Abstract
Multidrug-resistant bacterial (MDRB) infections have been difficult to treat clinically. Tigecycline (TIG) has several advantages, especially in the treatment of severe infections. Many clinicians have considered increasing the TIG dose to improve the efficacy of this molecule. The safety and efficacy of high-dose TIG in elderly patients with MDRB infections were investigated in this study.
We conducted a retrospective analysis of the elderly patients with MDRB infections who were treated at the First Affiliated Hospital. A total of 106 patients received a conventional dose (CD-TIG group: 50 mg every 12 hours) of TIG and 51 received a high dose (HD-TIG group: 100 mg every 12 hours). The data from all patients were collected for examining the clinical features and performing the microbiological analysis. The safety profile and efficacy of the HD regimen were investigated.
The clinical efficacy and microbiological eradication in the patients with MDRB infection were higher in the HD-TIG group than the CD-TIG group. The independent predictors of clinical cure were the use of TIG at HD (odd ratio [OR], 5.129; 95% confidence interval [CI] [1.890, 13.921]; P = .001) and microbiological eradication (OR, 3.049; 95% CI, [1.251, 7.430]; P = .014). In the ventilator-associated pneumonia (VAP) and bloodstream infection (BSI) subgroups, the sole independent predictor of clinical cure was the HD of TIG, and no significant adverse events were observed. The occurrence of multidrug-resistant Acinetobacter baumannii infection and an MIC value of 1 to 2 g/mL for TIG were independently associated with clinical failure in the VAP subgroup.
HDs of TIG was found to associate with better clinical efficacy and microbiological eradication than its CDs in the elderly patients with MDRB infections. In the VAP and BSIs subgroups, administration of HDs of TIG was associated with better outcomes.
Keywords: adverse events, bloodstream infections, elderly, high-dose tigecycline, multidrug-resistance, ventilator-associated pneumonia
1. Introduction
With the widespread use of broad-spectrum antibiotics and immunosuppressants, the therapeutic management of multidrug-resistant bacterial (MDRB) infections is currently facing a grim situation.[1] According to the report of CHINET Antimicrobial Surveillance Network in 2018,[2] the detection rate of methicillin-resistant Staphylococcus aureus (MRSA) was 30.9% and the resistance rate of Enterococcus faecium (E.fm) for vancomycin was 1.4%. The resistance rate of Klebsiella pneumoniae (K.P.) for carbapenems was 10.1% in 2018, which was 1.1% higher than that in 2017. The resistance rate of Acinetobacter baumannii (A.B.) for carbapenems was 56.1% in 2018, the same as that in 2017. For the therapeutic management of MDRB infections, the commonly used antibiotics were found to be resistant or were associated with higher minimum inhibitory concentrations (MICs). Thus, these antibiotics were not effective as anti-infective agents, which resulted in increased mortality rates. Previous studies have shown that increased MIC is associated with high mortality rates.[3] Bochud et al[4] found that the rational use of early antimicrobial therapy could significantly reduce the overall mortality rate in patients with gram-negative bacterial infection and sepsis (49% vs 28%, P < .001). Elderly patients were observed to be more susceptible to contract MDRB infections due to their low immunity status, impaired liver and kidney function, that can affect the metabolizing of the antibiotics, and thereby, decreasing their therapeutic efficacy as anti-infective agents.[5] The improper treatment with antibiotics can lead to development of new antibiotic-resistant strains, thus continuing a vicious cycle of treatment.[6] Thus, treatment of elderly patients with MDRB infections has become extremely challenging. Tigecycline (TIG) has been approved by FDA for the treatment of complicated skin and skin structure infections, complicated intra-abdominal infections (cIAIs), and community-acquired pneumonia in 18 years or older patients.[7] This antibiotic has also been found to be effective against ventilator-associated pneumonia (VAP) and bloodstream infections (BSIs).[8] TIG has broad-spectrum antimicrobial activity against the multidrug-resistant gram-negative and gram-positive bacteria as well as anaerobic bacteria.[9] TIG has demonstrated a wide range of pharmacokinetic properties, such as tissue distribution and long elimination half-life.[10] It has been reported that TIG is not subjected to resistance development through the mechanism of β-lactamases, target enzyme modification, and target change. TIG could overcome or limit the bacterial efflux and ribosomal protection; hence, it was not easy to develop drug resistance, organ toxicity, and drug interactions.[11] The recommended initial dose of TIG is 100 mg and the maintenance dose is 50 mg to be administered every 12 hours intravenously once daily; however, due to its low concentration observed in the lung, blood, and other organs, the clinicians have considered increasing the TIG dosage.[12] A multi-center study in children with severe infection was performed to obtain the optimal AUC0–24:MIC90 ratios, and it was found that a dose of 1.2 mg/kg TIG, administered every 12 hours, was effective for treatment.[13] Gandijini[14] used TIG as salvage therapy and found that the patients in the 100 mg every 12 hours group had a higher effective rate than those in the 75 mg every 12 hours group, with a bacterial clearance rate of up to 63.8%. The area under the steady blood concentration versus time (AUC) to microbial MIC ratio (AUC/MIC ratio) was mainly decided for the antibacterial activity; therefore, increasing the dose of TIG could improve the tissue concentration and the antibacterial activity against the MDRB infections, resulting in better clinical curative effects.[14–16]
Elderly patients are more prone to MDRB infections because of multiple underlying diseases and suppressed immune function. The distribution of antibiotics was found to be increased in patients with sepsis due to aggressive resuscitation, which made it difficult for the antimicrobial agents to reach the optimal concentration levels in blood that is required for them to be effective, and in turn, led to infection that was complex and hard to control.[17] Optimizing the antimicrobial pharmacokinetics could improve patient prognosis.[1] In order to decrease the mortality rate, high-dose TIG therapy can be used as an effective anti-infective agent. However, there is still lack of research on the efficacy and safety of HD-TIG in the elderly patients with slow metabolism and damaged functions of the liver and kidney, especially in VAP and BSIs subgroups. Thus, we performed a retrospective analysis of the efficacy and safety of HD- TIG in elderly patients with MDRB infection to optimize the clinical parameters for the effective application of TIG.
