Abstract
Schizophrenia (SCZ) is a chronic disability disorder related to oxidative stress. Glutathione S-transferase (GST) is a group enzyme that protects cells and tissues from oxidative stress damage. Among GSTs, GSTT1 and GSTM1 have well defined genetic polymorphisms. The purpose of our research was to explore the correlation between GSTT1 and GSTM1 polymorphism and SCZ risk in Chinese Han population.
A total of 650 subjects (386 SCZ patients and 264 healthy individuals) were included in this case–control designed study. The GSTT1 and GSTM1 polymorphisms were analyzed by multiplex polymerase chain reaction (PCR). We explored the relationship between these 2 polymorphisms and the risk of SCZ.
We found that the GSTT1 null genotype had a protective effect on the development of SCZ [odds ratio (OR) = 0.601, 95% confidence interval (95% CI) = 0.412–0.986, P = .031]. We also found that the combination of null genotypes of the GSTT1 and GSTM1 genes was made at a lower risk of SCZ (OR = 0.452, 95% CI = 0.238–0.845, P = .028). However, we found no correction between Positive and Negative Syndrome Scale score (PANSS) and GSTM1, GSST1 genotypes in SCZ patients.
Our finding revealed that GSTT1 null polymorphisms may be related to the reduced risk of SCZ in Chinese Han population, and this risk was further reduced with the combination of GSTT1 null polymorphisms and GSTM1 null polymorphisms.
Keywords: gene polymorphisms, glutathione S-transferases, oxidative stress, schizophrenia
1. Introduction
Schizophrenia (SCZ) is a chronic disability disorder with a lifetime prevalence estimate of 4 per 1000 individuals in developing countries.[1] SCZ is a complex polygenetic disorder with over 80% heritability.[2] To date, the underlying genetic and molecular mechanisms for SCZ are still not fully understood.
Increasing evidences support that the pathophysiology of SCZ involves oxidative stress.[3,4] Oxidative stress is general related to many disease, including vascular disease,[5] cancer,[6] neurodegenerative diseases,[7] and psychosis.[8] Oxidative stress refers to an increase in reactive oxygen species or a decrease in antioxidant capacity in cells.[4] Granulocyte colony-stimulating factor (G-CSF) is a growth factor that stimulates the proliferation, differentiation, and survival of myeloid hematopoietic cells.[9] More and more evidences show that G-CSF is easy to combine with its receptor through blood–brain barrier, and play a role in mobilizing hematopoietic stem cells and bone marrow mesenchymal stem cells, anti-apoptotic, anti-inflammatory, promoting neuron differentiation and angiogenesis.[10,11] Abnormal oxidative stress parameters in patients with SCZ have been found in blood,[12] platelets,[13] cerebrospinal fluid,[14] and red blood cells.[15]
Glutathione S-transferases (GSTs) are classified as α, μ, ω, π, θ, and ζ, and they are encoded by GSTA, GSTM, GSTO, GSTP, GSTT, and GSTZ genes, respectively.[16] GSTs are phase II biotransferase, involved in detoxification of various toxicants.[17] GSTT1 (OMIM: 600436) and GSTM1 (OMIM: 138350) have definite genetic polymorphisms.[18–21] There are studies showing that the frequencies of GSTT1 and GSTM1 genes variants are different among ethnic groups.[22,23] The genotype distributions of GSTT1 genes as well as GSTM1 genes variants in Chinese populations are similar to those of Korean populations,[24,25] but were different from the Europeans, American, and Africans.[26,27]
GSTT1 null genotype and GSTM1 null genotype could lead to complete absence of the enzyme,[28] further increasing the risks of various diseases, such as diabetes,[29] male infertility,[30] hypertension,[31] and some types of cancer.[22–34] To date, many researches have indicated the correlation between SCZ and gene polymorphisms of GSTT1, GSTM1.[35–38] However, the results were inconsistent. We found that there were few studies where the relationship between GSTT1, GSTM1 gene polymorphisms, and SCZ among Chinese people was investigated. Therefore, a case–control designed study, including 386 SCZ patients and 264 healthy individuals, was conducted to explore the relationship between susceptibility to SCZ among Chinese Han population and GSTT1 and GSTM1 polymorphism.
