Supplemental Digital Content is available in the text
Keywords: Ki-67, meningioma, meta-analysis, prognostic role
Abstract
Background:
Ki-67 is a typical immunohistochemical marker for cell proliferation. Higher expression of Ki-67 is correlated with poor clinical outcomes in several cancers. However, the prognostic value of Ki-67 on the prognosis of meningiomas is still controversial. The purpose of this meta-analysis was to evaluate the prognostic value of Ki-67 in meningiomas.
Methods and materials:
We searched Medline and EMBASE from inception to December 31, 2018, to identify relevant articles. Using a fixed or random effects model, pooled hazard ratios (HRs) for overall survival (OS) and disease/progression/recurrence-free survival (D/P/RFS) were estimated.
Results:
A total of 43 studies, comprising 5012 patients, were included in this analysis. Higher Ki-67 expression levels were significantly associated with worse OS (HR = 1.565; 95% CI: 1.217–2.013) and D/P/RFS (HR = 2.644; 95% CI: 2.264–3.087) in meningiomas. Subgroup analysis revealed that all the included factors (ethnicity, tumor grade, HR sources, definition of cutoffs, cutoff values) for heterogeneity investigation can affect the pooled results. Among them, the definitions of cutoffs and cutoff values factor are the two main contributors toward heterogeneity. Multivariable meta-regression analysis also showed that methodologies used for cutoff value definition contributed to the high inner-study heterogeneity.
Conclusions:
Higher Ki-67 expression levels negatively influenced survival in meningiomas. A higher cutoff value (>4%) is more appropriate for prognosis prediction. It is highly recommended that Ki-67 expression profile could be assessed in meningiomas treatment for predicting survival. And patients with elevated expression of Ki-67 need to have close follow-ups.
1. Introduction
Meningiomas are usually considered clinically benign tumors, which account for 36.4% of all central nervous system (CNS) neoplasms.[1,2] According to the 2016 World Health Organization (WHO) classification scheme, meningiomas are stratified into 3 groups: grade I (benign), grade II (atypical), and grade III (anaplastic).[3,4] The initial choice of treatment for meningioma is gross total microsurgical resection mostly with improved post-surgery outcomes.[5] However, tumor recurrence and progression after surgical treatment are frequent, and these patients are prone to associate with poor overall survival (OS).[2,6] Thus, identification of risk factors in predicting tumor recurrence and progression is needed. The histological grade and the extent of resection are reported as the two most important, widely accepted predictive factors of meningioma recurrence and progression.[7] Nevertheless, the recurrence rates for grade I patients were reported as high as 7% to 20%,[3] and those for patients who received complete resection were 10% to 30%.[6] Therefore, it is vital to find other prognostic parameters to improve evaluation of recurrence and progression in patients with meningioma.
Increased cell proliferation activity was considered the most important mechanism of oncogenesis.[8] Ki-67/MIB-1 is a typical immunohistochemical marker for cell proliferation. It is increasingly popular due to its minimal tissue requirements and suitability to routinely fixed tissues.[9] The negative effect of Ki-67 on clinical outcomes has been extensively identified in most solid tumors, such as gastrointestinal stromal tumors, renal cell carcinoma, thyroid cancer, prostate cancer, bladder cancer, and oral squamous cell carcinoma.[10–15] Moreover, Ki-67/MIB-1 is found to be more predictive of survival than expression of p53[16] and proliferating cell nuclear antigen (PCNA) in brain tumors.[17,18] And numerous studies have shown that Ki-67/MIB-1 is an independent predictor in meningiomas prognosis.[19–21] However, the prognostic role of Ki-67 in meningiomas remains unclear. Some studies[16,22,23] indicated negative association between Ki-67 expression level and meningiomas prognosis while others[19,24] reported insignificant results. The inconsistency was probably ascribed to the great diversity of cutoff values of Ki-67/MIB-1 index for analysis, definition of cutoff values, and sample's composition of tumor grade among studies. Therefore, we conduct this meta-analysis study to evaluate the prognostic value of Ki-67/MIB-1 in meningiomas.
2. Methods
2.1. Search strategy
This meta-analysis was registered in PROSPERO (registration CRD42018093940). There is no need for ethical approval of this meta-analysis because all the included studies have clearly stated ethical approval in their manuscripts. We performed a thorough search for available literatures in electronic databases of Medline and EMBASE until December 31, 2018. Medical Subject Headings and Emtree headings were searched and combined with the following keywords: “meningioma OR meningeal neoplasms” and “prognosis OR survival OR mortality OR outcome OR treatment OR recurrence OR predict∗.” We also manually scanned the references of included articles in order to check more potential studies. The full search strategies are presented in Supplementary Table S1 (see Table S1, Supplemental Content, which illustrates full search strategies).
The eligible studies were selected based on the following criteria:
-
1.
studies were published in English as a full essay;
-
2.
all patients were diagnosed with histologically confirmed meningioma;
-
3.
the Ki-67 expression was detected by immunohistochemistry (IHC);
-
4.
correlation between Ki-67/MIB-1 expression and prognosis of patients with meningioma was investigated;
-
5.
hazardous risks (HRs) with 95% confidence interval (CI) for survival analysis were provided or could be calculated from the provided data;
-
6.
Ki-67/MIB-1 expression level was analyzed as a dichotomous variable with cutoff values provided or data to calculate;
-
7.
for cohorts included in more than one publication, the most complete and recent study was selected for analysis.
Two reviewers (TJW and SYS independently screened the titles and abstracts of all initially identified studies according to the selection criteria. Full-text articles of studies that met all selection criteria were retrieved.
