out and only colonoscopic resection was employed.[20]
However, actual clinical experience showed that there is a very small minority of stage 0 colorectal cancer patients who experienced metastasis or recurrence. Several case reports in the literature also found distal metastases after resection in stage 0 colorectal cancer.[7,8] Why does metastasis or recurrence occur in stage 0 cancer, even though it should not occur in theory? Currently, there is no definite conclusion, but the authors of different papers have proposed possible reasons, such as tumor detachment during colonoscopic resection and implantation at nearby intestinal sites, resulting in recurrence.[21] In addition, sometimes there are differences between the pathology section report and the final surgical pathology report, resulting in an underestimation of the risk of metastasis when selecting the initial treatment for the patient.[22,23] According to the 1992 to 2004 AJCC statistics, a total of 5939 patients had a tumor invasion depth reaching the mucosal layer (Tis), which are staged as stage 0. It was found that 1.6% of these patients had lymph node metastasis in the final pathology report after they had undergone intestinal resection and lymph node dissection, showing that these patients would have an extremely high chance of metastasis if colonoscopic resection was used initially instead of intestinal resection by surgery.[24]
In this study, we calculated the 3- and 5-year recurrence rates for stage 0 colorectal cancer in 4808 patients, which were 1.18% and 1.68%, respectively. The 3- and 5-year mortality rates were 0.3% and 0.6%, respectively. In theory, metastasis and recurrence should not occur after treatment is completed for stage 0 colorectal cancer. There are very few papers that examined mortality or recurrence rate for stage 0 colorectal cancer, and most were statistics from a single hospital and published in the form of case reports.[8,25] However, from this study, nationwide statistics showed that the 5-year recurrence rate for stage 0 colorectal cancer was 1.68%, which does not fit the theory that metastasis should not occur. The reason for this may be that a nationwide cancer registry was used for data analysis in this study and 4808 stage 0 colorectal cancer patients were enrolled. The number of patients is higher than most single-center studies, and the follow-up period is longer (mean follow-up period: 69.8 months). Therefore, we were still able to identify 84 patients with metastasis and 31 patients who died due to colorectal cancer even though the recurrence and mortality rates are relatively low for stage 0 colorectal cancer. With regards to the mortality rate statistics, one paper used the US Surveillance, Epidemiology, and End Results Program (SEER) database to analyze the survival rate after colonoscopic resection or surgical resection of early colorectal cancer and found that the 5-year CRC-free survival rates for polypectomy and surgical resection were 96.3% and 95.9%, respectively.[26] In comparison, our study linked that National Cancer Registry with NHIRD and found that the 5-year cancer-specific survival for overall stage 0 colorectal cancer was 99.4%, regardless of whether surgical resection or colonoscopic polypectomy were carried out.
From the results of this study, we can see that different risk factors will affect the risk of recurrence and death in stage 0 colorectal cancer patients. Age and gender are intimately associated with the incidence of colorectal cancer, which tends to occur in males aged 50 years and above. Are prognostic factors for colorectal cancer after treatment associated with gender? Statistical data from the UK showed that, although gender affects incidence, there are no significant differences in prognosis and mortality rate in colorectal cancer patients between the sexes.[27] Another South Korean study also found that gender will not affect the prognosis of stage 1 colorectal cancer.[28] However, there are studies with different viewpoints: A Japanese study found that the prognosis of women with colorectal cancer is significantly better than that of men (HR: 0.87, 95% CI: 0.85–0.90).[29] Another meta-analysis found a similar conclusion: women with colorectal cancer have better prognosis (HR 0.87, 95% CI: 0.85–0.89).[30] However, this study showed that there were no statistically significant differences for stage 0 colorectal cancer patients, regardless of whether bivariate analysis or Cox model was used.
According to a large population study in the US, the probability of colorectal cancer occurring is higher in people with low education level or living in low socioeconomic places.[12] The reasons why cancer tends to occur in people with low socioeconomic status include fewer physical exercise, unhealthy diet, smoking, and a higher obesity rate.[31] In addition, few people with low socioeconomic status tend to undergo periodic stool examinations, which may be another reason for increased incidence of colorectal cancer.[32] However, this study showed that the urbanization of the area of residence does not have significant effects on prognosis (such as recurrence or death) after treatment in Taiwan.
