Supplemental Digital Content is available in the text
Keywords: biotin, thyroid function tests, diagnostic errors
Abstract
The aim of the study was to systematically characterize the interference of biotin on thyroid function tests and biotin washout periods.
Ten healthy adults were recruited with administration of 5 and 10 mg/d biotin for 7 days. Analyte concentrations of thyroid function tests were measured at baseline prior to starting biotin and from 2 hours to 2 days after withdrawal of 5 and 10 mg/d biotin. The outcomes were compared the baseline with the several points after taking biotin at Roche cobas e602, Beckman UniCel DxI 800, and Abbott Architect 2000 immunoassay platforms, respectively.
Ingesting 5 or 10 mg/d of biotin for 7 days could produce positive or negative interference among the thyroid function tests at Roche cobas e602 and Beckman UniCel DxI 800 systems, but no interference on Abbott Architect 2000. Interference duration of 5 mg/d biotin for Roche cobas e602 and Beckman UniCel DxI 800 of thyroid function tests lasted for 8 hours, while 10 mg/d biotin interfered with Roche cobas e602 or Beckman UniCel DxI 800 for 1 day or 2 days.
This study provides valuable guidance on biotin washout periods at doses common in over-the-counter supplements necessary to avoid false assay results.
Trial registration: ChiCTR1800020472
1. Introduction
Biotin (vitamin B7 or H), a water-soluble vitamin, is an essential coenzyme involved in carbon dioxide transfer in carboxylase reactions. Biotin is present in many foods, including organ meats, egg yolk, some vegetables, and cow's milk.[1] The recommended daily intake of biotin is 30 μg/d and biotin deficiency is rare as the majority of diets contain enough biotin for this to be reached.[2] Biotin supplements at doses from 5 to 20 mg daily are used as a therapy for rare inherited conditions such as holocarboxylase synthetase deficiency and biotin transporter deficiency.[3] However, for the past several years, biotin has been marketed as an over the counter remedy for reducing hair loss or improving nails or skin condition. In fact, 15% to 20% of individuals in the United States report consuming biotin-containing dietary supplements.[4] There are limited data on biotin supplementation to treat dermatologic conditions, especially in patients with normal biotin levels.[5] The only medical evidence in support of megadoses of biotin (100–300 mg/d) applies to patients with primary or secondary progressive multiple sclerosis, in which clinical outcomes have been improved by using biotin as a complementary medicine.[6]
Biotin is generally believed to have favorable tolerability and safety to individuals, even when individuals received pharmacological doses up to 300 mg/d. The potential medical issue is that supraphysiologic biotin intakes can cause spurious results in streptavidin/biotin-based immunoassays used for routine clinical laboratory tests, such as circulating thyroid, steroid, polypeptide hormones, tumor markers, and vitamins.[7] Briefly, excessive biotin in a blood sample competes with biotinylated antibody, which produces falsely decreased hormone concentrations in sandwich immunoassays and falsely increased hormone concentrations in competitive immunoassays.[8] Interferences have been reported in laboratory assays and particularly for thyroid function tests.[9–13] This may lead to a missed diagnosis and potentially serious clinical implications.
At present, it has been recognized that biotin can interfere with thyroid immunoassays using the biotin–streptavidin interaction. However, the experiments involving the washout periods of biotin interference were all spiking studies. Biotin metabolites may still interfere with the biotin–avidin system.[14] Therefore, the washout periods of interference are still controversial. The aim of this study was to investigate the impact of biotin ingestion (5 and 10 mg/d) on the performance of thyroid function tests on Roche cobas e602 (Roche Diagnostics GmbH, Mannheim, Germany), Beckman UniCel DxI 800 (Beckman Coulter, Fullerton, CA, USA), and Abbott Architect 2000 immunoassay platforms (Abbott Diagnostics, Abbott Park, IL, USA), respectively.. The time from the last ingestion to the disappearance of biotin interference was also investigated.
2. Materials and methods
The study was approved by the Ethics Committee of China-Japan Union Hospital of Jilin University. Ten healthy adults were recruited by study announcement fliers, and written informed consents were obtained before participation.
2.1. Participants
The inclusion criteria were: healthy adults between the ages of 18 to 50; normal liver and renal function.
The exclusion criteria were: pregnant or lactating; having known thyroid disease; working the night shift; history of smoking or alcohol abuse; having been treated with any medicine (including dietary or nutritional supplements) or significant iodine intake in the past 1 months; thyroid ultrasonography suggestive of Graves disease, Hashimoto thyroiditis, subacute thyroiditis, and other diseases that may cause thyroid function changes.