2. Materials and methods
2.1. Subjects
A total of 386 patients with SCZ (SCZ group) and 264 healthy individuals (control group) were included in our study. All subjects were between 18 and 80 years old. The demographic characteristics of the subjects are summarized in Table 1. Three hundred eighty-six SCZ patients were recruited from Shandong Mental Health Center. Diagnosis were assessed by at least 2 psychiatrists according to the Diagnostic and Statistical Manual of mental disorders (DSM)-5 criteria.[39] Moreover, Positive and Negative Syndrome Scale (PANSS) was used to assess the severity of SCZ.[40] These 386 SCZ patients had no other mental disorder, including bipolar disorder (BD), major depression disorder (MDD), substance abuse, or mental retardation. The study was approved by the Ethics committee of Shandong Mental Health Center. Informed consent were obtained from all subjects.
Table 1.
Characteristic data of SCZ patients and controls.
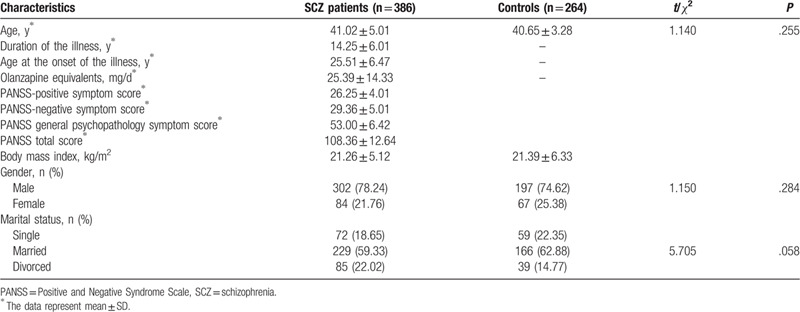
The control individuals were recruited in the same geographic area. They had no history of mental disorder including SCZ, MD, BD, substance abuse. Moreover, those who had a first-degree relative with SCZ were excluded. Their current psychological status and medical history were evaluated through unstructured interview.
2.2. Genotyping of GSTT1 and GSTM1 polymorphisms
For genotyping, 5 mL cubital venous blood was drawn into an ethylenediamine tetraacetic acid (EDTA) tube. Gnomic DNA was isolated from whole blood by DNA extraction kit (Jianlun Biotechnology Co., Ltd, Guangzhou, China; No. JL45852). DNA purity was determined by Epoch microplate spectrophotometer (Bio Tek). In this study, we used multiplex PCR assay to assess the null or presence of GSTT1 and GSTM1 gene null polymorphism. The β-globin gene was used as an internal reference. The amplification reaction was conducted at the final volume of 20 μL, containing 7 μL diethyl pyrocarbonate (DEPC) water, 1 μL DNA, 1 μL primer (10 μmol/L), and 10 μL Taq polymerase master mix (Hengfei Biotechnology Co., Ltd, Shanghai, China; No. PCR-TAQ-MX-1). PCR was done using Veriti Dx Thermal Cycler (ABI). The amplification condition in our study was as follows: introductory denaturation for 300 seconds at 94°C, amplification for 30 cycles, denaturation for 60 seconds at 92°C, annealing at 60°C for 0.5 minutes, and final extension at 72°C for 300 seconds. Electrophoretic amplification of PCR products was performed on 2% agarose gel (Huazhong Haiwei Gene Technology Co., Ltd, Beijing, China; No. S2016) and stained with ethidium bromide (Zhijie Fangyuan Technology Co., Ltd, Beijing, China; No. Amresco 0492). In this study, we took the gel imagings under UV transilluminator (Cleaver, UK). The primer sequences and the sizes of PCR products are summarized in Table 2 and Fig. 1, respectively.
Table 2.