2. Materials and methods
2.1. Study subjects
The study was conducted in the intensive care unit of the hematology and respiratory, and geriatric wards of the First Affiliated Hospital of Zhejiang Chinese Medical University. A total of 157 elderly patients with MDRB infection, who received TIG for a microbiologically documented infection, were evaluated between 1 February 2015 and 31 May 2018. Admission criteria were as follows: multidrug-resistant or pan drug-resistant bacterial infection; age ≥65 years; TIG monotherapy or in combination with other antibiotics (such as carbapenems and Cefoperazone/sulbactam) for at least 3 days, including the loading dose (LD). Exclusion criteria were as follows: <65 years of age; Patients with allergic reactions to antibiotics or tetracycline antibiotics; pregnant women; severe liver dysfunction (Child-Pugh class C); MDRB colonization. Severity of the disease was evaluated according to the Acute Physiology and Chronic Health Evaluation II (APACHE II)[18] and sequential organ failure assessment (SOFA) scores.[19] The mortality risk (R) formula used was ln (R/1-R) = −3.517 + (APACHE II score × 0.146) + (0.603, only for emergency surgery) + (diagnostic classification coefficient).[20] The R-value of each patient was added, and then divided by the total number of patients who showed the expected mortality in that group of patients. Patient's samples (sputum, blood, peritoneal fluid, and urine) were collected and submitted to the microbiology laboratory of the First Affiliated Hospital of Zhejiang Chinese Medicine University for bacterial semi quantitative culture analysis. Results of 2 times ≥ 3 + Colony forming units were considered as positive bacterial culture. TIG susceptibility breakpoints were determined according to the FDA standard (MIC ≤2 mg/L, sensitive; MIC ≥8 mg/L, resistant). The MDRB cases were those that acquired nonsusceptibility to at least 1 agent in 3 or more antimicrobial categories. The MRB infection was determined according to the results of the bacterial culture and clinical symptoms.[21] Cluster of differentiation 14+ monocyte human leukocyte antigen-DR (Human leukocyte antigen DR/ Cluster of differentiation 14+) <30% was defined as an immunosuppressive state.[22] The diagnosis of VAP, cIAIs, cSSTIs, BSIs, and urinary tract infections (UTIs) were respectively according to the current guidelines and treatment strategy.[23–27] This study was approved by the medical ethics committee of the First Affiliated Hospital of Zhejiang Chinese Medicine University that waived the need for informed consent due to its retrospective design.
2.2. Treatment plan
Elderly patients with MDRB infections received TIG monotherapy or TIG combination therapy with other antibiotics (such as carbapenems and cefoperazone/sulbactam) for at least 3 days, including LD. Patients who received TIG 50 mg every 12 hours after a 100 mg LD were defined as the conventional dose group (CD-TIG). Those who received 100 mg every 12 hours after a 200 mg LD were classified as the high dose group (HD-TIG).
2.3. Data collection
The data was extracted from the patient's medical records and the hospital database, which included the information on demographic characteristics, medical history, clinical, and laboratory findings, APACHE II and SOFA scores, TIG dosage and duration of treatment, bacterial type, infection site, clinical outcomes, bacterial eradication, and adverse reactions. The clinical effective rate, microbial clearance rate, and 28-day mortality rate were calculated, and the VAP and BSIs subgroups were separately analyzed.
2.4. Observed indicators
The results of clinical manifestations and auxiliary examination before TIG administration were gathered, which included body temperature measurements, routine blood work, assessment of liver and kidney function, determination of the serum amylase, procalcitonin (PCT), and C-reactive protein (CRP) levels, pathogen culture and drug susceptibility results, imaging of the examination results, and tracking the changes of the above indicators during TIG treatment to evaluate the clinical curative effect. The day of withdrawal or death was the end point of the observation, and the follow-up end point was 28 days after the TIG administration.
The clinical and microbiological efficacies were evaluated in the elderly patients with multidrug resistance after TIG treatment.[28] The infection that was cured or improved was defined as the clinical efficacy with improvement in fever, cough, and other clinical symptoms, the negative results for the bacterial culture in the infected foci, and imaging the lesions after anti-infection treatment.
Based on the bacterial clearance, the microbiological efficacy was divided into eradication, hypothetical eradication, no eradication, replacement, and re-infection. Eradication was defined as the absence of the original pathogens from the culture of the specimens subsequently collected from the original site. Hypothetical eradication referred to the success of clinical treatment but where the bacterial culture results were not obtained. Eradication, hypothetical eradication, and replacement were considered as effective to calculate microbial eradication rate.[29] Adverse reactions during TIG treatment were included according to the adverse reaction list of TIG instructions.[30] The following clinical information and laboratory data was recorded: gastrointestinal reactions (nausea, vomiting, and diarrhea), and the levels of serum creatinine (Cr), blood urea nitrogen (Bun), blood amylase (AMY), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and activated partial thromboplastin time (APTT). If the values of the laboratory data were observed to more than the normal value after TIG treatment, they were included in the adverse reactions.