2.2. Intervention
Participants were scheduled to take 5 mg of biotin (Nature Made) orally at 7:00 am for 7 consecutive days. The process of blood samples’ collection is shown in Figure 1A. The blood specimens were collected by nurses at the department of endocrinology using an inert separation gel vacuum procoagulant collective tube with a volume of 5 mL. The gel tubes were allowed to clot for 30 minutes at room temperature (22–24°C) before centrifugation at 4000 rpm for 5 minutes (with a centrifuge radius of 15 cm). Serum after centrifugation was collected in polypropylene tubes identified by identification number and date, which were stored at −80°C until further analysis. After 2 weeks of biotin discontinuation, the participants took 10 mg/d of biotin again at 7:00 am for 7 consecutive days. The process of blood samples collection is shown in Figure 1B. After the last sample was taken, every sera was shipped to 3 different automated platforms (Roche cobas e602 and Abbott Architect 2000 in nuclear medicine department, Beckman UniCel DxI 800 in clinical Laboratory) for evaluation of thyroid-stimulating hormone (TSH), total triiodothyronine (T3), total thyroxine (T4), free T3, free T4, anti-TSH receptor (anti-TSHR), antithyroid peroxidase antibodies (anti-TPO), anti-thyroglobulin (anti-Tg), and Tg. All tests on the same automated platform were operated by the same experienced laboratory technologist. All assays were performed using calibrators and controls for each corresponding manufacturer's instructions (see Supplementary Table 1, which illustrates the internal quality control of the 3 automated platforms). The clinical laboratory technologists were blinded to the nature of the study.
Figure 1.
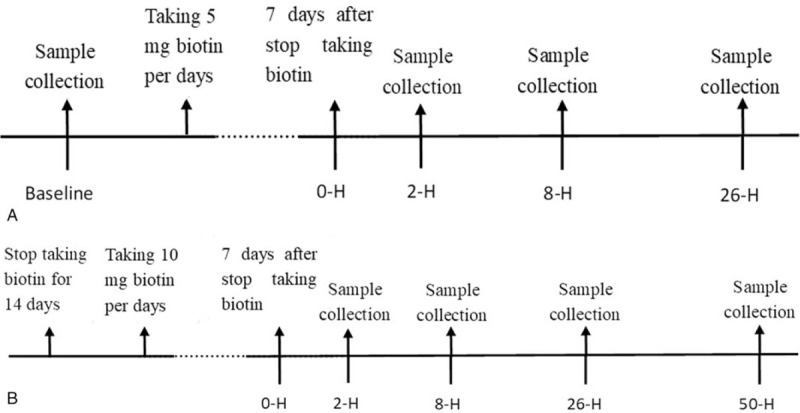
Flow diagram of sample collection before and after taking biotin. 2-H = 2 hours postdose, 8-H = 8 hours postdose, 26-H = 26 hours postdose, 50-H = 50 hours postdose.
2.3. Statistical analysis
Date analysis was performed with SPSS statistical analysis software (version 24.0; IBM-SPSS, Inc, Chicago, IL). Mixed linear model was used to evaluate different combinations of an analyte and a system, where the random effect was participant and the within-participant fixed effect was time. For each combination of an analyte and a system, a post hoc between-group comparison according to different time points was conducted using the LSD test. P < .05 was considered with statistical significance.
3. Results
A total of 10 healthy adults (5 women and 5 men) were included in our study between December 2018 and January 2019. The median age was 29 years (range 21–37 years). The baseline levels of thyroid immunoassays in all participants were within the manufacturer's reference ranges for 3 systems, except for 4 analytes: free T3 by Beckman UniCel DxI 800 (6.76 pmol/L, reference range 3.28–6.47 pmol/L); total T3 by Beckman UniCel DxI 800 (2.68 nmol/L, reference range 1.01–2.48 nmol/L); anti-Tg by Abbott Architect 2000 (4.67 IU/mL, reference range 0–4.11 IU/mL); Tg by Roche cobas e602 (2.64 ng/mL, reference range 3.5–77 ng/mL). These abnormal values were slightly out of the normal range and for each analyte, existed in no more than 1 system.
3.1. Roche cobas e602
The changes of thyroid function tests after ceasing biotin at various sample collection time points for Roche cobas e602 are shown in Figure 2. All indexes were compared with baseline, which was before starting taking biotin.
Figure 2.