Many factors will affect survival in colorectal cancer patients after treatment. One study examined the effects of different factors on survival in colorectal cancer, and statistical results showed that age at diagnosis is the only influencing factor, as older patients have a poorer prognosis.[33] In comparison, prognosis is better in younger patients.[34] However, a South Korean study on prognosis after stage 1 colorectal cancer treatment found that age will not affect recurrence rate after treatment.[28] Another paper pointed out that the age and comorbidities of colon cancer patients are independent and important prognostic risk factors.[14] Low physical activity and frailty in elderly people with colorectal cancer are also markers for poor prognosis: frail patients have a significantly reduced 5-year survival rate.[35] Comorbidity severity is also an important marker that affects colorectal cancer prognosis.[36] The CCI score can clearly affect prognosis: the higher the CCI score, the poorer the prognosis.[13,34] Identical conclusions were obtained in this study: age is a risk factor for stage 0 recurrence and is also simultaneously a risk factor for death due to stage 0 colorectal cancer. In addition, a comorbidity index >4 points will significantly increase the risk of death due to stage 0 colorectal cancer.
In theory, both colonoscopic polypectomy and surgical resection (laparoscopic or conventional abdominal surgery) can be used to treat stage 0 colorectal cancer, and this conforms to current routine clinical guidelines.[37] However, in this study, statistics showed that although the recurrence rate was higher when polypectomy was carried out than after surgery, the difference was not statistically significant (HR: 2.07, 95% CI: 0.98–4.33). Mortality rate analysis found that the mortality rate for traditional open surgery (14/709, 1.94%) was higher than that of colonoscopic polypectomy (17/3784, 0.45%), whereas no deaths occurred in patients who underwent laparoscopic surgery (0/284). Cox analysis was used to analyze stage 0 colorectal cancer death, but there was no statistical difference between surgical resection and colonoscopic polypectomy. From these results, we can see that good prognosis can be achieved with both surgery and colonoscopic polypectomy.
A paper on South Korean hospitals examined the tumor sites of 712 early (stage 1 and 2) colorectal cancer patients and the probability of recurrence after treatment. They found that the recurrence rate was lower in patients with lesions at the proximal colon, while the probability of recurrence was higher in patients with lesions at the distal colon and rectum (distal colon: HR 9.213, rectum: HR 15.366, P < .05).[38] A study also found that the prognosis for rectal cancer is significantly poorer than colon cancer.[34] In this study, although statistics showed that the risk of death is higher for stage 0 rectal cancer than for colon cancer, this difference was not statistically significant (HR: 2.74, 95% CI: 0.96–7.83).
Periodic colonoscopies were found to affect the risk of death from colorectal cancer. One study pointed out that patients who underwent colonoscopy within 10 years have a 67% reduced risk of death from colorectal cancer compared with those who did not undergo colonoscopy, regardless of whether it was right colon or left colon (including the rectum) cancer (OR = 0.33; CI 0.21–0.52).[39] With regards to the effects of whether postoperative colonoscopy can affect colorectal cancer prognosis, a U.S. paper showed that undergoing at least one colonoscopy within 5 years after surgery can increase the 5-year survival rate for colorectal cancer (76.8% vs 52.2%, P < .001).[40] Another study using the U.S. database showed the postoperative colonoscopy can reduce mortality rate by 43% (HR = 0.57, 95% CI: 0.51–0.64).[41] Similar conclusions were obtained in this study: periodic colonoscopies after surgery in stage 0 colorectal cancer patients can effectively reduce the risk of death from colorectal cancer. However, there is no significant difference in undergoing postoperative CEA test or not in reducing the risk of death from colorectal cancer.
The definition and name of pathological diagnosis for stage 0 colorectal cancer changes with the AJCC version. In versions preceding version 7, stage 0 colorectal cancer includes severe or high-grade dysplasia, intraepithelial carcinoma, and intramucosal carcinoma, in which cancer cells did not penetrate the mucosal layer and reach the submucosal layer. However, there is no consistency between Eastern and Western pathologists in the determination of stage 0 colorectal cancer as this differs according to subjectivity.[42] Moreover, after 2018, the 8th AJCC defined stage 0 colorectal cancer as TisN0M0. Tis (intramucsosal carcinoma) means that the tumor is only limited to the mucosal layer. Although there is invasion of the lamina propria, the tumor did not penetrate the lamina propria. Other categories that do not belong to Tis are no longer considered to be stage 0 colon cancer.[43] As this is a retrospective study that examined stage 0 colorectal cancer patients that are registered in the National Cancer Registry of Taiwan from 2007 to 2012, the 2010 7th AJCC edition was used. Hence, stage 0 colorectal cancer in the statistical data includes those histological subtypes that were described above. Whether different histological subtypes that are considered to be stage 0 colorectal cancer will affect patient prognosis is a direction of future studies.