Primer sequencing for GSTT1, GSTM1, and β-globin and amplicon size.

Figure 1.
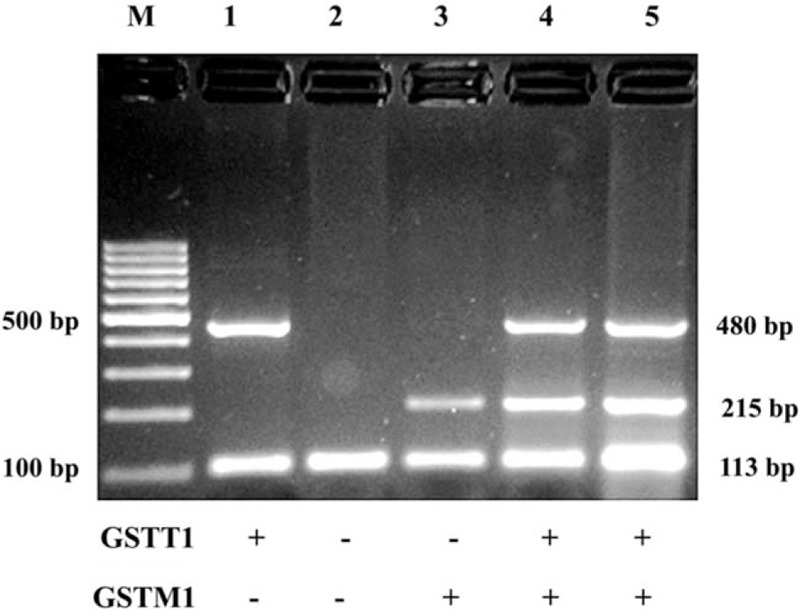
Genotyping of GSTT1, GSTM1, and β-globin polymorphisms. There are 4 types of genotype showed as null genotypes of GSTT1 (−) and GSTM1 (−) and functional GSTT1 (+) and GSTM1 (+).
2.3. Statistical analysis
SPSS 19.0 was used for statistical analyses. Comparison between SCZ group and controls group were performed using Student t test and χ2 test. In order to evaluate the combined effects of GSTT1 and GSTM1, we used logistic regression analysis. All tests in this study were 2-tailed. P < .05 was statistically significant.
3. Results
A total of 650 subjects were included in present research. Three hundred eighty-six were patients with SCZ who aged 41.02 ± 5.01 years, and 264 were healthy individuals aged 41.02 ± 5.01 years. There was no statistical difference in demographic data between SCZ patients and healthy individuals (P > .05).
In our study, Hardy–Weinberg test was not performed to GSTT1 and GSTM1 genotypes, due to that multiplex PCR assay could not distinguish the heterozygous presence of the allele (null/presence) from the homozygous presence. Nevertheless, the distributions of GSTT1-null and GSTM1-null polymorphism in our study were not different from the previous studies in Chinese Han population.[41–43]
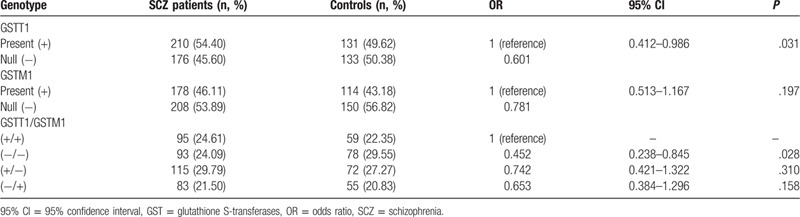
Genotype frequencies of SCZ patients and healthy individuals are summarized in Table 3. The frequencies of GSTM1 null genotype were 53.89% and 56.82% for SCZ patients and healthy individuals, respectively. The frequencies of GSTT1 null genotype were 45.60% and 50.38% for SCZ patients and healthy individuals, respectively. The GSTT1 null polymorphism showed a reduced risk of SCZ (P = .031). However, the GSTM1 null genotype tended to reduce the risk of SCZ, but there was no significant difference (P = .197). Furthermore, we found that 24.09% of SCZ patients and 29.55% of healthy individuals carried both null genotypes of GSTT1 and GSTM1 genes, which reduced the risk of SCZ (P = .028).