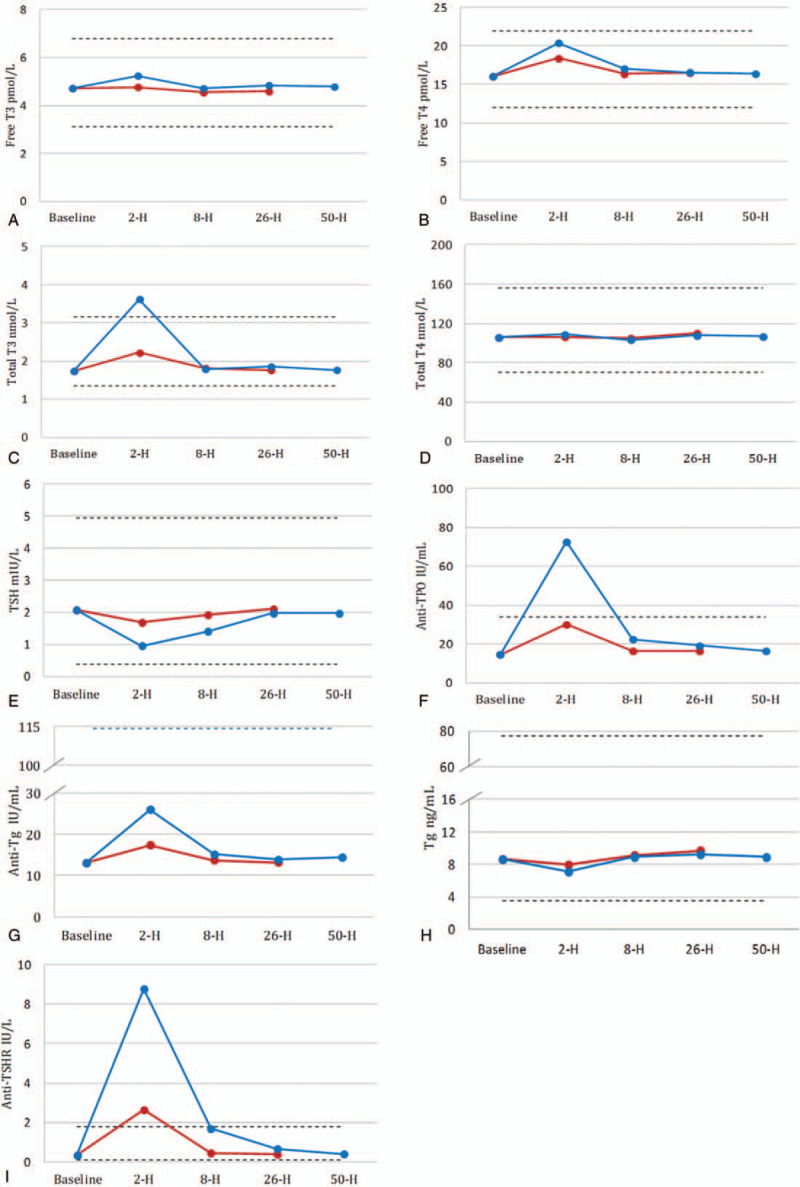
Changes in mean concentration of thyroid function tests on Roche cobas e602 before and after the withdrawal of biotin from 10 participants. The red polylines represented changes before and after taking 5 mg of biotin; the blue polylines represented changes before and after taking 10 mg of biotin; the dotted lines represent the lower and the upper reference range for each assays. Anti-TPO = antithyroid peroxidase antibodies, anti-TSHR = TSH receptor antibody, T3 = total triiodothyronine, T4 = total thyroxine, Tg = thyroglobulin.
Two hours after ceasing 5 mg biotin, the levels of free T4, total T3, anti-TPO, anti-Tg, and anti-TSHR significantly increased (all P < .001), while the level of TSH significantly decreased (P = .005). Two hours after ceasing 10 mg biotin, the levels of free T3, free T4, total T3, anti-TPO, anti-Tg, and anti-TSHR significantly increased (all P < .001), while the levels of TSH and Tg significantly decreased (all P < .001).
Eight hours after ceasing 10 mg biotin, the levels of free T4, anti-TPO, and anti-TSHR significantly increased (all P < .05), while the level of TSH significantly decreased (P < .001).
Twenty-six and 50 hours after ceasing 5 or 10 mg biotin, all thyroid function tests were not significantly different from the baseline.
3.2. Beckman DxI 800
The changes of thyroid function tests after ceasing biotin at various sample collection time points for Beckman DxI 800 are shown in Figure 3. All indexes were compared with baseline, which was before starting taking biotin.
Figure 3.