2.2. Data extraction and quality assessment
The data were extracted from the identified studies by two investigators independently. The following variables were captured from all included studies: first author, publication year, ethnicity, number of cases, grade, grade criteria, patient age, definition of cutoffs, cutoff values, outcome measures, and risk estimates; any disagreement was resolved by discussion between the two reviewers or consultation with a third reviewer. HR with its 95% CI of each included study was extracted or estimated from Kaplan–Meier survival curves by the open digitizing program (Engauge Digitizer).[25,26] If results of both univariate and multivariate analysis were reported, the latter was used first as it offered a more accurate risk estimate. We used a set of modified predefined criteria to evaluate the quality of all included studies.[27,28]
2.3. Statistical analysis
Data were analyzed by using Stata SE14.0 (Stata Corp LP, College Station, TX). Weights for each study in the analysis were calculated using the method reported by Mizuki.[29] The HR with its 95% CI and redefined weight was used to define the prognostic value of Ki-67 expression in meningiomas. Inter-study heterogeneity was evaluated using the Chi-Squared test and expressed as I2 index. A fixed effects model was used when the value of Pheterogeneity was > .05 and I2 < 50%; otherwise, a random effects model was applied. To investigate the potential origin of the heterogeneity, subgroup analysis and meta-regression were performed for OS or disease/progression/recurrence-free survival (D/P/RFS) analysis according to the ethnicity, tumor grade, HR sources, and definitions of cutoffs and cutoffs. In addition, sensitivity analysis was carried out to investigate the influence of each included study on the pooled HR. Begg funnel plot and Egger's linear regression test were conducted for evaluating publication bias. Additionally, Duval and Tweedie's “Trim and Fill” method was applied to estimate a corrected effect size after adjustment for publication bias.[30] Two-tailed value of P < .05 was considered statistically significant.
3. Results
3.1. Search results
The detailed study selection process is presented as a flowchart in Figure 1. Potentially relevant citations were initially retrieved through initial search of relevant databases. After duplicates were removed and title/abstract screened, 138 articles remained for full-text assessment. Ninety-five articles were further excluded for lack of HRs with estimates of 95% CIs or data to calculate.
Figure 1.
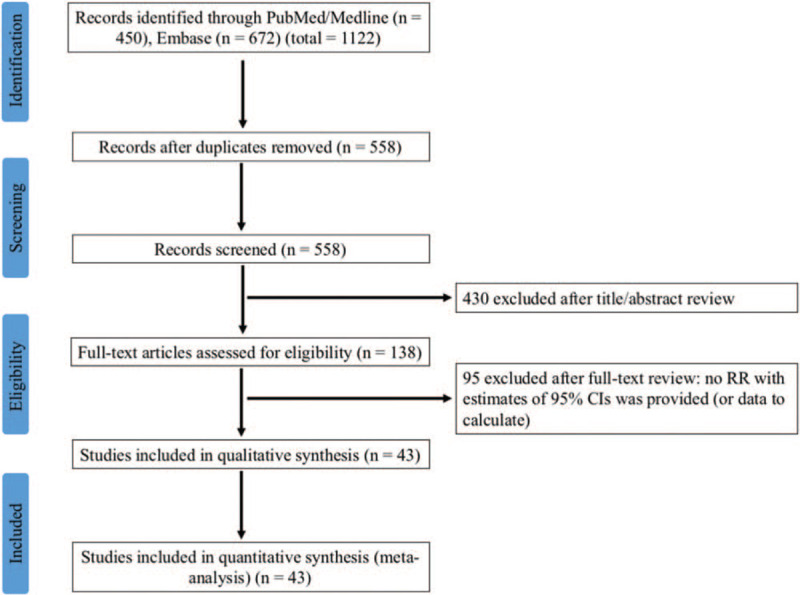
Flowchart of screening strategy for included studies.
3.2. Study characteristics
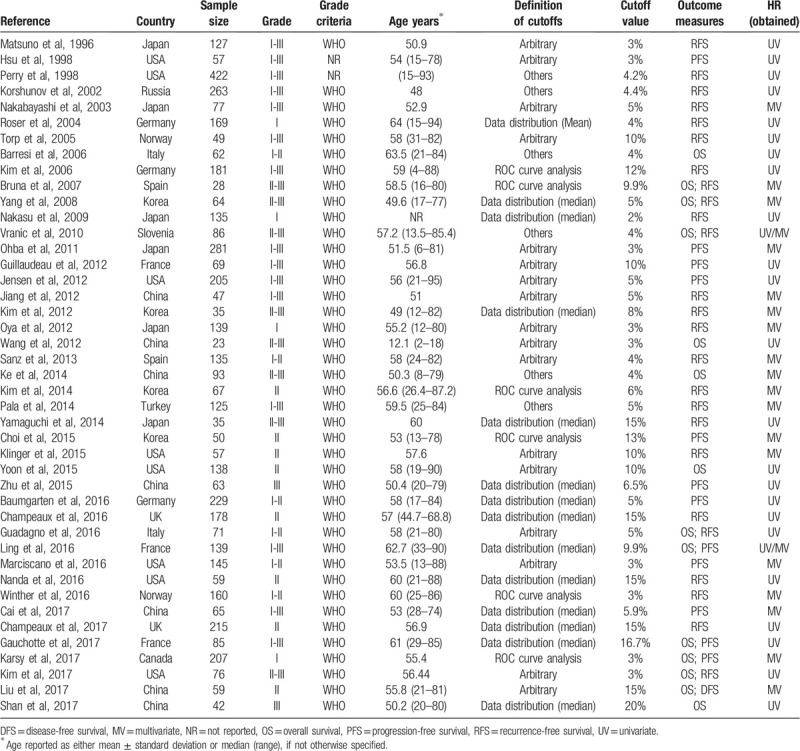
Summary of major characteristics of included studies is shown in Table 1. A total of 43 studies published from 1996 to 2017 with 5012 patients were included in the final meta-analysis.[16,19–24,31–66] Among these studies, 17 studies were conducted in Eastern countries and 26 studies in Western countries; the sample size ranged from 23 to 422; 4 studies comprised only low-grade (grade I) meningioma patients, 18 studies only high-grade (grade II/III) patients, and 21 studies both low- and high-grade patients; 14 articles reported OS and 38 articles reported D/P/RFS; HR and 95% CIs data were extracted directly from 25 studies, or were calculated from Kaplan–Meier survival curves in 18 studies. According to the quality assessment, 7 studies had quality scores of 7 or less, and the rest 36 studies had a score of more than 7 (see Table S2, Supplemental Content, which illustrates the quality assessment of included studies).