Table 3.
Genotype distribution of GSTM1 and GSTT1 in SCZ patients and controls.
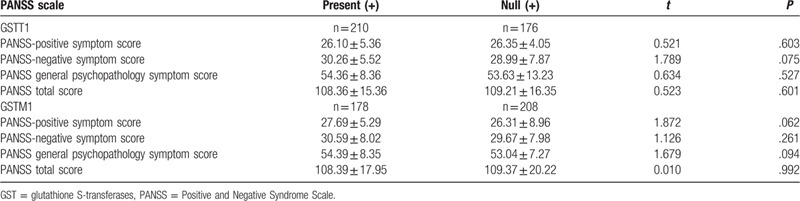
We found no association between PANSS score and GSTM1, GSST1 genotypes in SCZ patients (Table 4).
Table 4.
PANSS score (mean ± SD) according to GSTM1 and GSST1 genotypes in SCZ patients.
4. Discussion
SCZ is a complex polygenetic disorder. Increasing evidences support that the pathophysiology of SCZ involves oxidative stress.[3,4] The relationship between GST gene polymorphism and SCZ susceptibility had been studied in many previous studies, but the results were inconclusive. GSTT1 (OMIM: 600436) and GSTM1 (OMIM: 138350) have definite genetic polymorphisms.[18–21] In our research, we evaluated the relationship between SCZ susceptibility and polymorphism of GSTT1 and GSTM1. Our research is first to explore the relationship between these 2 genetic polymorphisms and SCZ in Chinese Han population. We found that GSTT1 null polymorphism may be related to reduce the risk of SCZ in Chinese people. Furthermore, when GST1 null polymorphisms and GSTM1 null polymorphisms are combined, the risk of SCZ was decreased.
Our results indicated that GSTT1 active genotype was associated with SCZ. Previous study has shown that GSTT1 gene maps on region 2qq11.2, and this gene is associated with susceptibility to SCZ by genome scanning analysis.[44] Moreover, several reports showed that GSTT1 gene increased the risk of certain types of cancer.[32–34,45] However, there is not a common rule, the disease protection effect of GSTT1 null genotype has also been found.[29,46] GSTT1 is associated with the metabolism of detoxification. In mammals, the empty genotype encoding GSTT1 protein leads to a lack of active protein,[47] reduces the detoxification ability of specific substrate induced by oxidative stress damage, and increases sensitivity to oxidative DNA damage.[48] Previous studies have also found that SCZ patients had a higher proportion of GSTT1 enzymes than healthy individuals.[44] The fact that GSTT1 active genotype is a risk factor for SCZ has been confirmed in many previous studies.[35,37,49] In this study, the null genotype proportion of GSTT1 were 45.60% and 50.38% for SCZ patients and healthy individuals, respectively. The results indicated that GSTT1 could be a candidate gene for predisposing to SCZ in Chinese Han population. The results of our study are supported by Raffa et al,[37] and they suggested that GSTT1 null genotype might decrease the risk of SCZ in Tunisian. However, Pejovic-Milovancevic et al[35] and Matsuzawa et al[50] have demonstrated that GSTT1 polymorphism is not associated with susceptibility to SCZ independently.
GSTM1 gene has also been emphasized in genetic epidemiologic studies in SCZ patients. GSTM1 null genotype is the result of a 1500 base pairs deletion. Previous study indicated that GSTM1 null genotype resulted in a complete lack of GSTM1 activity.[51] Thus, GSTM1 null genotype could lead to the decrease of oxidative stress. Present study showed that GSTM1 null genotype in SCZ patients and healthy individuals was 53.89% and 56.82%, respectively, although there was no statistical difference between the 2 groups. However, Harada et al[52] found that GSTM1 null genotype was more present in disorganized-subtype schizophrenia individuals than normal individuals.