2.5. Statistical analysis
The Kolmogorov–Smirnov test was used to evaluate the distribution of the variables. The data with non-normal distribution were assessed with Mann–Whitney test and the values for the median and selected centiles (25th–75th) has been provided. The data with normal distribution were assessed with the Student t test. Categorical variables are presented as proportions and were analyzed with Chi-square test or Fisher exact test, as appropriate. A P-value < .05 was considered significant. The crude odds ratio (OR) and 95% confidence interval (CI) were calculated for each variable. We included all the variables in the multivariable logistic regression if they achieved a P-value of less than or equal to .2 at the univariate analysis. A stepwise selection procedure was used to select the variables for inclusion in the final model. The Hosmer–Lemeshow goodness-of-fit test and the receiver operating characteristic curve were used to assess the goodness of the final logistic model. All statistical analyses were performed using SPSS software, version23.0 (SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Patient's clinical characteristics
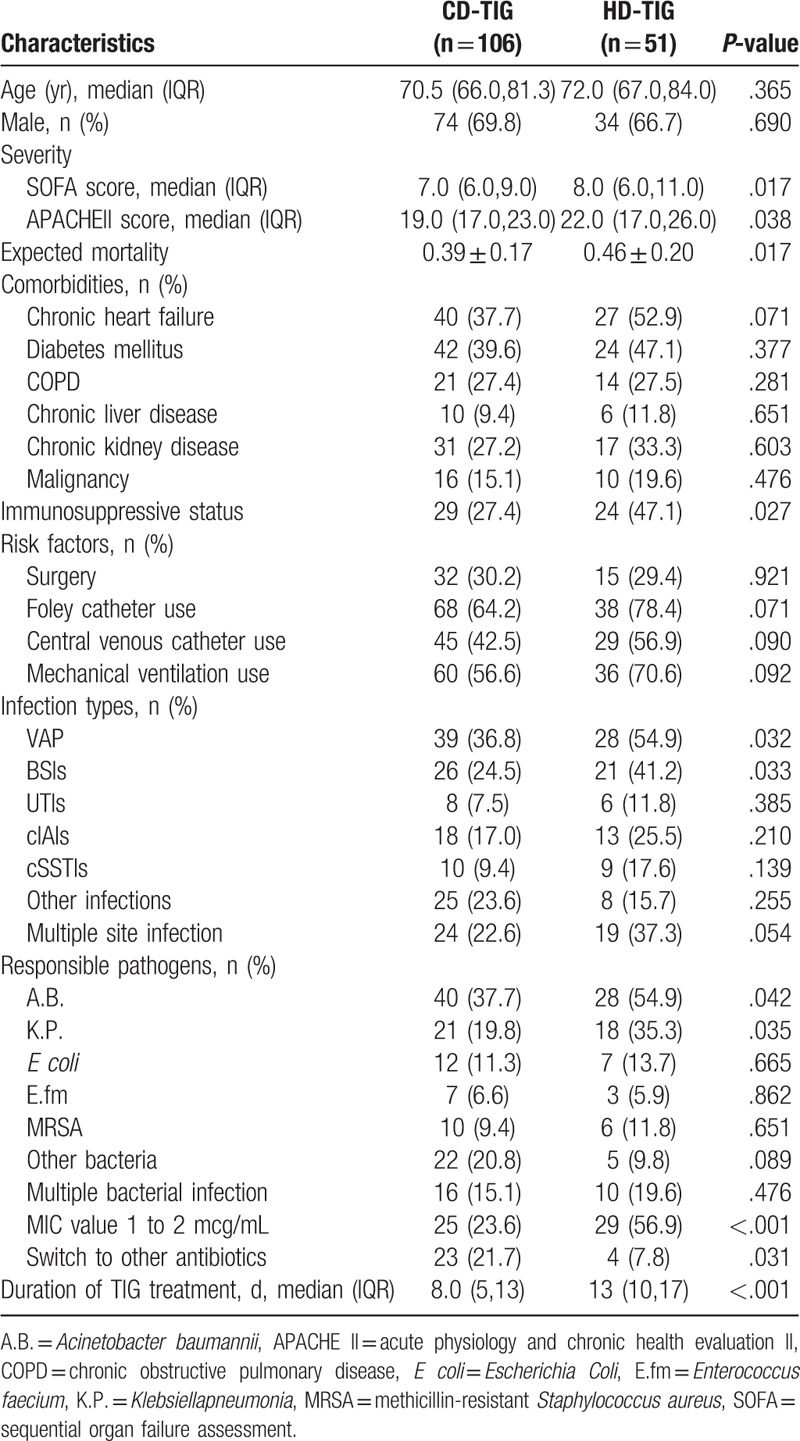
A total of 157 elderly patients with MDRB infection who fulfilled the inclusion criteria were considered for the retrospective analysis. Of these, 106 elderly patients received CD-TIG and 51 received HD-TIG. The elderly patients with MDRB infection who were treated with CD- or HD-TIG demonstrated similar baseline clinical characteristics, such as age, gender, underlying diseases, surgery, principal comorbidities, indwelling catheter and central venous catheterization, mechanical ventilation, and combined use of antibiotics (P > .05), but the APACHE II and SOFA scores were significantly higher in the HD-TIG group as compared to those in the CD-TIG group (P < .05). The number of patients with immunosuppression in the HD-TIG group was also significantly higher as compared to that in the CD-TIG group (P < .05). These results showed that patients with severe illness and higher expected mortality were present in the HD-TIG group (Table 1).
Table 1.
Clinical characteristics of 157 elderly patients with severe MDRB infection in the CD-TIG group and HD-TIG group.