Table 1.
Main characteristics of 43 eligible studies included in the meta-analysis.
3.3. Overall survival
There were 14 studies with 1173 patients taken for analysis for OS. No significant association between the Ki-67/MIB-1 expression and OS was found (HR = 1.009; 95% CI: 0.999–1.019; P = .073; I2 = 77.2%; Pheterogeneity < .001) (see Figure S1, Supplemental Content, which illustrates the association between the Ki-67/MIB-1 expression and OS). However, it is noticed that the pooled HR was mainly ascribed to 2 studies with extremely large weight.[56,62] In order to reduce the contribution of these 2 studies, recalculated weights were applied to get the adjusted HR and 95% CI. Consequently, a negative prognostic value of Ki-67 was testified, whereas a marked heterogeneity was observed among these studies (HR = 1.565; 95% CI: 1.217–2.013; P = .000; I2 = 100.0%; Pheterogeneity < .001, Fig. 2).
Figure 2.
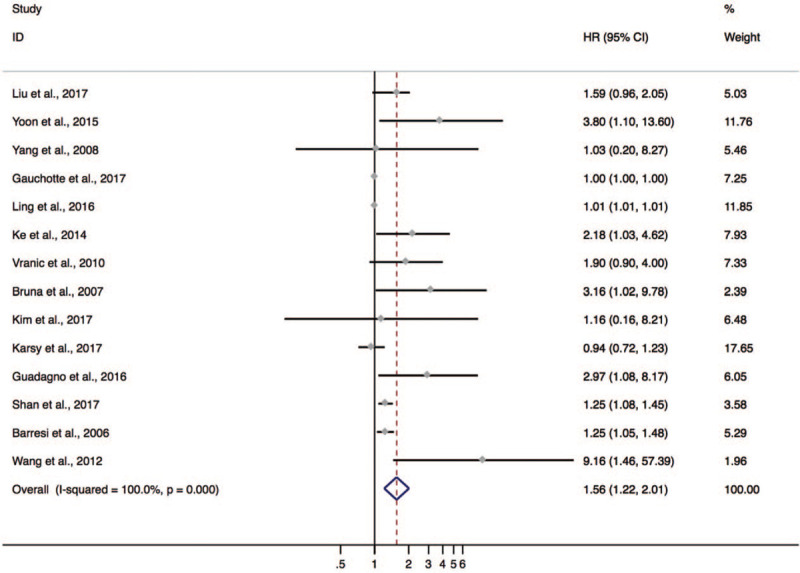
The hazard ratio (HR) of Ki-67 expression associated with OS in meningioma patients calculated by redefined weight.
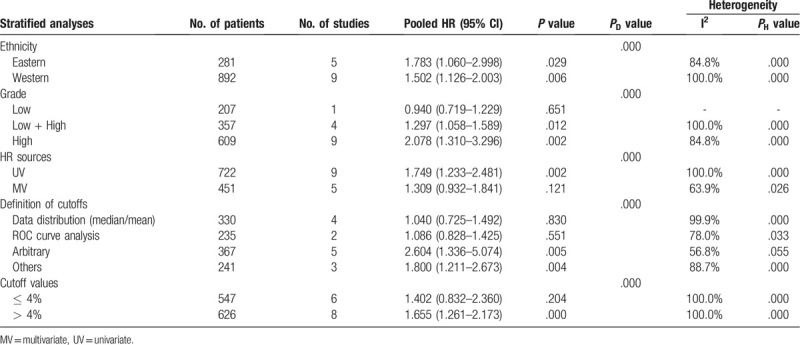
To explore the source of heterogeneity, subgroup analysis on OS was conducted by ethnicity, tumor grade, HR sources (HR calculated from univariate analysis or multivariate analysis), and definitions of cutoffs and cutoff values (Table 2). The results showed that
Table 2.
Subgroup analyses of the associations between Ki-67 and OS.
-
1.
a negative effect of Ki-67 on OS was shown in both Eastern (HR = 1.783; 95% CI: 1.060–2.998; P = .029; I2 = 84.8%) and Western (HR = 1.502; 95% CI: 1.126–2.003; P = .006; I2 = 100.0%) subgroups;
-
2.
for HR sources subgroup analysis, only HR estimated from UV method was adversely correlated with OS (HR = 1.749; 95% CI: 1.233–2.481; P = .002; I2 = 100.0%);
-
3.
in the definition of cutoffs subgroup analysis, higher Ki-67 expression was associated with poor OS in “arbitrary” (HR = 2.604; 95% CI: 1.336–5.074; P = .005; I2 = 56.8%) and “others” (HR = 1.800; 95% CI: 1.211–2.673; P = .004; I2 = 88.7%) subgroups;
-
4.
when it came to cutoff values subgroups, higher Ki-67 reactivity was significantly associated with deteriorated OS only in the “ > 4%” subgroup (HR = 1.655; 95% CI: 1.261–2.173; P = .000; I2 = 100.0%);
-
5.
regarding tumor grade subgroup analysis, the negative prognostic value of higher Ki-67 expression level was demonstrated only in the “Low + High” subgroup (HR = 1.297; 95% CI: 1.058–1.589; P = .012; I2 = 100.0%) and “High” subgroup (HR = 2.078; 95% CI: 1.310–3.296; P = .000; I2 = 84.8%).
3.4. Disease/progression/recurrence-free survival
Thirty-eight studies comprising 4717 patients were included for D/P/RFS analysis. The Ki-67 expression had a significant association with poor D/P/RFS (HR = 1.090; 95% CI: 1.057–1.124; P < .001; I2 = 85.0%; Pheterogeneity = .000) (see Fig. S2, Supplemental Content, which illustrates the association between Ki-67 expression and D/P/RFS). However, it is noticed that the pooled HR was mainly determined by four studies with extremely large weight.[35,56,62,63] Hence, redefined weights according to Mizuki method of the included studies were applied to get the adjusted HR and 95% CI.[29] Intriguingly, the significant association was also identified with a marked between-study heterogeneity (HR = 2.644; 95% CI: 2.264–3.087; P < .001; I2 = 100.0%, Pheterogeneity < .001, Fig. 3).