Previous study of combined effects of GSTM1 genotypes and GSTT1 genotypes was still inconsistent. This present study showed that combined GSTM1-null/GSTT1-null genotypes may reduce the susceptibility to SCZ in Chinese Han population. However, most previous studies found that the combination of GSTT1 and GSTM1 polymorphisms (null or presence) was not related to SCZ.[36–38,50] These discrepancies may be due to the sample size of study and ethnicity.
We also studied the relationship between SCZ severity and GSTM1 polymorphisms as well as GSTT1 polymorphisms. However, Student t test showed that there was no association between PANSS score and GSTM1 genotypes as well as GSST1 genotypes in SCZ patients. Raffa et al[37] found that there was no significant correlation between GSTM1 polymorphisms, GSTT1 polymorphisms, and SCZ subtypes (Undifferentiated, Paranoid, Disorganized). These results indicated that GSTM1 polymorphisms and GSTT1 polymorphisms may only be associated with the susceptibility to SCZ, but not with severity and type.
The advantage of our study lies in its comparatively large sample size as well as genetic homogeneity of the subjects. The limitation of our research is that GST enzyme activity has not been measured. Therefore, there is no direct evidence that GSTT1 and GSTM1 polymorphisms alter enzyme activity. Besides, we did not examine whether treatment response and prognosis are associated with gene polymorphism.
SCZ is a complex polygenetic disorder. Here, we demonstrated that GSTT1 null polymorphisms may be related to decrease the risk of SCZ in Chinese Han population, and that the risk was further reducing with the combination of both GSTT1 null polymorphisms and GSTM1 null polymorphisms. The relationship between these 2 gene polymorphisms and drug response and disease prognosis needs to be further explored in the future.
Author contributions
XXXX.
Footnotes
Abbreviations: BD = bipolar disorder, DEPC = diethyl pyrocarbonate, DSM = diagnostic and Statistical Manual of mental disorders, EDTA = ethylenediamine tetraacetic acid, GSTs = Glutathione S-transferase, MDD = major depression disorder, PANSS = Positive and Negative Syndrome Scale, PCR = polymerase chain reaction, SCZ = Schizophrenia.
How to cite this article: Zhang X, Yang J, Liu X, Zhao G, Li X, Xun G. Glutathione S-transferase gene polymorphisms (GSTT1 and GSTM1) and risk of schizophrenia: A case–control study in Chinese Han population. Medicine. 2020;99:36(e21918).
Xin Zhang and Jinmei Yang contributed equally to this work.
The study was approved by the Ethics committee of Shandong Mental Health Center. Informed consent was obtained.
There are no potential conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
The datasets generated during and/or analyzed during the current study are publicly available.
References
- [1].Esan OB, Ojagbemi A, Gureje O. Epidemiology of schizophrenia: an update with a focus on developing countries. Int Rev Psychiatry 2012;24:387–92.. [DOI] [PubMed] [Google Scholar]
- [2].Foley C, Corvin A, Nakagome S. Genetics of schizophrenia: ready to translate? Curr Psychiatry Rep 2017;19:61. [DOI] [PubMed] [Google Scholar]
- [3].Solberg DK, Refsum H, Andreassen OA. A five-year follow-up study of antioxidants, oxidative stress and polyunsaturated fatty acids in schizophrenia. Acta Neuropsychiatr 2019;31:202–12.. [DOI] [PubMed] [Google Scholar]
- [4].Upthegrove R, Khandaker GM. Cytokines, oxidative stress and cellular markers of inflammation in schizophrenia. Curr Top Behav Neurosci 2019;44:49–66.. [DOI] [PubMed] [Google Scholar]