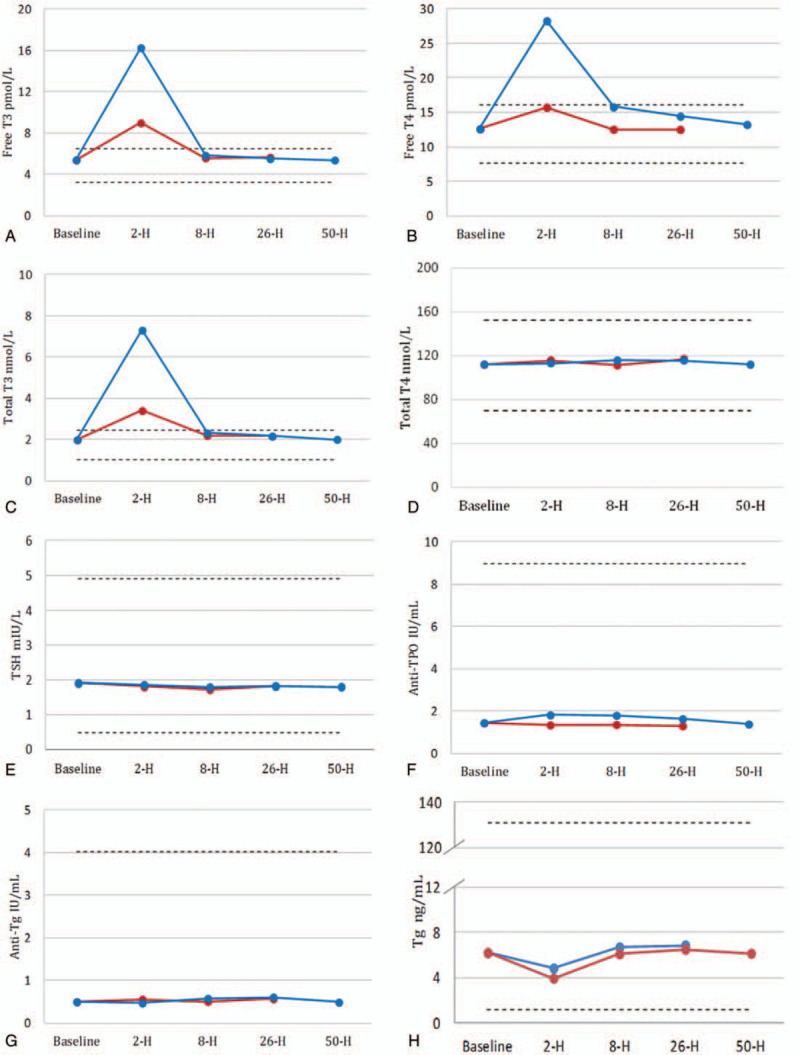
Changes in mean concentration of thyroid function tests on Beckman UniCel DxI 800 before and after the withdrawal of biotin from 10 participants. Anti-thyroid-stimulating hormone receptor (anti-TSHR) is no available in the Beckman UniCel DxI 800 test menu. Anti-TPO = antithyroid peroxidase antibodies, T3 = total triiodothyronine, T4 = total thyroxine, Tg = thyroglobulin.
Two hours after ceasing 5 mg biotin, the levels of free T3, free T4, and total T3 increased (all P < .001). Two hours after ceasing 10 mg biotin, the levels of free T3, free T4, total T3, and anti-TPO increased (all P < .001), while the levels of Tg decreased (all P < .001).
Eight and 26 hours after ceasing 10 mg biotin, the levels of free T4, anti-TPO increased (all P < .05).
Fifty hours after ceasing 5 or 10 mg biotin, all thyroid function tests were not significantly different from the baseline.
3.3. Abbott architect 2000
The changes of thyroid function tests after ceasing biotin at various sample collection time points for Abbott Architect 2000 are shown in Figure 4. All thyroid function tests were not significantly different from the baseline for either 5 or 10 mg biotin.
Figure 4.