Figure 3.
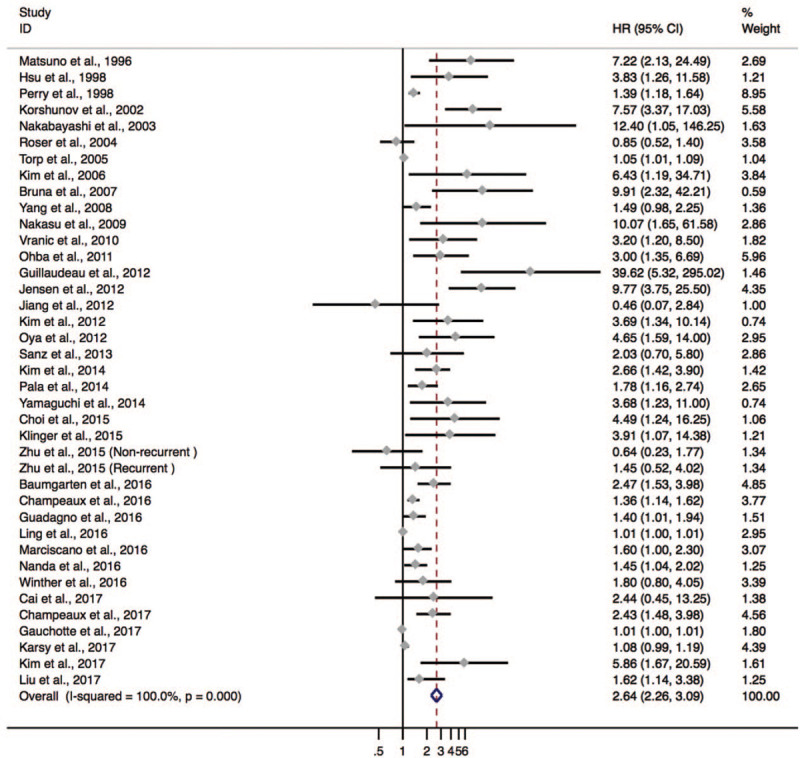
The hazard ratio (HR) of Ki-67 expression associated with D/P/RFS in meningioma patients calculated by redefined weight.
To investigate the potential origin of the between-study heterogeneity, subgroup analysis was performed according to the following factors: ethnicity, tumor grade, HR sources, and definitions of cutoffs and cutoff values (Table 3). The results displayed that
Table 3.
Subgroup analyses of the associations between Ki-67 and D/P/RFS.

-
1.
a negative effect of Ki-67 on D/P/RFS was shown in both Eastern (HR = 3.355; 95% CI: 2.323–4.846; P = .000; I2 = 68.7%) and Western subgroups (HR = 2.413; 95% CI: 2.052–2.837; P = .000; I2 = 100.0%);
-
2.
for HR sources subgroup analysis, HRs estimated from both UV (HR = 2.585; 95% CI: 1.929–3.464; P = .000; I2 = 100.0%) and MV (HR = 2.567; 95% CI: 2.167–3.042; P = .000; I2 = 100.0%) methods were adversely correlated with D/P/RFS;
-
3.
in the definition of cutoffs subgroup analysis, higher Ki-67 expression was associated with poor D/P/RFS in all subgroups;
-
4.
when it came to cutoff values subgroup, higher Ki-67 reactivity was significantly associated with deteriorated D/P/RFS in both the “≤ 4%” (HR = 2.603; 95% CI: 1.974–3.433; P = .000; I2 = 97.1%) and “ > 4%” (HR = 2.667; 95% CI: 2.215–3.211; P = .000; I2 = 100.0%) subgroups;
-
5.
regarding tumor grade subgroup analysis, the negative prognostic value of higher Ki-67 expression level was demonstrated in all subgroups.
3.5. Meta-regression analysis
We also conducted a meta-regression analysis to investigate the impact of various study characteristics on the study estimates of HR. For the OS subset, ethnicity, HR sources, tumor grade, and definitions of cutoffs and cutoff values were entered as explanatory factors. As shown in Table S3, only the geographical origin and definition of cutoffs presented statistically significant association with poor OS in the univariate regression analysis (see Table S3, Supplemental Content, which illustrates the meta-regression analysis in OS subset). Further multivariate regression analysis was conducted by geographical origin and definitions of cutoffs. In multivariate model, the significant association was only presented in definition of cutoffs variable. Nevertheless, for the D/P/RFS subset, all included variables failed to reach statistical significance in univariate analysis (see Table S4, Supplemental Content, which illustrates the meta-regression analysis in D/P/RFS subset).
3.6. Sensitivity analysis
Sensitivity analysis was performed to examine the stability of the current meta-analysis. The result for the OS subset is presented in Table S5, and indicated that studies reported by Ling et al[56] and Gauchotte et al[62] were not stable and significantly influenced the pooled HR (see Table S5, Supplemental Content, which illustrates the sensitivity analysis in OS subset). After excluding these two studies, the meta-analysis of the remaining studies was stable. The pooled HRs (random effect model) for OS changed from 1.565 (95% CI: 1.217–2.013; P = .000) to 1.737 (95% CI: 1.272–2.371; P = .000), and the I2 changed from 100.0% to 82.10%%. For D/P/RFS analysis, 4 studies[35,56,62,63] were found attributed to the instability of the result (see Table S6, Supplemental Content, which illustrates the sensitivity analysis in D/P/RFS subset). Their exclusion made combined HRs under a random effects model alter from 2.644 (95% CI: 2.264–3.087; P = .000) to 2.937 (95% CI: 2.472–3.491; P = .000), and the I2 decreased from 100.0% to 88.30%. The combined HR for D/P/RFS was similar after the exclusion of selected studies, with a stability of meta-analysis confirmed.