- [5].Kim YW, Byzova TV. Oxidative stress in angiogenesis and vascular disease. Blood 2014;123:625–31.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chikara S, Nagaprashantha LD, Singhal J, et al. Oxidative stress and dietary phytochemicals: role in cancer chemoprevention and treatment. Cancer Lett 2018;413:122–34.. [DOI] [PubMed] [Google Scholar]
- [7].Chen ZH, Xian JF, Luo LP. Association between GSTM1, GSTT1, and GSTP1 polymorphisms and gastric cancer risk, and their interactions with environmental factors. Genet Mol Res 2017;16: [DOI] [PubMed] [Google Scholar]
- [8].Barron H, Hafizi S, Andreazza AC, et al. Neuroinflammation and oxidative stress in psychosis and psychosis risk. Int J Mol Sci 2017;18:651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kojima H, Otani A, Oishi A, et al. Granulocyte colony-stimulating factor attenuates oxidative stress-induced apoptosis in vascular endothelial cells and exhibits functional and morphologic protective effect in oxygen-induced retinopathy. Blood 2011;117:1091–100.. [DOI] [PubMed] [Google Scholar]
- [10].Solaroglu I, Digicaylioglu M, Keles GE, et al. New missions for an old agent: granulocyte-colony stimulating factor in the treatment of stroke patients. Curr Med Chem 2015;22:1302–9.. [DOI] [PubMed] [Google Scholar]
- [11].Dwivedi P, Greis KD. Granulocyte colony stimulating factor receptor (G-CSFR) signaling in severe congenital neutropenia, chronic neutrophilic leukemia and related malignancies. Exp Hematol 2016;9: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bai ZL, Li XS, Chen GY, et al. Serum oxidative stress marker levels in unmedicated and medicated patients with schizophrenia. J Mol Neurosci 2018;66:428–36.. [DOI] [PubMed] [Google Scholar]
- [13].Dietrich-Muszalska A, Olas B, Rabe-Jablonska J. Oxidative stress in blood platelets from schizophrenic patients. Platelets 2005;16:386–91.. [DOI] [PubMed] [Google Scholar]
- [14].Lleo A, Parnetti L, Belbin O, et al. Has the time arrived for cerebrospinal fluid biomarkers in psychiatric disorders? Clin Chim Acta 2019;491:81–4.. [DOI] [PubMed] [Google Scholar]
- [15].Prabakaran S, Wengenroth M, Lockstone HE, et al. 2-D DIGE analysis of liver and red blood cells provides further evidence for oxidative stress in schizophrenia. J Proteome Res 2007;6:141–9.. [DOI] [PubMed] [Google Scholar]
- [16].Lee JY, Hwang IW, Lim MH, et al. Association of glutathione S-transferases M1, T1 and P1 gene polymorphisms with attention deficit and hyperactivity disorder in Korean children. Gene 2016;586:228–33.. [DOI] [PubMed] [Google Scholar]
- [17].Glisic B, Mihaljevic I, Popovic M, et al. Characterization of glutathione-S-transferases in zebrafish (Danio rerio). Aquat Toxicol 2015;158:50–62.. [DOI] [PubMed] [Google Scholar]
- [18].Ghorbel R, Ben Salah G, Ghorbel R, et al. Do GSTM1 and GSTT1 polymorphisms influence the risk of developing mitochondrial diseases in a Tunisian population? Environ Sci Pollut Res Int 2018;25:5779–87.. [DOI] [PubMed] [Google Scholar]
- [19].Kalacas NA, Garcia JA, Sy Ortin T, et al. GSTM1 and GSTT1 genetic polymorphisms and breast cancer risk in selected Filipino cases. Asian Pac J Cancer Prev 2019;20:529–35.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Singh MM, Kumar R. Association of GSTT1/GSTM1 and ApoE variants with left ventricular diastolic dysfunction in thalassaemia major patients. Hematology 2019;24:20–5.. [DOI] [PubMed] [Google Scholar]