5. Limitations
In this study, NHIRD and National Cancer Registry Data were used. However, some tumor-related variables are not registered in the database, such as dietary lifestyle, smoking, physical activity, and alcohol consumption. Second, some important prognostic factors, for example, tumor size, R0 resection, and serum tumor markers cannot be obtained as well. These factors may affect the prognosis of stage 0 colorectal cancer patients after treatment. Besides, we can only know that the patients have received colonoscopies or CEA tests and the number of the examinations, but these results cannot be obtained. Therefore, we were unable to know whether patients came for follow-up due to physical discomfort or due to periodic follow-up examinations.
6. Conclusion
This study showed that the 5-year recurrence rate and 5-year mortality rate for stage 0 colorectal cancer were 1.68% and 0.6%, respectively. Age was the only risk factor that affected stage 0 recurrence, and the recurrence risk was higher for patients aged 61 to 74 years. Examination of the risk factors for stage 0 colorectal cancer death showed that age >75 years and CCI score ≥4 points had a higher risk of death. On the other hand, periodic colonoscopy (once or ≥2 times) within 2 years can reduce the risk of death. In addition, the recurrence rate for colonoscopic polypectomy and the probability of death for rectal cancer were higher, but these differences were not statistically significant.
Acknowledgments
We are grateful for using the National Health Insurance Research Database provided by the Science Center of the Ministry of Health and Welfare, Taiwan. We are also grateful to Health Data Science Center, China Medical University Hospital for providing administrative, technical, and funding support.
Author contributions
Analyzed the data: Ming-Hao Hsieh Wen-Yin Kuo Wen-Chen Tsai
Conceived and designed the experiments: Ming-Hao Hsieh Pei-Tseng Kung Tao-Wei Ke Wen-Chen Tsai.
Contributed reagents/materials/analysis tools: Ming-Hao Hsieh Pei-Tseng Kung Wen-Yin Kuo Tao-Wei Ke Wen-Chen Tsai.
Performed the experiments: MHH Wen-Yin Kuo Wen-Chen Tsai.
Wrote the paper: Ming-Hao Hsieh Pei-Tseng Kung Wen-Chen Tsai.
Footnotes
Abbreviations: AJCC = American Joint Committee on Cancer, CCI = Charlson's comorbidity index, CEA = carcinoembryonic antigen, CI = confidence interval, CRC = colorectal cancer, HR = hazards ratio, ICD-O-3 = International Classification of Diseases for Oncology, 3rd edition, NHIRD = National Health Insurance Research Database, NTD = new Taiwan dollar, SEER = the U.S. surveillance, epidemiology, and end results program database.
How to cite this article: Hsieh MH, Kung PT, Kuo WY, Ke TW, Tsai WC. Recurrence, death risk, and related factors in patients with stage 0 colorectal cancer: a nationwide population-based study. Medicine. 2020;99:36(e21688).
P-TK and W-CT had equal contributions to this work.
This study was supported by the grant (CMU108-S-10) from China Medical University and Asia University.
The authors have no conflicts of interest to disclose.
The data that support the findings of this study are available from a third party, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are available from the authors upon reasonable request and with permission of the third party.