3.7. Publication bias
Publication bias is also a potential factor that influenced the pooled results. Funnel plots were drawn to evaluate possible publication bias. The shapes of the funnel plots of both OS and D/P/RFS did obviously show asymmetry (Fig. 4A and B). Quantitative assessment by Egger test for the OS and D/P/RFS subset suggested that our analysis was not stable (P = .001 and P = .000, respectively) (Fig. 4C and D). After refilling “missing” studies by Trim and Fill method, the adjusted pooled HR was still significant for OS (HR = 1.005; 95% CI: 1.004–1.007) and D/P/RFS (HR = 1.008; 95% CI: 1.005–1.010) (see Table S7, Supplemental Content, which illustrates the publication bias assessment in OS subset and D/P/RFS subset).
Figure 4.
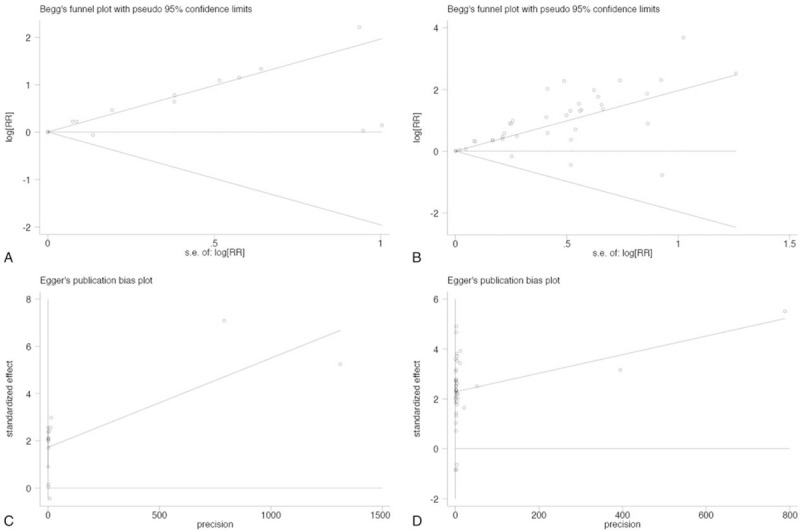
Begg and Egger tests were used to evaluate publication bias on OS (A, C) and D/P/RFS (B, D).
4. Discussion
Negative prognostic factors in meningiomas include young age, male gender, low Karnofsky performance status, high grade, high mitotic rate, subtotal surgical resection, and involvement of the optic nerve.[67] Among these factors, histological grading is the most important determinant of prognosis. Nevertheless, it has been proved inadequate in evaluating the survival outcomes.[68,69] Thus, development of biomarkers may be a promising strategy to improve the prognostic accuracy.[70]
This current study contained of 43 studies and 5012 individuals. To our best knowledge, this was probably the most comprehensive meta-analysis to evaluate the prognostic value of Ki-67 in patients with meningiomas. Our results showed that higher Ki-67 expression was negatively associated with OS as well as D/P/RFS in meningiomas. This negative effect widely existed regardless of different clinical characteristic (ethnicity, tumor grade) and methodological difference between studies (cutoff definition, cutoff value, and HR calculation). Taken together, Ki-67 was a promising biomarker for meningiomas prognosis prediction.
In the present study, we did comprehensive investigation on the between-study heterogeneity through subgroup analysis, meta-regression, and sensitivity analysis. It is suggested that various cutoff values between different studies may be a major contributor to the high heterogeneity in subgroup analysis and meta-regression analysis. We chose 4% as a threshold based on a literature reviewing.[71] As a result of subgroup analysis, higher Ki-67 expression (> 4%) was associated with poor OS and D/P/RFS. Moreover, cutoff value (> 4%) also contributed to a majority of heterogeneity in meta-regression. One possible explanation for these findings may be due to an obvious variety of the original cutoff values (2%–20%) among our included studies. An appropriate cutoff value of Ki-67 was important to predict clinical outcomes in patients with meningiomas. Therefore, we suggested that cutoff value more than 4% would be a reasonable choice for further studies.
In addition, we found that methodological difference in the definition of cutoff value was another factor that influenced the pooled HR estimation. According to the included studies in this meta-analysis, we observed 40% studies defined cutoff value by arbitrary method. Both subgroup analysis and meta-regression analysis showed that arbitrarily defined cutoff value was ascribed to high inter-study heterogeneity. Cutoff value may be changeable due to various factors, such as clinical characteristic (ethnicity, tumor grade) and methodological difference (cutoff definition, cutoff value, and HR calculation). Thus, we suggested that a uniform method to define cutoff value was necessary for future study to reduce the method error between studies.
It is a pity that although we have done comprehensive investigation, the source of heterogeneity is still not completely explained. The significant heterogeneity may be due to the following reasons: first, the tumor grading criteria adopted for each study were different; secondly, the concomitant variables for outcome analysis of each study varied a lot; thirdly, cohorts with small scales would bring sample error.
The systematic evidence provided by the present meta-analysis has far-reaching clinical implications. First, a close follow-up is highly recommended for patients with high Ki-67 expression. Despite the existing publication bias, the pooled results still reached a significant statistical level after refilling “missing” studies. Thus, it is reliable for the conclusion that higher Ki-67 expression level is strongly associated with unfavorable prognosis in meningiomas. Second, Ki-67 is a typical and widely used biomarker, which can be easily incorporated into daily clinical work. What's more, Ki-67 has been reported to be positively correlated with the meningioma grade.[72,73] Therefore, Ki-67 with the combination of traditional predictive factors, such as histological grade, may help the diagnosis with progression and recurrence.
Several limitations of this study must be acknowledged. All of the included studies are retrospective studies. What's more, a large portion of HR was extracted from univariate analysis results other than multivariate analysis results. Besides, non-English studies, unpublished studies, and studies without sufficient data to calculate HRs were not included in the final assessment. Therefore, more well-designed and large-scale prospective studies are needed to confirm our findings.