- [21].Yu C, Hequn C, Longfei L, et al. GSTM1 and GSTT1 polymorphisms are associated with increased bladder cancer risk: evidence from updated meta-analysis. Oncotarget 2017;8:3246–58.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Saitou M, Ishida T. Distributions of the GSTM1 and GSTT1 null genotypes worldwide are characterized by latitudinal clines. Asian Pac J Cancer Prev 2015;16:355–61.. [DOI] [PubMed] [Google Scholar]
- [23].Senthilkumar KP, Thirumurugan R. GSTM1 and GSTT1 allele frequencies among various Indian and non-Indian ethnic groups. Asian Pac J Cancer Prev 2012;13:6263–7.. [DOI] [PubMed] [Google Scholar]
- [24].Uhm YK, Yoon SH, Kang IJ, et al. Association of glutathione S-transferase gene polymorphisms (GSTM1 and GSTT1) of vitiligo in Korean population. Life Sci 2017;81:223–7.. [DOI] [PubMed] [Google Scholar]
- [25].Wang D, Zhang LM, Zhai JX, et al. GSTM1 and GSTT1 polymorphisms and colorectal cancer risk in Chinese population: a meta-analysis. Int J Colorectal Dis 2012;27:901–9.. [DOI] [PubMed] [Google Scholar]
- [26].Gaspar PA, Hutz MH, Salzano FM, et al. Polymorphisms of CYP1a1, CYP2e1, GSTM1, GSTT1, and TP53 genes in Amerindians. Am J Phys Anthropol 2002;119:249–56.. [DOI] [PubMed] [Google Scholar]
- [27].Piacentini S, Polimanti R, Porreca F, et al. GSTT1 and GSTM1 gene polymorphisms in European and African populations. Mol Biol Rep 2011;38:1225–30.. [DOI] [PubMed] [Google Scholar]
- [28].Setiawan VW, Zhang ZF, Yu GP, et al. GSTT1 and GSTM1 null genotypes and the risk of gastric cancer: a case-control study in a Chinese population. Cancer Epidemiol Biomarkers Prev 2000;9:73–80.. [PubMed] [Google Scholar]
- [29].Cilensek I, Mankoc S, Petrovic MG, et al. GSTT1 null genotype is a risk factor for diabetic retinopathy in Caucasians with type 2 diabetes, whereas GSTM1 null genotype might confer protection against retinopathy. Dis Markers 2012;32:93–9.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hu CY, Lu DL, Wu T, et al. Glutathione-S-transferases M1/T1 gene polymorphisms and male infertility risk in Chinese populations: a meta-analysis. Medicine (Baltimore) 2019;98:e14166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Rong SL, Zhou XD, Wang ZK, et al. Glutathione S-Transferase M1 and T1 polymorphisms and hypertension risk: an updated meta-analysis. J Hum Hypertens 2019;33:454–65.. [DOI] [PubMed] [Google Scholar]
- [32].Chen L, Liu B. Relationships between stress granules, oxidative stress, and neurodegenerative diseases. Oxid Med Cell Longev 2017;2017:1809592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Hasan S, Hameed A, Saleem S, et al. The association of GSTM1 and GSTT1 polymorphisms with squamous cell carcinoma of cervix in Pakistan. Tumour Biol 2015;36:5195–9.. [DOI] [PubMed] [Google Scholar]
- [34].Saravani S, Miri-Moghaddam M, Bazi A. Association of glutathione-S-transferases M1 and T1 deletional variants with development of oral squamous cell carcinoma: a study in the South-East of Iran. Asian Pac J Cancer Prev 2019;20:1921–6.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Pejovic-Milovancevic MM, Mandic-Maravic VD, Coric VM, et al. Glutathione S-transferase deletion polymorphisms in early-onset psychotic and bipolar disorders: a case-control study. Lab Med 2016;47:195–204.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Pinheiro DS, Santos RDS, de Brito RB, et al. GSTM1/GSTT1 double-null genotype increases risk of treatment-resistant schizophrenia: a genetic association study in Brazilian patients. PLoS One 2017;12:e0183812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Raffa M, Lakhdar R, Ghachem M, et al. Relationship between GSTM1 and GSTT1 polymorphisms and schizophrenia: a case-control study in a Tunisian population. Gene 2013;512:282–5.. [DOI] [PubMed] [Google Scholar]