3.2. Infection types and microbiological analysis
Of the 157 cases, 67 (43%) were suffering from VAP and 90 were suffering from the other infections, such as BSIs (n = 47, 30%), cIAIs (n = 30, 19%), cSSTIs (n = 19, 12%), UTIs (n = 14, 9%), and other site infections (n = 33, 21%). The incidence of VAP and BSIs in the HD-TIG group was significantly higher than that in the CD-TIG group (54.9% vs 36.8%, 41.2% vs 24.5%, both P < .05), but there were no significant differences observed among cIAIs, cSSTIs, and UTIs (P > .05). Of the multidrug-resistant pathogenic bacteria, A.B. (n = 68), K.P. (n = 39), Escherichia coli (E coli; n = 19), E.fm (n = 10), MRSA (n = 16), 27 cases of other bacteria, 26 cases of multiple bacterial infections, and 54 cases of TIG MIC of 1 to 2 mg/L were included. A.B. and K.P. infection rates were significantly higher in the HD-TIG group than that of the CD-TIG group (54.9% vs 37.7%, 35.3% vs 19.8%, P < .05). E coli, MRSA, E.fm, and the other bacteria and multiple bacterial infections demonstrated no significant differences between the 2 groups (P > .05). The incidence of TIG MIC of 1 to 2 mg/L was higher in the HD-TIG group than in the CD-TIG group (56.9% vs 23.6%, P < .05, Table 1).
3.3. Efficacy evaluation according to TIG dosage
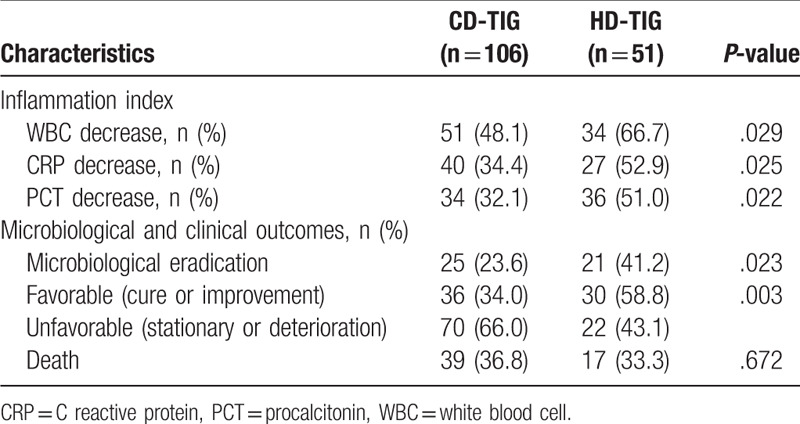
Duration of TIG therapy was significantly longer in the HD-TIG group than that in the CD-TIG alone (13 days vs 8 days, P < .05). The effective rate (cure or improvement) and microbiological eradication percentage were higher when TIG was used at a higher dose (58.8% vs 34%; P = .003 and 41.2% vs 23.6%; P = .023, respectively). The decreased incidence of white blood cell, CRP, and PCT in the CD-TIG group were significantly lower than that in the HD-TIG group (48.1% vs 66.7%, 34.5% vs 52.9%, 32.1% vs 51%, respectively; P < .05). However, the overall mortality in the HD-TIG group was 36.8%, without any significant differences between the 2 groups (36.8% vs 33.3%, P = .672, Table 2).
Table 2.
Summary of treatments and outcomes among in the CD-TIG group and HD-TIG group.
3.4. Adverse events
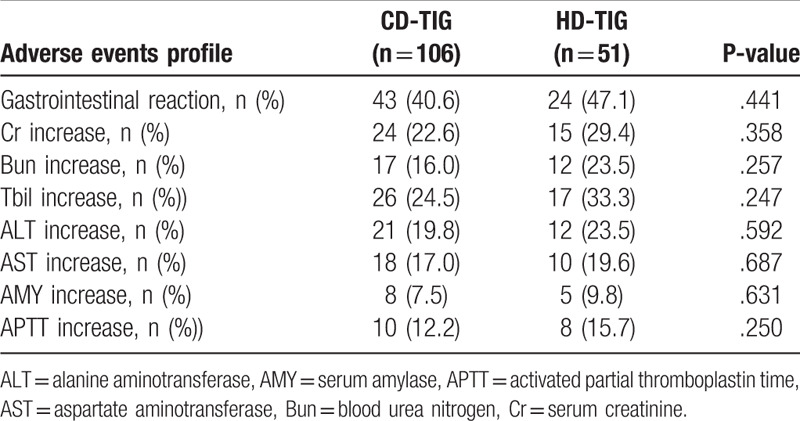
A total of 67 patients (42.6%) had adverse events associated with the use of TIG, which included gastrointestinal symptoms, such as nausea and vomiting. There was no significant difference observed in the incidence of adverse events between the HD-TIG group and the CD-TIG group (47.1% vs 40.6%, P = .441). At the end of the TIG therapy, the incidence of Cr, BUN, AMY, ALT, AST, and APTT were similar in the 2 groups (P > .05, Table 3). None of the patients required TIG discontinuation or dose reduction. Similar results were also obtained on stratifying the patients by the type of infection, that is, VAP versus infections other than VAP.
Table 3.
Comparison of adverse events profile of TIG in the CD-TIG group and HD-TIG group.