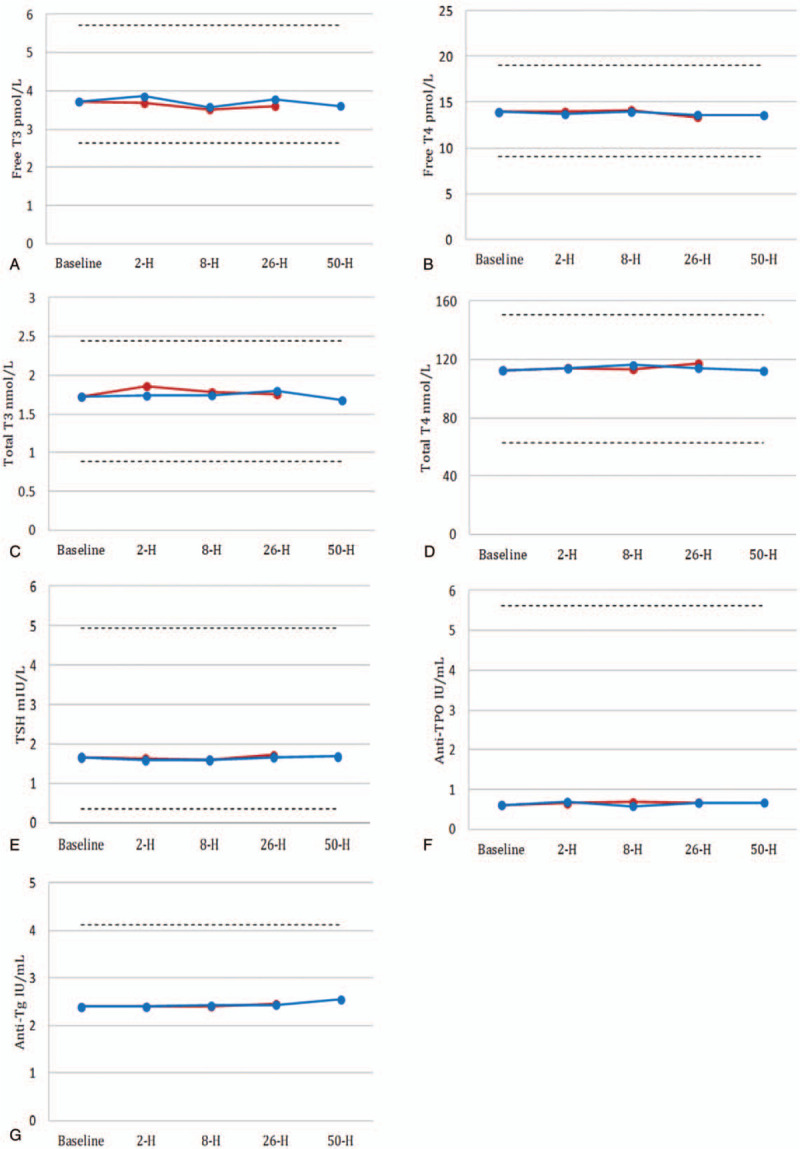
Changes in mean concentration of thyroid function tests on Abbott Architect 2000 before and after the withdrawal of biotin from 10 participants. Tg and anti-TSHR are no available in the Abbott Architect 2000 test menu. To convert free T3 from pg/mL to pmol/L, multiply by 1.54; free T4 from ng/dL to pmol/L, multiply by 12.871; total T3 from ng/mL to nmol/L, multiply by 1.54; total T4 from μg/dL to nmol/L, multiply by 12.871. Anti-TPO = antithyroid peroxidase antibodies, anti-TSHR = thyroid-stimulating hormone receptor antibody, T3 = total triiodothyronine, T4 = total thyroxine, Tg = thyroglobulin.
4. Discussion
This study of 1 healthy volunteers confirmed that thyroid function tests were interfered by oral biotin. This interference was present in some but not all assays and analysis about thyroid immunoassays (Table 1). In the case of competitive immunoassays based on biotin–streptavidin interaction, such as free T3, free T4, total T3, anti-TPO assays on Beckman UniCel DxI 800 and free T3, free T4, total T3, anti-TSHR, anti-TPO, anti-Tg assays on Roche cobas e602, excessive biotin in the blood sample competed with the biotinylated immunocomplex for binding to the streptavidin-coated magnetic particles resulting in falsely high values. Meanwhile, for the sandwich assay format, biotin in the sample displaced biotinylated antibodies resulting in falsely low results. This could be seen for the Beckman UniCel DxI 800 Tg and Roche cobas e602 TSH, Tg. For the free T3, free T4, total T3 concentrations, biotin interfered with the Beckman UniCel DxI 800 assay systems more strongly than the Roche cobas e602 systems. The Beckman DxI free T3, free T4, total T3 results were significantly higher than their respective reference ranges after oral 10 mg/d biotin for 1 week in healthy people. For anti-TSHR, anti-TPO, anti-Tg, the interference of biotin on Roche cobas e602 was greater.
Table 1.
Biotin interference in thyroid function tests performed by three automated clinical assay systems.