- [38].Saruwatari J, Yasui-Furukori N, Kamihashi R, et al. Possible associations between antioxidant enzyme polymorphisms and metabolic abnormalities in patients with schizophrenia. Neuropsychiatr Dis Treat 2013;9:1683–98.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Tandon R, Gaebel W, Barch DM, et al. Definition and description of schizophrenia in the DSM-5. Schizophr Res 2013;150:3–10.. [DOI] [PubMed] [Google Scholar]
- [40].Wolthaus JE, Dingemans PM, Schene AH, et al. Component structure of the positive and negative syndrome scale (PANSS) in patients with recent-onset schizophrenia and spectrum disorders. Psychopharmacology (Berl) 2000;150:399–403.. [DOI] [PubMed] [Google Scholar]
- [41].Liu K, Lin X, Zhou Q, et al. The associations between two vital GSTs genetic polymorphisms and lung cancer risk in the Chinese population: evidence from 71 studies. PLoS One 2014;9:e102372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Qi R, Gu Z, Zhou L. The effect of GSTT1, GSTM1 and GSTP1 gene polymorphisms on the susceptibility of age-related cataract in Chinese Han population. Int J Clin Exp Med 2015;8:19448–53.. [PMC free article] [PubMed] [Google Scholar]
- [43].Wang X, Li W, Liu W, et al. GSTM1 and GSTT1 gene polymorphisms as major risk factors for bronchopulmonary dysplasia in a Chinese Han population. Gene 2014;533:48–51.. [DOI] [PubMed] [Google Scholar]
- [44].Saadat M, Mobayen F, Farrashbandi H. Genetic polymorphism of glutathione S-transferase T1: a candidate genetic modifier of individual susceptibility to schizophrenia. Psychiatry Res 2007;153:87–91.. [DOI] [PubMed] [Google Scholar]
- [45].Rebbeck TR. Molecular epidemiology of the human glutathione S-transferase genotypes GSTM1 and GSTT1 in cancer susceptibility. Cancer Epidemiol Biomarkers Prev 1997;6:733–43.. [PubMed] [Google Scholar]
- [46].Guan L, Fan P, Liu X, et al. Association study between GSTT1 and GSTM1 polymorphisms and risk of preeclampsia in Chinese population. Eur J Obstet Gynecol Reprod Biol 2016;204:31–5.. [DOI] [PubMed] [Google Scholar]
- [47].Pemble S, Schroeder KR, Spencer SR, et al. Human glutathione S-transferase theta (GSTT1): cDNA cloning and the characterization of a genetic polymorphism. Biochemical Journal 1994;300:271–6.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Song Y, Shan Z, Luo C, et al. Glutathione S-transferase T1 (GSTT1) null polymorphism, smoking, and their interaction in coronary heart disease: a comprehensive meta-analysis. Heart Lung Circ 2017;26:362–70.. [DOI] [PubMed] [Google Scholar]
- [49].Kim SK, Kang SW, Chung JH, et al. Genetic polymorphisms of glutathione-related enzymes (GSTM1, GSTT1, and GSTP1) and schizophrenia risk: a meta-analysis. Int J Mol Sci 2015;16:19602–11.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Matsuzawa D, Hashimoto K, Hashimoto T, et al. Association study between the genetic polymorphisms of glutathione-related enzymes and schizophrenia in a Japanese population. Am J Med Genet B Neuropsychiatr Genet 2009;150b:86–94.. [DOI] [PubMed] [Google Scholar]
- [51].de Oliveira E, de Aquino Castro R, Vieira Gomes MT, et al. Role of glutathione S-transferase (GSTM1) gene polymorphism in development of uterine fibroids. Fertil Steril 2009; 91:1496-1498. [DOI] [PubMed] [Google Scholar]
- [52].Harada S, Tachikawa H, Kawanishi Y. Glutathione S-transferase M1 gene deletion may be associated with susceptibility to certain forms of schizophrenia. Biochem Biophys Res Commun 2001;281:267–71.. [DOI] [PubMed] [Google Scholar]