3.5. Predictive factors of clinical efficacy
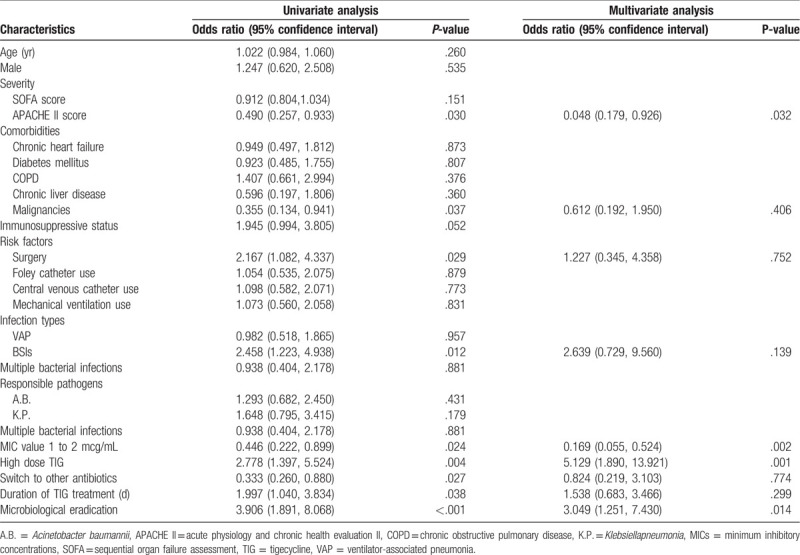
Univariate analysis of MDRB patients showed that the individuals with clinical failure included malignancy, surgery, high APACHE II score, BSIs, switching to other antibiotics, and TIG MIC of 1 to 2 g/mL, whereas the patients with successful clinical outcome had high-dose TIG, longer duration of TIG treatment, and high microbiological eradication. The multivariate analysis of logistic regression indicated that the use of HD TIG (OR 5.129; 95% CI [1.890, 13.921]; P = .001) and microbiological eradication (OR 3.049, 95%CI [1.251, 7.430], P = .014) were 2 independent predictors of clinical cure, while a higher APACHE II score (OR 0.048, 95% CI [0.179, 0.926]; P = .032) and TIG MIC of 1 to 2 g/mL (OR 0.169, 95% CI [0.055, 0.524]; P = .002) were significantly associated with clinical failure (Table 4).
Table 4.
Univariate and multivariate analysis of factors associated with clinical cure in 157 elderly patients with MDRB infection.
3.6. Predictors of clinical efficacy in VAP
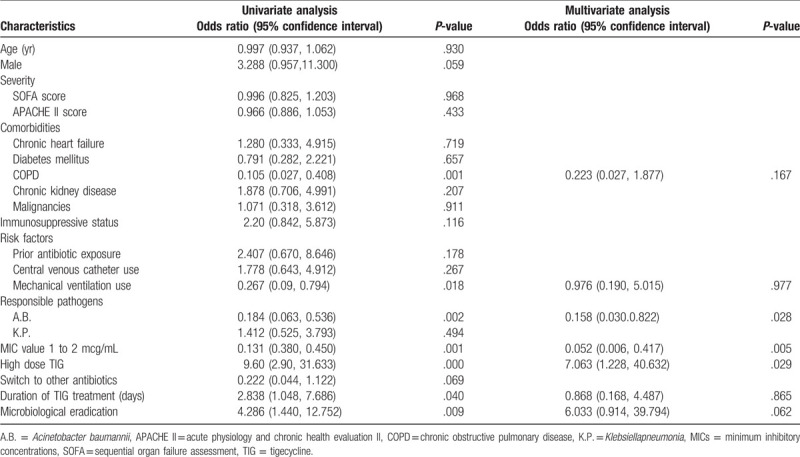
The clinical efficacy of VAP was 44.7%. The univariate analysis of the 67 patients with VAP showed that the individuals with clinical failure had chronic obstructive pulmonary disease, mechanical ventilation, A.B. infection, and TIG MIC of 1-2 g/mL, whereas the patients with a successful clinical outcome had high-dose TIG, longer duration of TIG treatment, and higher microbiological eradication. The multivariate analysis of logistic regression indicated that the use of high-dose TIG was the sole independent predictor of clinical efficacy (OR 7.063, 95% CI [1.228, 40.632]), while a high MDR A.B. infection (OR 0.158, 95% CI [0.030, 0.822]), and TIG MIC 1 to 2 g/mL (OR 0.052, 95% CI [0.006, 0.417]) were significantly associated with clinical failure (Table 5).
Table 5.
Logistic regression analysis of factors associated with clinical cure in 67 elderly patients with VAP.
3.7. Predictors of clinical efficacy in BSIs
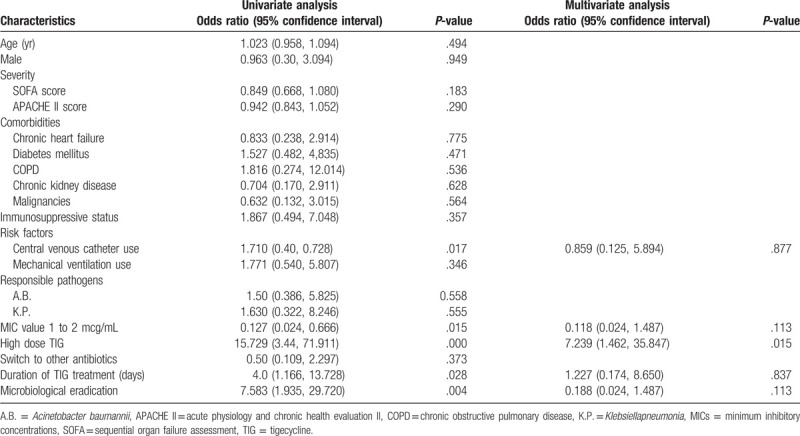
The clinical effective rate of BSIs was 46.8%. The univariate analysis of the 47 patients with BSIs showed that the individuals that showed clinical failure had the central venous catheter and TIG MIC of 1 to 2 g/mL, whereas the patients with a successful clinical outcome had high-dose TIG, longer duration of TIG treatment, and higher microbiological eradication. The multivariate analysis of logistic regression indicated that the high-dose TIG was the sole independent predictor of clinical efficacy (OR 7.239, 95% CI [1.462, 35.847], Table 6).