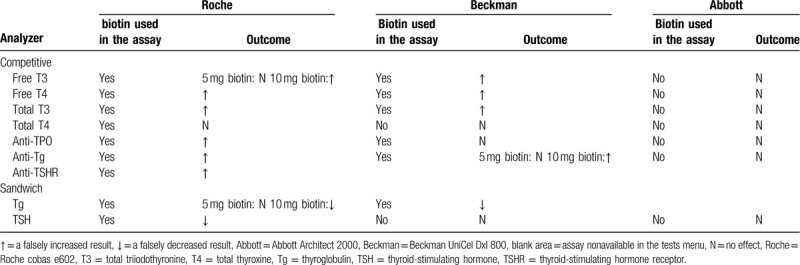
However, the Roche cobas e602 total T4 biotinylated assay was not affected by oral administration of 5 and 10 mg/d biotin in this study, which has been reported for biotin interference.[15] The pharmacokinetic graphs after taking 5 and 10 mg/d biotin is shown in Figure 5, according to the pharmacokinetics model described by Grimsey and colleagues. It was observed that the residual biotin serum concentration was 32.3 or 75 ng/mL after 2 hours of 5 or 10 mg biotin intake following consecutive dosing over several days.[16] But Roche cobas package pointed out that the biotin interference threshold for total T4 is 100 ng/mL.[17] We speculated that higher doses of biotin may have an effect in the result. No interference was seen for the Beckman UniCel DxI 800 TSH, total T4, and Abbott Architect assay about all the thyroid immunoassays as biotin–streptavidin interaction was not used in the assay format.[18,19]
Figure 5.
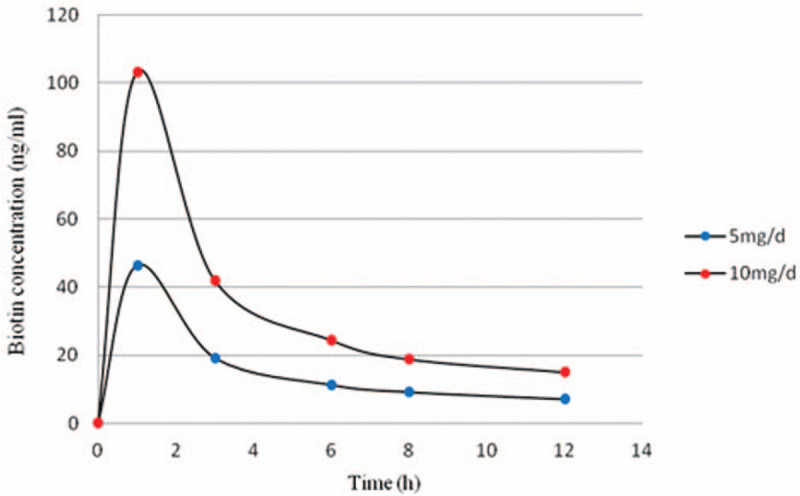
Biotin concentration in serum with time following its administration, from Grimsey et al.[16]
Biotin interference is particularly worrisome when the treating physician does not know whether the patient was taking high doses of biotin or not. First, the positive and negative interference of biotin in the Roche Diagnostics thyroid immunoassays paint a picture of Graves disease in patients. There have been a large number of reports with regard to misdiagnosed Graves disease due to biotin interference.[11] Second, falsely elevated free T3 and free T4 levels associated with normal TSH level may be misdiagnosed as rare causes such as central hyperthyroidism or thyroid hormone resistance, resulting in unnecessary as well as costly investigations.[20] Finally, a biotin-related underestimation of Tg result poses a challenge in patients with thyroid cancer in whom Tg level may aid in determining persistent or recurrent disease.[21] Therefore, it is important to know the washout periods of this potential assay interference.
The washout period of biotin interference with laboratory tests is closely related to the dosage of biotin. The Roche cobas package insert recommends that samples should not be drawn from patients with oral biotin more than 5 mg/d within 8 hours following the last administration.[22] In this study, we demonstrated that 8 hours of washout period was sufficient to remove the risk of biotin interference for patients taking 5 mg biotin per day (Supplementary Table 2). However, for patients taking biotin doses of up to 10 mg/d, falsely abnormal thyroid immunoassays levels could return to normal 1 day after ceasing biotin on Roche cobas e602 and 2 days on Beckman UniCel DxI 800 (Supplementary Table 3). Taking a larger dose of biotin would require even longer periods of cessation. One study showed a single oral dose of 30 mg biotin may persistently interfere with free T4, free T3, and Tg on Beckman DxI lasted for 24 hours.[23] Grimsey and colleagues reported that to avoid the risk of false assay results, a 73 hours washout period was sufficient following biotin intake with daily doses ≥10 mg for Roche Diagnostics system.[16] Kummer and colleagues reported that anti-TSHR abnormalities disappeared after withdrawing the vitamin for up to 7 days in some patients taking 2 to 15 mg/kg/d biotin.[9] Clearly, further investigation is required to clarify washout periods needed for a range of biotin doses according to different diagnostic assay systems.