5. Conclusion
In conclusion, our meta-analysis indicated the significant negative prognostic value of high Ki-67 expression level in the prognosis of meningiomas, especially for patients with Ki-67 index higher than 4%. It is strongly advised for patients with higher Ki-67 expression level to have close follow-ups. However, the sources of heterogeneity were still not completely explained and obvious publication bias was also observed. Further well-designed studies are therefore needed to enhance the robustness of this conclusion.
Author contributions
Data curation: Tingjian Wang, Siying Song, Jiabao Jiang.
Methodology: Tingjian Wang.
Supervision: Changxiang Yan.
Writing – original draft: Tingjian Wang, Ning Liu.
Writing – review & editing: Ning Liu.
Changxiang Yan orcid: 0000-0001-8764-1596.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, CNS = central nervous system, D/P/RFS = Disease /Progression /Recurrence-free survival, HR = Hazard ratios, IHC = immunohistochemistry, OS = Overall survival, PCNA = proliferating cell nuclear antigen, WHO = World Health Organization.
How to cite this article: Liu N, Song Sy, Jiang Jb, Wang Tj, Yan Cx. The prognostic role of Ki-67/MIB-1 in meningioma: a systematic review with meta-analysis. Medicine. 2020;99:9(e18644).
NL and SSY contributed equally to the work.
The authors have no conflicts of interest to disclose.
References
- [1].Ostrom QT, Gittleman H, Fulop J, et al. CBTRUS Statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro Oncol 2015;17: Suppl 4: iv1–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Domingues PH, Sousa P, Otero A, et al. Proposal for a new risk stratification classification for meningioma based on patient age, WHO tumor grade, size, localization, and karyotype. Neuro Oncol 2014;16:735–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Saraf S, McCarthy BJ, Villano JL. Update on meningiomas. Oncologist 2011;16:1604–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 2016;131:803–20. [DOI] [PubMed] [Google Scholar]
- [5].Alexiou GA, Gogou P, Markoula S, et al. Management of meningiomas. Clin Neurol Neurosurg 2010;112:177–82. [DOI] [PubMed] [Google Scholar]
- [6].Stafford SL, Perry A, Suman VJ, et al. Primarily resected meningiomas: outcome and prognostic factors in 581 Mayo Clinic patients, 1978 through 1988. Mayo Clin Proc 1998;73:936–42. [DOI] [PubMed] [Google Scholar]
- [7].Hortobagyi T, Bencze J, Varkoly G, et al. Meningioma recurrence. Open Med (Wars) 2016;11:168–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].van Diest PJ, Brugal G, Baak JP. Proliferation markers in tumours: interpretation and clinical value. J Clin Pathol 1998;51:716–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Spyratos F, Ferrero-Pous M, Trassard M, et al. Correlation between MIB-1 and other proliferation markers: clinical implications of the MIB-1 cutoff value. Cancer 2002;94:2151–9. [DOI] [PubMed] [Google Scholar]
- [10].Zhou Y, Hu W, Chen P, et al. Ki67 is a biological marker of malignant risk of gastrointestinal stromal tumors: a systematic review and meta-analysis. Medicine 2017;96:e7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Xie Y, Chen L, Ma X, et al. Prognostic and clinicopathological role of high Ki-67 expression in patients with renal cell carcinoma: a systematic review and meta-analysis. Sci Rep-UK 2017;7:44281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Pan DH, Wen DY, Luo YH, et al. The diagnostic and prognostic values of Ki-67/MIB-1 expression in thyroid cancer: a meta-analysis with 6,051 cases. Onco Targets Ther 2017;10:3261–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Berlin A, Castro-Mesta JF, Rodriguez-Romo L, et al. Prognostic role of Ki-67 score in localized prostate cancer: a systematic review and meta-analysis. Urol Oncol 2017;35:499–506. [DOI] [PubMed] [Google Scholar]
- [14].Tian Y, Ma Z, Chen Z, et al. Clinicopathological and prognostic value of Ki-67 expression in bladder cancer: a systematic review and meta-analysis. PLoS One 2016;11:e0158891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Xie S, Liu Y, Qiao X, et al. What is the prognostic significance of Ki-67 positivity in oral squamous cell carcinoma? J Cancer 2016;7:758–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Perry A, Stafford SL, Scheithauer BW, et al. The prognostic significance of MIB-1, p53, and DNA flow cytometry in completely resected primary meningiomas. Cancer 1998;82:2262–9. [PubMed] [Google Scholar]
- [17].Cunningham JM, Kimmel DW, Scheithauer BW, et al. Analysis of proliferation markers and p53 expression in gliomas of astrocytic origin: relationships and prognostic value. J Neurosurg 1997;86:121–30. [DOI] [PubMed] [Google Scholar]
- [18].Carvalho LH, Smirnov I, Baia GS, et al. Molecular signatures define two main classes of meningiomas. Mol Cancer 2007;6:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Vranic A, Popovic M, Cör A, et al. Mitotic count, brain invasion, and location are independent predictors of recurrence-free survival in primary atypical and malignant meningiomas: a study of 86 patients. Neurosurgery 2010;67:1124–32. [DOI] [PubMed] [Google Scholar]
- [20].Oya S, Kawai K, Nakatomi H, et al. Significance of Simpson grading system in modern meningioma surgery: integration of the grade with MIB-1 labeling index as a key to predict the recurrence of WHO Grade I meningiomas. J Neurosurg 2012;117:121–8. [DOI] [PubMed] [Google Scholar]