Table 6.
Logistic regression analysis of factors associated with clinical cure in 47 patients with BSIs.
4. Discussion
In this retrospective study, we found that HD-TIG group (100 mg q12 hours after 200 mg LD) had higher SOFA and APACHE II scores, and the patients in this group showed more immunosuppression (P < .05), with serious and complicated conditions. According to the aforementioned formula,[20] the expected mortality rate of HD-TIG group was 46%, which was significantly higher than the CD-TIG group (39%, P = .017). So, HD-TIG group patients must have controlled infections more effectively. The clinical efficacy and microbiological eradication in the HD-TIG group was significantly higher than that of the CD-TIG group (58.8% vs 34%, P = .003 and 41.2% vs 23.6%, P = .023, respectively), but there was no significant difference in the mortality rates between the 2 groups (36.8% vs 33.3%, P = .672). This retrospective data has highlighted 3 features of the HD-TIG group: greater severity of illness, the high-expected mortality, and antibiotic exposure to stronger bacterial resistance that was more difficult to control. The elderly patients with higher disease severity often had symptoms of hypoalbuminemia, edema, and hyperdynamic circulation, which increased the antibiotic volume of distribution. The tissue drug concentration in the patients was affected by many factors.[5] The response was not necessarily strong when a higher dose of TIG was administered or the administration time was increased. Therefore, the severity of the disease and the long duration of TIG treatment were not the determining factors for the improvement of the efficacy of the antibiotics. However, in our study, TIG administered at a higher dose could improve the clinical efficacy and microbiological eradication, thus, reducing the high mortality rate in the elderly. A randomized phase II trial in the United States found that the HD-TIG (100 mg q12 hours) had higher clinical efficacy than the middle-dose (75 mg q12 hours) and the control group for hospital acquired pneumonia (HAP, 85%, 69.6%, and 75%, respectively). The blood concentration of high-dose TIG had a larger AUC/MIC ratio, so, the HD-TIG was essential for the treatment of HAP infection.[14] Our study has also confirmed the efficacy of HD-TIG in the elderly patients.
TIG has strong lipophilicity and large apparent volume of distribution (7–10 L/kg). It is more effectively distributed in the fatty tissues and organs, such as skin and soft tissues, and intra-abdominal organs. The elderly patients who underwent long-term hospitalization were susceptible to pneumonia and BSIs by MDRB. The elderly patients with sepsis often had symptoms of hypoalbuminemia, edema, and hyperdynamic circulation, which increased the antibiotic volume of distribution. The low concentration of TIG in the lungs and blood, and the high MIC value of TIG caused poor antibacterial effect.[1,17] The study found that a MIC of 1 to 2 g/mL for TIG was associated with clinical failure, and HD-TIG was the independent predictor of clinical efficacy. This indicated the need for higher doses of TIG for the treatment of elderly patients with MDRB infection, especially for the pathogenic bacteria that had high MIC for TIG, which necessitated increasing the therapeutic dose of TIG to improve clinical outcomes.
VAP is not included in the range of TIG instructions, and TIG is generally used as an anti-infective agent against MDRB in VAP because of ineffectiveness of the other antibiotics. The TIG concentration in the lung tissues was not high, so, the drug concentration decreased rapidly and the AUC appeared to be low.[31,32] Thus, more TIG was needed to be administered to increase the concentration in the lung tissues to improve the clinical efficacy in VAP. This study suggests that the use of HD-TIG was the sole independent predictor of the clinical efficacy and it increased the clinical efficacy rate by 60.7%. Pascale et al[33] reported on 63 VAP of MDR A.B. and Klebsiella, which showed that the clinical efficacy rate and the pathogen clearance rate (100 mg q12 hours) in the HD group were higher than those in the standard dose group (P < .05).
The therapeutic application of TIG for BSIs is also not in the range of TIG instructions and its clinical value is still controversial. TIG can be quickly absorbed into the tissues from the blood due to its lipophilic nature, leading to low TIG blood concentrations and not achieving the best antibacterial activity. The highest concentration that has been observed in the serum is only 1.5 g/mL with the administration of TIG at the CD; the HD-TIG in combination with other antibiotics could successfully treat the pan-drug resistant K.P. BSIs.[34,35] Our study found that the HD-TIG was the sole independent predictor of clinical efficacy, indicating that the HD-TIG treatment can improve the clinical efficacy of MDRB infection in blood.
TIG is metabolized in both the liver and kidneys. Therefore, any single organ dysfunction of either the liver or kidney did not affect the overall metabolism of TIG in the body. The application of HD-TIG aggravated the metabolic load in the body, especially in the elderly patients who often had inadequate compensation for the organ function, and thus more attention should have been paid to the adverse events of the HD-TIG. Previous studies have shown that HD-TIG did not increase functional damage to the patient's liver and kidneys, and abnormal coagulation as compared with that of the effects of the standard dose.[33] The most common adverse events of TIG were the gastrointestinal reaction, but the adverse events were not aggravated in the HD-TIG group, including gastrointestinal symptoms, drug-induced liver and kidney dysfunction, pancreatitis, and abnormal blood coagulation. Thus, the use of HD-TIG in elderly patients with MDRB infection is considered to be safe.
However, this study has some limitations. First, it was a single-center retrospective analysis and thus, the sample size was small. Second, we did not monitor the plasma and tissue concentrations of TIG and different doses of PK/PD data analysis were lacking. However, we believe that this is a meaningful comparative clinical study, where we have analyzed the use of HDs of TIG in elderly patients with MDRB infection with subgroup analysis for VAP and BSIs. In future, a prospective multicenter study should be conducted to determine the efficacy and safety of HDs of TIG at different time points.