Streptavidin-biotin based technology is not only used for thyroid function tests but also widely for routine clinical laboratory tests, such as parathyroid, cortisol, oestradiol, progesterone, testosterone, prolactin, C-peptide, insulin, hCG, and so on.[24] The FDA has warned that high doses of biotin can interfere with lab tests, and even reported the death of 1 patient who took supraphysiologic dose of biotin that presents a falsely low troponin test result.[25] At the same time, this methodology is extensively used in routine immunoassays and multiple platforms, including: all Roche Elecsys immunoassays; the Ortho Clinical Diagnostics Vitros platform; Dimension Vista, Centaur, Immulite platforms (Siemens Healthineers); Access, DxI, and DxC (Beckman Coulter); and Isys platforms (Immuno Diagnostic System).[24,26] However, different instruments have different sensitivities to biotin interference due to different signal detection methods and other assay design considerations.[27] Notably, Abbott Architect systems do not utilize the biotin–streptavidin capture method during the sample testing process.[19] Above all, this methodology is widely used in laboratory assays and automated platform. However, patients and physicians may be unaware of biotin interference in laboratory assays. Therefore, clinicians and laboratory professionals need to further strengthen the understanding of biotin interference and keep close collaboration to reduce misdiagnosis and waste of medical resources.
According to our search, there are few in vivo studies about the washout period of biotin on thyroid function tests. This study has several limitations. First, we did not explore other immunoassays and other automated platforms which also use the streptaxidin–biotin interaction. Moreover, the study population was limited, which did not include patients with hepatic and kidney disease or thyroid dysfunction. Finally, the sample size was small. Because the study was designed with the method of repeated measurements eliminating the variability among people, and the biotin ingestion-associated changes were relatively obvious, this small sample size could still indicate the interference. At present, there is only 1 in vivo experiment on biotin interference, and the sample size of this experiment is only 6 cases.[28] Future research is needed to develop recommendation in the biotin washout periods for different laboratory tests which used biotin technology.
5. Conclusion
In summary, this preliminary study provides guidance for patients using biotin on thyroid function tests in 3 different platforms. According to our results, Abbott Architect platform could be recommended for patients irrespective of biotin withdrawal. Roche cobas e602 platform should be recommended for patients after ceasing 5 to 10 mg/d biotin at least 1 day, and Beckman UniCel DxI 800 platform for at least 2 days.
Author contributions
Conceptualization: Renjie Wang.
Data curation: Ying Dong, Guanning Huang.
Formal analysis: Youjia Zhang, Ying Dong.
Funding acquisition: Guanning Huang, Qing Wang.
Investigation: Youjia Zhang, Guanning Huang, Bin Ji.
Methodology: Youjia Zhang, Renjie Wang, Qing Wang.
Project administration: Bin Ji.
Resources: Youjia Zhang, Renjie Wang, Qing Wang.
Software: Ying Dong, Qing Wang.
Validation: Renjie Wang, Ying Dong, Guanning Huang.
Visualization: Bin Ji.
Writing – review & editing: Youjia Zhang, Qing Wang.
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: Anti-Tg = antithyroglobulin antibodies, anti-TPO = antithyroid peroxidase antibodies, anti-TSHR = TSH receptor antibody, T3 = total triiodothyronine, T4 = total thyroxine, Tg = thyroglobulin, TSH = thyroid-stimulating hormone.
How to cite this article: Zhang Y, Wang R, Dong Y, Huang G, Ji B, Wang Q. Assessment of biotin interference in thyroid function tests. Medicine. 2020;99:9(e19232).
YZ and RW contributed equally to the work and share the first authorship.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Said HM. Biotin: biochemical, physiological and clinical aspects. Subcell Biochem 2012;56:1–9. [DOI] [PubMed] [Google Scholar]
- [2].Institute of Medicine Standing Committee on the Scientific Evaluation of Dietary Reference I, its Panel on Folate OBV Choline. The National Academies Collection: Reports funded by National Institutes of Health. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington, DC: National Academies Press (US), National Academy of Sciences; 1998. [PubMed] [Google Scholar]
- [3].Wolf B. Clinical issues and frequent questions about biotinidase deficiency. Mol Genet Metab 2010;100:6–13. [DOI] [PubMed] [Google Scholar]