- [21].Kim MS, Kim KH, Lee EH, et al. Results of immunohistochemical staining for cell cycle regulators predict the recurrence of atypical meningiomas. J Neurosurg 2014;121:1189–200. [DOI] [PubMed] [Google Scholar]
- [22].Barresi V, Cerasoli S, Paioli G, et al. Caveolin-1 in meningiomas: expression and clinico-pathological correlations. Acta Neuropathol 2006;112:617–26. [DOI] [PubMed] [Google Scholar]
- [23].Baumgarten P, Gessler F, Schittenhelm J, et al. Brain invasion in otherwise benign meningiomas does not predict tumor recurrence. Acta Neuropathol 2016;132:479–81. [DOI] [PubMed] [Google Scholar]
- [24].Roser F, Samii M, Ostertag H, et al. The Ki-67 proliferation antigen in meningiomas. experience in 600 cases. Acta Neurochir 2004;146:37–44. [DOI] [PubMed] [Google Scholar]
- [25].Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998;17:2815–34. [DOI] [PubMed] [Google Scholar]
- [27].Hayden JA, Cote P, Bombardier C. Evaluation of the quality of prognosis studies in systematic reviews. Ann Inter Med 2006;144:427–37. [DOI] [PubMed] [Google Scholar]
- [28].Altman DG. Systematic reviews of evaluations of prognostic variables. BMJ (Clinical research ed) 2001;323:224–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Nishino M, Giobbie-Hurder A, Hatabu H, et al. Incidence of programmed cell death 1 inhibitor-related pneumonitis in patients with advanced cancer: a systematic review and meta-analysis. JAMA Oncol 2016;2:1607–16. [DOI] [PubMed] [Google Scholar]
- [30]. Higgins J, Green SR. Cochrane Handbook for Systematic Review of InterventionsVersion 5.1.0. 2011. [Google Scholar]
- [31].Matsuno A, Fujimaki T, Sasaki T, et al. Clinical and histopathological analysis of proliferative potentials of recurrent and non-recurrent meningiomas. Acta Neuropathol 1996;91:504–10. [DOI] [PubMed] [Google Scholar]
- [32].Hsu DW, Efird JT, Hedley-Whyte ET. MIB-1 (Ki-67) index and transforming growth factor-alpha (TGF alpha) immunoreactivity are significant prognostic predictors for meningiomas. Neuropath Appl Neuro 1998;24:441–52. [DOI] [PubMed] [Google Scholar]
- [33].Korshunov A, Shishkina L, Golanov A. DNA topoisomerase II-( and cyclin A immunoexpression in meningiomas and its prognostic significance: an analysis of 263 cases. Arch Pathol Lab Med 2002;126:1079–86. [DOI] [PubMed] [Google Scholar]
- [34].Nakabayashi H, Shimizu K, Hara M. Prognostic significance of cyclin A expression in meningiomas. Appl Immunohisto MM 2003;11:9–14. [DOI] [PubMed] [Google Scholar]
- [35].Torp SH, Lindboe CF, Gronberg BH, et al. Prognostic significance of Ki-67/MIB-1 proliferation index in meningiomas. Clin Neuropathol 2005;24:170–4. [PubMed] [Google Scholar]
- [36].Kim Y-J, Ketter R, Henn W, et al. Histopathologic indicators of recurrence in meningiomas: correlation with clinical and genetic parameters. Virchows Archiv 2006;449:529–38. [DOI] [PubMed] [Google Scholar]
- [37].Bruna J, Brell M, Ferrer I, et al. Ki-67 proliferative index predicts clinical outcome in patients with atypical or anaplastic meningioma. Neuropathology 2007;27:114–20. [DOI] [PubMed] [Google Scholar]
- [38].Yang SY, Park CK, Park SH, et al. Atypical and anaplastic meningiomas: prognostic implications of clinicopathological features. J Neurol Neurosurg PS 2008;79:574–80. [DOI] [PubMed] [Google Scholar]
- [39].Nakasu S, Fukami T, Jito J, et al. Recurrence and regrowth of benign meningiomas. Brain Tumor Pathol 2009;26:69–72. [DOI] [PubMed] [Google Scholar]
- [40].Ohba S, Kobayashi M, Horiguchi T, et al. Long-term surgical outcome and biological prognostic factors in patients with skull base meningiomas. J Neurosurg 2011;114:1278–87. [DOI] [PubMed] [Google Scholar]
- [41].Guillaudeau A, Durand K, Bessette B, et al. Egfr soluble isoforms and their transcripts are expressed in meningiomas. PLoS One 2012;7:e37204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Jensen R, Lee J. Predicting outcomes of patients with intracranial meningiomas using molecular markers of hypoxia, vascularity, and proliferation. Neurosurgery 2012;71:146–56. [DOI] [PubMed] [Google Scholar]
- [43].Jiang L, Wang T, Bao Y, et al. A study of UbcH10 expression and its association with recurrence of meningiomas. J Surg Onco 2012;106:327–31. [DOI] [PubMed] [Google Scholar]
- [44].Kim JW, Kim DG, Paek SH, et al. Radiosurgery for atypical and anaplastic meningiomas: histopathological predictors of local tumor control. Stereot Func Neuro 2012;90:316–24. [DOI] [PubMed] [Google Scholar]
- [45].Wang XQ, Jiang CC, Zhao L, et al. Clinical features and treatment of World Health Organization grade II and III meningiomas in childhood: report of 23 cases. J Neurosurg-Pediatr 2012;10:423–33. [DOI] [PubMed] [Google Scholar]
- [46].Sanz J, Ruiz J, Hernández S, et al. Chromosome 1p36 loss and COX-2 overexpression predict recurrence-free survival in completely removed meningioma grade I and II. Rev Esp Patol 2013;46:14–25. [Google Scholar]
- [47].Ke RH, Wang Y, Mao Y, et al. Decreased expression of LASS2 is associated with worse prognosis in meningiomas. J Neuro-Oncol 2014;118:369–76. [DOI] [PubMed] [Google Scholar]
- [48].Pala EE, Kucuk U, Bayol U, et al. Histopathological review of meningiomas: 125 Cases. J Neurol Sci-Turk 2014;31:699–708. [Google Scholar]