5. Conclusions
Our study has shown that administering HD-TIG (100 mg every 12 hours after a 200 mg LD) is more effective in improving the outcomes of elderly patients with MDRB infection than the CD, and with minimal adverse events. In VAP and BSIs subgroups of, the high-dose TIG proved to be safe and effective. The HD-TIG was the most important predictor of clinical efficacy in elderly patients with MDRB infection. This study provides clinical evidence for the treatment of MDRB infections in elderly patients with HD- TIG.
Acknowledgments
The authors thank Dr JM Cao from the Microbiology Department for providing the microbiological data and Dr QS Li from the Clinical evaluation center for her assistance with the statistical analyses. The authors also thank the reviewers for valuable suggestions and comments. The authors would like to thank Editage (www.editage.com) for English language editing.
Author contributions
Conceptualization: Ronglin Jiang.
Data curation: Ronglin Jiang.
Formal analysis: Ronglin Jiang.
Funding acquisition: Ronglin Jiang.
Investigation: Guolian Xia.
Methodology: Guolian Xia.
Project administration: Guolian Xia.
Resources: Guolian Xia.
Software: Guolian Xia.
Supervision: Guolian Xia.
Validation: Guolian Xia.
Visualization: Guolian Xia, Ronglin Jiang.
Writing – original draft: Guolian Xia.
Writing – review and editing: Ronglin Jiang.
Footnotes
Abbreviations: A.B. = Acinetobacter Baumannii, ALT = alanine aminotransferase, AMY = amylase, APACHEII = acute physiology and chronic health evaluation II, APTT = activated partial thromboplastin time, AST = aspartate aminotransferase, AUC = area under the steady blood concentration versus time, BSIs = bloodstream infection, Bun = blood urea nitrogen, CD = conventional dose, CHINET = China high speed information network, cIAIs = complicated intra-abdominal infections, Cr = serum creatinine, CRP = C-reactive protein, cSSIs = complicated skin and skin-structures infections, E coli = Escherichia coli, E.fm = Enterococcus faecium, HAP = hospital acquired pneumonia, HD = high dose, K.P. = Klebsiella pneumonia, LD = loading dose, MDRB = multidrug-resistant bacterial, MICs = minimum inhibitory concentrations, MRSA = methicillin-resistant Staphylococcus aureus, PCT = procalcitonin, SOFA = the sequential organ failure assessment, TIG = tigecycline, UTIs = urinary tract infections, VAP = ventilator-associated pneumonia.
How to cite this article: Xia G, Jiang R. Clinical study on the safety and efficacy of high-dose tigecycline in the elderly patients with multidrug-resistant bacterial infections: A retrospective analysis. Medicine. 2020;99:10(e19466).
Our study was approved by the medical ethics committee of the First Affiliated Hospital of Zhejiang Chinese Medicine University that waived the need for informed consent, due to its retrospective design.
Consent for publication was obtained from the participants.
The raw dataset is not available publicly due to China privacy regulations. Applicants for any data must be prepared to conform to China privacy regulations.
This work was supported in part by the Construct Program of the Key Discipline of Chinese Medicine in Zhejiang Province (CN), the Department of Health Zhejiang Province (No. 2012ZGG001), the Chinese Medicine Research Program of Zhejiang Province (No. 2016ZA071, No. 2015ZB060) and the Natural Science Foundation of Zhejiang Province (No.LY14H290006).
The authors have no conflicts of interest to disclose.
References
- [1].Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med 2017;43:304–77. [DOI] [PubMed] [Google Scholar]
- [2].Hu F, Guo Y, Yang Y, et al. Resistance reported from China antimicrobial surveillance network (CHINET) in 2018. Eur J Clin Microbiol Infect Dis 2019;38:2275–81. [DOI] [PubMed] [Google Scholar]
- [3].Hsieh CC, Lee CH, Li MC, et al. Empirical third-generation cephalosporin therapy for adults with community-onset Enterobacteriaceae bacteraemia: Impact of revised CLSI breakpoints. Int J Antimicrob Agents 2016;47:297–303. [DOI] [PubMed] [Google Scholar]
- [4].Bochud PY, Bonten M, Marchetti O, et al. Antimicrobial therapy for patients with severe sepsis and septic shock: an evidence-based review. Crit Care Med 2004;32: 11 Suppl: S495–512. [DOI] [PubMed] [Google Scholar]
- [5].Pea F. Pharmacokinetics and drug metabolism of antibiotics in the elderly. Expert Opin Drug Metab Toxicol 2018;14:1087–100. [DOI] [PubMed] [Google Scholar]
- [6].Merli M, Lucidi C, Di Gregorio V, et al. The spread of multi drug resistant infections is leading to an increase in the empirical antibiotic treatment failure in cirrhosis: a prospective survey. PloS One 2015;10:e0127448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wyeth Pharmaceutics. Tygacil (Tigecycline) for Injection [Package Insert]. Philadelphia, PA, USA: Wyeth Pharmaceuticals Inc; 2005. [Google Scholar]
- [8].Jean SS, Hsieh TC, Hsu CW. Comparison of the clinical efficacy between tigecycline plus extended-infusion imipenem and sulbactam plus imipenem against ventilator-associated pneumonia with pneumonic extensively drug-resistant Acinetobacter baumannii bacteremia, and correlation of clinical efficacy with in vitro synergy tests. J Microbiol Immunol Infect 2016;49:924–33. [DOI] [PubMed] [Google Scholar]
- [9].Barbour A, Schmidt S, Ma B, et al. Clinical pharmacokinetics and pharmacodynamics of tigecycline. Clin Pharm 2009;48:575–84. [DOI] [PubMed] [Google Scholar]