- [4].Zempleni J, Wijeratne SS, Hassan YI. Biotin. BioFactors (Oxford, England) 2009;35:36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lipner SR. Rethinking biotin therapy for hair, nail, and skin disorders. J Am Acad Dermatol 2018;78:1236–8. [DOI] [PubMed] [Google Scholar]
- [6].Sedel F, Papeix C, Bellanger A, et al. High doses of biotin in chronic progressive multiple sclerosis: a pilot study. Mult Scler Relat Disord 2015;4:159–69. [DOI] [PubMed] [Google Scholar]
- [7].Samarasinghe S, Meah F, Singh V, et al. Biotin interference with routine clinical immunoassays: understand the causes and mitigate the risks. Endocr Pract 2017;23:989–98. [DOI] [PubMed] [Google Scholar]
- [8].Piketty ML, Polak M, Flechtner I, et al. False biochemical diagnosis of hyperthyroidism in streptavidin-biotin-based immunoassays: the problem of biotin intake and related interferences. Clin Chem Lab Med 2017;55:780–8. [DOI] [PubMed] [Google Scholar]
- [9].Kummer S, Hermsen D, Distelmaier F. Biotin treatment mimicking Graves’ disease. N Engl J Med 2016;375:704–6. [DOI] [PubMed] [Google Scholar]
- [10].Trambas CM, Sikaris KA, Lu ZX. More on biotin treatment mimicking Graves’ disease. N Engl J Med 2016;375:1698–9. [DOI] [PubMed] [Google Scholar]
- [11].Elston MS, Sehgal S, Du Toit S, et al. Factitious Graves’ disease due to biotin immunoassay interference-a case and review of the literature. J Clin Endocrinol Metab 2016;101:3251–5. [DOI] [PubMed] [Google Scholar]
- [12].De Roeck Y, Philipse E, Twickler TB, et al. Misdiagnosis of Graves’ hyperthyroidism due to therapeutic biotin intervention. Acta Clin Belg 2018;73:372–6. [DOI] [PubMed] [Google Scholar]
- [13].Batista MC, Ferreira CES, Faulhaber ACL, et al. Biotin interference in immunoassays mimicking subclinical Graves’ disease and hyperestrogenism: a case series. Clin Chem Lab Med 2017;55:e99–103. [DOI] [PubMed] [Google Scholar]
- [14].Meany DL, Jan de Beur SM, Bill MJ, et al. A case of renal osteodystrophy with unexpected serum intact parathyroid hormone concentrations. Clin Chem 2009;55:1737–9. [DOI] [PubMed] [Google Scholar]
- [15].Minkovsky A, Lee MN, Dowlatshahi M, et al. High-dose biotin treatment for secondary progressive multiple sclerosis may interfere with thyroid assays. AACE Clin Case Rep 2016;2:e370–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Grimsey P, Frey N, Bendig G, et al. Population pharmacokinetics of exogenous biotin and the relationship between biotin serum levels and in vitro immunoassay interference. Int J Pharmacokinetics 2017;2:247–56. [Google Scholar]
- [17]. Roche Diagnostics. Cobas T4 assay package insert. Roche Diagnostics, GmbH 2013-05, V3.0 English. [Google Scholar]
- [18]. Access Immunoassay Product Sheets. Beckman Coulter Inc, Brea. 2016. [Google Scholar]
- [19]. Architect Immunoassay Product Sheets. Abbott Diagnostics, Abbott Park. 2016. [Google Scholar]
- [20].Lim SK, Pilon A, Guechot J. Biotin interferes with free thyroid hormone and thyroglobulin, but not TSH measurements using Beckman-Access immunoassays. Ann Endocrinol 2017;78:186–7. [DOI] [PubMed] [Google Scholar]
- [21].Barbesino G. Misdiagnosis of Graves’ disease with apparent severe hyperthyroidism in a patient taking biotin megadoses. Thyroid 2016;26:860–3. [DOI] [PubMed] [Google Scholar]
- [22].Roche Elecsys Method Sheets, Vol 2016. Manheim: Roche Diagnostics GmbH; 2016. [Google Scholar]
- [23].Wijeratne NG, Doery JCG, Lu ZX. Positive and negative interference in immunoassays following biotin ingestion: a pharmacokinetic study. Pathology 2012;44:674–5. [DOI] [PubMed] [Google Scholar]
- [24].Trambas C, Lu Z, Yen T, et al. Characterization of the scope and magnitude of biotin interference in susceptible Roche Elecsys competitive and sandwich immunoassays. Ann Clin Biochem 2018;55:205–15. [DOI] [PubMed] [Google Scholar]
- [25]. The FDA Warns that Biotin May Interfere with Lab Tests: FDA Safety Communication. November 28, 2017; https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm586505.htm. [Google Scholar]
- [26].Clerico A, Plebani M. Biotin interference on immunoassay methods: sporadic cases or hidden epidemic? Clin Chem Lab Med 2017;55:777–9. [DOI] [PubMed] [Google Scholar]
- [27].Ardabilygazir A, Afshariyamchlou S, Mir D, et al. Effect of high-dose biotin on thyroid function tests: case report and literature review. Cureus 2018;10:e2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Li D, Radulescu A, Shrestha RT, et al. Association of biotin ingestion with performance of hormone and nonhormone assays in healthy adults. JAMA 2017;318:1150–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