References
- [1].Allen C, Barber RM, et al. Global Burden of Disease Cancer Collaboration. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol 2017;3:524–48.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Cronin KA, Lake AJ, Scott S, et al. Annual Report to the Nation on the Status of Cancer, part I: National cancer statistics. Cancer 2018;124:2785–800.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Health Promotion Administration. Cancer registry annual report, 2015, Taiwan [Chinese]. 2017; Available from: 2018; Available from: https://www.hpa.gov.tw/Pages/Detail.aspx?nodeid=269&pid=10227. [Google Scholar]
- [4].Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471–4.. [DOI] [PubMed] [Google Scholar]
- [5].Lewin MR, Fenton H, Burkart AL, et al. Poorly differentiated colorectal carcinoma with invasion restricted to lamina propria (intramucosal carcinoma): a follow-up study of 15 cases. Am J Surg Pathol 2007;31:1882–6.. [DOI] [PubMed] [Google Scholar]
- [6].Kim MN, Kang JM, Yang JI, et al. Clinical features and prognosis of early colorectal cancer treated by endoscopic mucosal resection. J Gastroenterol Hepatol 2011;26:1619–25.. [DOI] [PubMed] [Google Scholar]
- [7].Lee HJ, Ye BD, Byeon JS, et al. Unusual local recurrence with distant metastasis after successful endoscopic submucosal dissection for colorectal mucosal cancer. Clin Endosc 2017;50:91–5.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lee HK, Kim JS, Cheon KS, et al. TisN0M1 sigmoid colon cancer: a case report. Ann Coloproctol 2014;30:141–6.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chan AT, Giovannucci EL. Primary prevention of colorectal cancer. Gastroenterology 2010;138:2029–43.. e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Corman ML. Corman's colon and rectal surgery. sixth edition ed. Philadelphia: Lippincott Williams & Wilkins, a Wolters Kluwer business; 2013: 745–748. [Google Scholar]
- [11].Townsend CR, Beauchamp DB, Evers M, et al. , “Colon and rectum” in Sabiston Textbook of Surgery: The Biological Basis of Modern Practical surgical Practice, Townsend C, Ed. 2004, Philadelphia: Saunders, 17 edition, pp 1401-1483. [Google Scholar]
- [12].Doubeni CA, Laiyemo AO, Major JM, et al. Socioeconomic status and the risk of colorectal cancer: an analysis of more than a half million adults in the National Institutes of Health-AARP Diet and Health Study. Cancer 2012;118:3636–44.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Erichsen R, Horvath-Puho E, Iversen LH, et al. Does comorbidity interact with colorectal cancer to increase mortality? A nationwide population-based cohort study. Br J Cancer 2013;109: p 2005–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].van Eeghen EE, Bakker SD, van Bochove A, et al. Impact of age and comorbidity on survival in colorectal cancer. J Gastrointest Oncol 2015;6:605–12.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Liu CY, Hung YT, Chuang YL, et al. Incorporating development stratification of Taiwan townships into sampling design of large scale health interview survey. (in Chinese) J Health Manag 2006;4:1–22.. [Google Scholar]
- [16].Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613–9.. [DOI] [PubMed] [Google Scholar]
- [17].Sung JJ, Lau JY, Goh KL, et al. Increasing incidence of colorectal cancer in Asia: implications for screening. Lancet Oncol 2005;6:871–6.. [DOI] [PubMed] [Google Scholar]
- [18].Onyoh EF, Hsu WF, Chang LC, et al. The rise of colorectal cancer in asia: epidemiology, screening, and managemen. Curr Gastroenterol Rep 2019;21:36. [DOI] [PubMed] [Google Scholar]
- [19].Sung JJY, Chiu HM, Jung KW, et al. Increasing trend in young-onset colorectal cancer in asia: more cancers in men and more rectal cancers. Am J Gastroenterol 2019;114:322–9.. [DOI] [PubMed] [Google Scholar]
- [20].Lan YT, Yang SH, Li AF, et al. Conflicting finding on intramucosal colon cancers based on national survival outcomes data. J Clin Oncol 2010;28:e469.author reply e470. [DOI] [PubMed] [Google Scholar]
- [21].Shinhata H, Yamamoto H, Sunada K, et al. Advanced rectal carcinoma caused by tumor cell implantation after curative endoscopic submucosal dissection of an intramucosal rectal carcinoma. Endoscopy 2015;47: Suppl 1 UCTN: E192–4.. [DOI] [PubMed] [Google Scholar]
- [22].Kojima M, Shimazaki H, Iwaya K, et al. Intramucosal colorectal carcinoma with invasion of the lamina propria: a study by the Japanese Society for Cancer of the Colon and Rectum. Hum Pathol 2017;66:230–7.. [DOI] [PubMed] [Google Scholar]