- [10].Schedlbauer A, Kaminishi T, Ochoa-Lizarralde B, et al. Structural characterization of an alternative mode of TIG binding to the bacterial ribosome 2015;59:2849–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kim WY, Moon JY, Huh JW, et al. Comparable efficacy of TIG versus colistin therapy for multidrug-resistant and extensively drug-resistant Acinetobacter baumannii pneumonia in critically Ill patients. PloS One 2016;11:e0150642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Garnacho-Montero J, Corcia-Palomo Y, Amaya-Villar R, et al. How to treat VAP due to MDR pathogens in ICU patients. BMC Infect Dis 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Purdy J, Jouve S, Yan JL, et al. Pharmacokinetics and safety profile of TIG in children aged 8 to 11 years with selected serious infections: a multicenter, open-label, ascending-dose study. Clin Therap 2012;34:496–507. e491. [DOI] [PubMed] [Google Scholar]
- [14].Ramirez J, Dartois N, Gandjini H, et al. Randomized phase 2 trial to evaluate the clinical efficacy of two high-dosage TIG regimens versus imipenem-cilastatin for treatment of hospital-acquired pneumonia. Antimicrob Agents Chemother 2013;57:1756–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Barbour A, Schmidt S, Ma B, et al. Clinical pharmacokinetics and pharmacodynamics of TIG. Clin Pharmacokinet 2009;48:575–84. [DOI] [PubMed] [Google Scholar]
- [16].Xie J, Wang T, Sun J, et al. Optimal TIG dosage regimen is urgently needed: results from a pharmacokinetic/pharmacodynamic analysis of TIG by Monte Carlo simulation. Int J Infect Dis 2014;18:62–7. [DOI] [PubMed] [Google Scholar]
- [17].Asbell PA, Sanfilippo CM, Pillar CM, et al. Antibiotic resistance among ocular pathogens in the United States: five-year results from the antibiotic resistance monitoring in ocular microorganisms (ARMOR) surveillance study. JAMA Ophthalmol 2015;133:1445–54. [DOI] [PubMed] [Google Scholar]
- [18].Man SY, Chan KM, Wong FY, et al. Evaluation of the performance of a modified acute physiology and chronic health evaluation (APACHE II) scoring system for critically ill patients in emergency departments in Hong Kong. Resuscitation 2007;74:259–65. [DOI] [PubMed] [Google Scholar]
- [19].Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016;315:801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med 1985;13:818–29. [PubMed] [Google Scholar]
- [21].Ren Y, Ma G, Peng L, et al. Active screening of multi-drug resistant bacteria effectively prevent and control the potential infections. Cell Biochem Biophys 2015;71:1235–8. [DOI] [PubMed] [Google Scholar]
- [22].Volk HD, Reinke P, Krausch D, et al. Monocyte deactivation--rationale for a new therapeutic strategy in sepsis. Intensive Care Med 1996;22: Suppl 4: S474–81. [DOI] [PubMed] [Google Scholar]
- [23].Klompas M, Branson R, Eichenwald EC, et al. Strategies to prevent ventilator-associated pneumonia in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol 2014;35: Suppl 2: S133–54. [DOI] [PubMed] [Google Scholar]
- [24].Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Surg Infect 2010;11:79–109. [DOI] [PubMed] [Google Scholar]
- [25].Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis 2014;59:e10–52. [DOI] [PubMed] [Google Scholar]
- [26].Marschall J, Mermel LA, Fakih M, et al. Strategies to prevent central line-associated bloodstream infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol 2014;35:753–71. [DOI] [PubMed] [Google Scholar]
- [27].Flores-Mireles AL, Walker JN, Caparon M, et al. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol 2015;13:269–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Bhavnani SM, Rubino CM, Hammel JP, et al. Pharmacological and patient-specific response determinants in patients with hospital-acquired pneumonia treated with TIG. Antimicrob Agents Chemother 2012;56:1065–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kuo SC, Wang FD, Fung CP, et al. Clinical experience with TIG as treatment for serious infections in elderly and critically ill patients. J Microbiol Immunol Infect 2011;44:45–51. [DOI] [PubMed] [Google Scholar]
- [30].Kadoyama K, Sakaeda T, Tamon A, et al. Adverse event profile of TIG: data mining of the public version of the U.S. Food and Drug Administration adverse event reporting system. Biol Pharm Bull 2012;35:967–70. [DOI] [PubMed] [Google Scholar]
- [31].Scaglione F. Comment on: efficacy and safety of TIG: a systematic review and meta-analysis. J Antimicrob Chemother 2011;66:2892–3. [DOI] [PubMed] [Google Scholar]
- [32].Freire AT, Melnyk V, Kim MJ, et al. Comparison of TIG with imipenem/cilastatin for the treatment of hospital-acquired pneumonia. Diagnos Microbiol Infect Dis 2010;68:140–51. [DOI] [PubMed] [Google Scholar]
- [33].De Pascale G, Montini L, Pennisi M, et al. High dose TIG in critically ill patients with severe infections due to multidrug-resistant bacteria. Crit Care 2014;18:R90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Cunha BA. Pharmacokinetic considerations regarding TIG for multidrug-resistant (MDR) Klebsiella pneumoniae or MDR Acinetobacter baumannii urosepsis. J Clin Microbiol 2009;47:1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Humphries RM, Kelesidis T, Dien Bard J, et al. Successful treatment of pan-resistant Klebsiella pneumoniae pneumonia and bacteraemia with a combination of high-dose TIG and colistin. J Med Microbiol 2010;59:1383–6. [DOI] [PMC free article] [PubMed] [Google Scholar]