- [49].Yamaguchi S, Terasaka S, Kobayashi H, et al. Prognostic factors for survival in patients with high-grade meningioma and recurrence-risk stratification for application of radiotherapy. PLoS One 2014;9:e97108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Choi Y, Lim DH, Yu JI, et al. Prognostic value of Ki-67 labeling index and postoperative radiotherapy in WHO grade II meningioma. Am J Clin Oncol 2015;41:18–23. [DOI] [PubMed] [Google Scholar]
- [51].Klinger DR, Flores BC, Lewis JJ, et al. Atypical reningiomas: recurrence, reoperation, and radiotherapy. World Neurosurg 2015;84:839–45. [DOI] [PubMed] [Google Scholar]
- [52].Yoon H, Mehta MP, Perumal K, et al. Atypical meningioma: randomized trials are required to resolve contradictory retrospective results regarding the role of adjuvant radiotherapy. J Cancer Res Ther 2015;11:59–66. [DOI] [PubMed] [Google Scholar]
- [53].Zhu H, Xie Q, Zhou Y, et al. Analysis of prognostic factors and treatment of anaplastic meningioma in China. J Clin Neurosci 2015;22:690–5. [DOI] [PubMed] [Google Scholar]
- [54].Champeaux C, Dunn L. World Health Organization grade II meningioma: A 10-year retrospective study for recurrence and prognostic factor assessment. World Neurosurg 2016;89:180–6. [DOI] [PubMed] [Google Scholar]
- [55].Guadagno E, Del Basso De Caro M, Pignatiello S, et al. Expression of p40 ((Np63) protein in meningiomas, an unexpected finding: immunohistochemical study and evaluation of its possible prognostic role. J Neuro-Oncol 2016;129:405–13. [DOI] [PubMed] [Google Scholar]
- [56].Ling C, Pouget C, Rech F, et al. Endothelial cell hypertrophy and microvascular proliferation in meningiomas are correlated with higher histological grade and shorter progression-free survival. J Neuropath Exp Neur 2016;75:1160–70. [DOI] [PubMed] [Google Scholar]
- [57].Marciscano AE, Stemmer-Rachamimov AO, Niemierko A, et al. Benign meningiomas (WHO Grade I) with atypical histological features: correlation of histopathological features with clinical outcomes. J Neurosurg 2016;124:106–14. [DOI] [PubMed] [Google Scholar]
- [58].Nanda A, Bir SC, Konar S, et al. Outcome of resection of WHO Grade II meningioma and correlation of pathological and radiological predictive factors for recurrence. J Clin Neurosci 2016;31:112–21. [DOI] [PubMed] [Google Scholar]
- [59].Winther TL, Arnli MB, Salvesen O, et al. Phosphohistone-H3 proliferation index is superior to mitotic index and MIB-1 expression as a predictor of recurrence in human meningiomas. Am J Clin Pathol 2016;146:510–20. [DOI] [PubMed] [Google Scholar]
- [60].Cai Z, Zhang C, Zou Y, et al. Tissue thioredoxin-interacting protein expression predicted recurrence in patients with meningiomas. Int J Clin Oncol 2017;22:660–6. [DOI] [PubMed] [Google Scholar]
- [61].Champeaux C, Houston D, Dunn L. Atypical meningioma. A study on recurrence and disease-specific survival. Neuro-Chirurgie 2017;63:273–81. [DOI] [PubMed] [Google Scholar]
- [62].Gauchotte G, Hergalant S, Vigouroux C, et al. Cytoplasmic overexpression of RNA-binding protein HuR is a marker of poor prognosis in meningioma, and HuR knockdown decreases meningioma cell growth and resistance to hypoxia. J Pathol 2017;242:421–34. [DOI] [PubMed] [Google Scholar]
- [63].Karsy M, Burnett B, Di Ieva A, et al. Microvascularization of grade I meningiomas: effect on tumor volume, blood loss, and patient outcome. J Neurosurg 2017;128:1–0. [DOI] [PubMed] [Google Scholar]
- [64].Kim D, Niemierko A, Hwang WL, et al. Histopathological prognostic factors of recurrence following definitive therapy for atypical and malignant meningiomas. J Neurosurg 2017;128:1–0. [DOI] [PubMed] [Google Scholar]
- [65].Liu H, Li Y, Li Y, et al. STMN1 as a candidate gene associated with atypical meningioma progression. Clin Neurol Neurosur 2017;159:107–10. [DOI] [PubMed] [Google Scholar]
- [66].Shan B, Zhang J, Song Y, et al. Prognostic factors for patients with World Health Organization grade III meningiomas treated at a single center. Medicine 2017;96:e7385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Apra C, Peyre M, Kalamarides M. Current treatment options for meningioma. Expert Rev Neurother 2018;18:1–9. [DOI] [PubMed] [Google Scholar]
- [68].Combs SE, Schulz-Ertner D, Debus J, et al. Improved correlation of the neuropathologic classification according to adapted world health organization classification and outcome after radiotherapy in patients with atypical and anaplastic meningiomas. Int J Radiat Oncol 2011;81:1415–21. [DOI] [PubMed] [Google Scholar]
- [69].Pearson BE, Markert JM, Fisher WS, et al. Hitting a moving target: evolution of a treatment paradigm for atypical meningiomas amid changing diagnostic criteria. Neurosurg focus 2008;24:E3. [DOI] [PubMed] [Google Scholar]
- [70].Rogers CL, Perry A, Pugh S, et al. Pathology concordance levels for meningioma classification and grading in NRG Oncology RTOG Trial 0539. Neuro Oncol 2016;18:565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Abry E, Thomassen IO, Salvesen OO, et al. The significance of Ki-67/MIB-1 labeling index in human meningiomas: a literature study. Pathol Res Pract 2010;206:810–5. [DOI] [PubMed] [Google Scholar]
- [72].Tang Y, Dundamadappa SK, Thangasamy S, et al. Correlation of apparent diffusion coefficient with Ki-67 proliferation index in grading meningioma. Am J Roentgenol 2014;202:1303–8. [DOI] [PubMed] [Google Scholar]
- [73].Pavelin S, Becic K, Forempoher G, et al. Expression of Ki-67 and p53 in meningiomas. Neoplasma 2013;60:480–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