- [23].MacDonald AW, Tayyab M, Arsalani-Zadeh R, et al. Intramucosal carcinoma on biopsy reliably predicts invasive colorectal cancer. Ann Surg Oncol 2009;16:3267–70.. [DOI] [PubMed] [Google Scholar]
- [24].Gunderson LL, Jessup JM, Sargent DJ, et al. categorization for colon cancer based on national survival outcomes data. J Clin Oncol 2010;28:264–71.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bracey T, Mathew J. Metastatic intramucosal colorectal adenocarcinoma: a case to support review of current concepts (and staging) of early colorectal cancer. Histopathology 2015;66:906–7.. [DOI] [PubMed] [Google Scholar]
- [26].Mounzer R, Das A, Yen RD, et al. Endoscopic and surgical treatment of malignant colorectal polyps: a population-based comparative study. Gastrointest Endosc 2015;81:733–40.. e2. [DOI] [PubMed] [Google Scholar]
- [27].White A, Ironmonger L, Steele RJC, et al. A review of sex-related differences in colorectal cancer incidence, screening uptake, routes to diagnosis, cancer stage and survival in the UK. BMC Cancer 2018;18:906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lee JH, Lee JL, Park IJ, et al. Identification of recurrence-predictive indicators in stage I colorectal cancer. World J Surg 2017;41:1126–33.. [DOI] [PubMed] [Google Scholar]
- [29].Kotake K, Asano M, Ozawa H, et al. Gender differences in colorectal cancer survival in Japan. Int J Clin Oncol 2016;21:194–203.. [DOI] [PubMed] [Google Scholar]
- [30].Yang Y, Wang G, He J, et al. Gender differences in colorectal cancer survival: a meta-analysis. Int J Cancer 2017;141:1942–9.. [DOI] [PubMed] [Google Scholar]
- [31].Doubeni CA, Major JM, Laiyemo AO, et al. Contribution of behavioral risk factors and obesity to socioeconomic differences in colorectal cancer incidence. J Natl Cancer Inst 2012;104:1353–62.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Klabunde CN, Cronin KA, Breen N, et al. Trends in colorectal cancer test use among vulnerable populations in the United States. Cancer Epidemiol Biomarkers Prev 2011;20:1611–21.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Baghestani AR, Daneshvar T, Pourhoseingholi MA, et al. Survival of colorectal cancer patients in the presence of competing-risk. Asian Pac J Cancer Prev 2014;15:6253–5.. [DOI] [PubMed] [Google Scholar]
- [34].McKay A, Donaleshen J, Helewa RM, et al. Does young age influence the prognosis of colorectal cancer: a population-based analysis. World J Surg Oncol 2014;12:370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ommundsen N, Wyller TB, Nesbakken A, et al. Frailty is an independent predictor of survival in older patients with colorectal cancer. Oncologist 2014;19:1268–75.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Marventano S, Grosso G, Mistretta A, et al. Evaluation of four comorbidity indices and Charlson comorbidity index adjustment for colorectal cancer patients. Int J Colorectal Dis 2014;29:1159–69.. [DOI] [PubMed] [Google Scholar]
- [37].Taiwan cooperative Oncology group. Clinical guideline of colorectal cancer in Taiwan (in Chinese). Taiwan: National Health Research Institutes, Ed. 2010: 19-22. [Google Scholar]
- [38].Kang SI, Kim DW, Kwak Y, et al. The prognostic implications of primary tumor location on recurrence in early-stage colorectal cancer with no associated risk factors. Int J Colorectal Dis 2018;33:719–26.. [DOI] [PubMed] [Google Scholar]
- [39].Doubeni CA, Corley DA, Quinn VP, et al. Effectiveness of screening colonoscopy in reducing the risk of death from right and left colon cancer: a large community-based study. Gut 2018;67:291–8.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Rulyak SJ, Lieberman DA, Wagner EH, et al. Outcome of follow-up colon examination among a population-based cohort of colorectal cancer patients. Clin Gastroenterol Hepatol 2007;5:470–6.. quiz 407. [DOI] [PubMed] [Google Scholar]
- [41].Fisher DA, Jeffreys A, Grambow SC, et al. Mortality and follow-up colonoscopy after colorectal cancer. Am J Gastroenterol 2003;98:901–6.. [DOI] [PubMed] [Google Scholar]
- [42].Stolte M. The new Vienna classification of epithelial neoplasia of the gastrointestinal tract: advantages and disadvantages. Virchows Arch 2003;442:99–106.. [DOI] [PubMed] [Google Scholar]
- [43].Weiser MR. AJCC 8th Edition: colorectal cancer. Ann Surg Oncol 2018;25:1454–5.. [DOI] [PubMed] [Google Scholar]


