Abstract
There are no studies on time to test since notification among identified sexual contacts of HIV-positive index clients using program data in Siaya County and Kenya. We sought to understand time to HIV testing by contact characteristics after identification to inform targeted testing interventions. We retrospectively analyzed data from adult (aged ≥18 years) sexual contacts identified by HIV-positive index clients from 117 health facilities in Siaya County (June 2017–August 2018). We used Chi-square tests to assess for differences in characteristics of contacts by HIV testing. We performed Cox proportional hazards analysis and time to HIV testing of contacts analysis including time-varying covariates (cluster-adjusted by facility) to assess characteristics (age, sex, and relationship to index client) associated with time to HIV-testing since notification. Sexual contacts not tested were right censored at last follow-up date. We calculated hazard ratios with 95% confidence intervals to evaluate characteristics associated with time to testing. Of the 6,845 contacts included in this analysis, 3,858 (56.4%) were men. Most were aged 25–34 years (3,209 [46.9%]). Median time to contact testing was 14.5 days (interquartile range, 2.5–62). On multivariable analysis, contacts aged 18–24 years (aHR, 1.32 [95% CI: 1.01–1.73], p = 0.040) and 25–34 years (aHR, 1.18 [95% CI: 1.01–1.39], p = 0.038) had shorter time to HIV testing than those aged 35–44 years. Married polygamous (aHR, 1.12 [95% CI: 1.01–1.25], p = 0.039) and single contacts (aHR, 1.17 [95% CI: 1.08–1.27], p <0.001) had shorter time to HIV testing than married monogamous contacts. Non-spouse sexual contacts had shorter time to HIV testing than spouses, (aHR, 1.23 [95% CI: 1.15–1.32], p <0.001). We recommend enhanced differentiated partner services targeting older adults, married monogamous, and spouse sexual contacts to facilitate early diagnosis, same day treatment, and prevention in Western Kenya and sub-Saharan Africa at large.
Introduction
Targeted HIV case-finding interventions such as partner services [1, 2], also referred to as assisted partner services or contact tracing, focus on ensuring that sexual partners of people living with HIV (PLHIV) are notified of their exposure, are offered testing, and are engaged in care [3]. Notification could be done through a passive or assisted approach, with the latter implemented through provider, contract, or dual referral methods [4]. Through partner services, screening efforts are targeted within specific networks of PLHIV going beyond family or household contacts, which previously were the focus of testing efforts in sub-Saharan Africa [2, 5].
With a high proportion of HIV serodiscordance in sub-Saharan Africa, timely HIV testing facilitates diagnosis and early access to treatment and care for those with new HIV diagnoses, access to prevention services for HIV-negative individuals, and support for various HIV services for both HIV-positive and HIV-negative individuals [6–10]. Reaching sexual contacts of HIV positive index clients could help prevent HIV transmission in serodiscordant relationships, especially among contacts of individuals with new HIV diagnoses [11]. Findings from a recent observational study in San Diego, CA, reported acute or early HIV infection in over one-third of contacts tested through partner services [12]. This suggests the need for early access to HIV-testing services for sexual contacts of index clients.
Early access to HIV testing has been demonstrated as feasible and successful. A cluster randomized controlled trial in Kenya demonstrated that sexual contacts of index clients using immediate assisted partner services were five times more likely to test for HIV within 6 weeks than the passive notification control group [13]. Sexual contacts who were randomized to immediate partner services had a higher proportion tested and receiving an HIV diagnosis for the first time compared to passive referral [13]. Partner services are cost-effective and a safe approach to decrease HIV morbidity and mortality rates in Kenya [14, 15]. However, losses after notification could limit access to HIV testing for sexual contacts. Some studies show losses between identification of sexual contacts to acceptance of testing, though some studies suggest improved linkage to care among those identified through partner services [16–18].
Understanding the time it takes to test notified contacts can help assess the effectiveness of partner services, but few studies have evaluated time to testing for sexual contacts of patients with a new HIV diagnosis among sexual contacts of HIV positive index clients notified. Partner services were implemented in program settings at health facilities in Siaya County in 2017. Siaya County has a high prevalence of HIV reported as between 15.3% (0–64 years) and 21% (15–49 years) [19, 20]. We evaluated time to HIV testing among sexual contacts notified through routine program implementation.
Materials and methods
Study design and setting
This cross-sectional retrospective study included 117 health facilities located in six sub-counties (Alego Usonga [Population in 2019: 224343], Bondo [Population in 2019: 197883], Gem [Population in 2019: 179792], Rarieda [Population in 2019: 152570], Ugenya [Population in 2019: 134354], and Ugunja [Population in 2019: 104241]) within Siaya County [Total Population in 2019: 993183] in the former Nyanza Province in Kenya [21]. Majority of facilities, 107, were government owned while six were privately owned and four were owned by faith based organizations. The health facilities were supported to provide HIV treatment and prevention interventions by Centre for Health Solutions—Kenya (CHS) through funding and technical assistance from the US Centers for Disease Control and Prevention (CDC) in partnership with the Ministry of Health and the Siaya County Department of Health. HIV testing services were provided at outpatient and inpatient service delivery points as well as at comprehensive care centers by HIV testing officers per Kenya’s HIV testing guidelines.
Study population
The study population included adult (aged ≥18 years) sexual contacts identified by index clients with either a new or previous HIV diagnosis. Excluded from the analysis were adults living with HIV who did not identify sexual contacts or whose known sexual contacts had already undergone HIV testing. This analysis focused on the sexual contacts identified by index clients.
Data collection
Routine data were collected by clinical and data officers using partner services registers (June 1, 2017–August 27, 2018). Variables included age, sex, marital status, relationship to index client (spouse or non-spouse), HIV test results, contact HIV test results, date contact was identified by the index client, date of contact’s HIV test, time to HIV test from the date of identification (if tested), and last follow-up at analysis dataset creation (August 27, 2018), facility and sub-county location. Spouse was used to mean the index client was married to the contact elicited while non spouse referred to contacts who were not married to the index from whom the elicitation was done. Data were entered into Epi Info Database, version 7.2 (CDC, Atlanta, GA), were exported into Microsoft Excel (Redmond, WA), and were imported for analysis into Stata version 15.1 (2017; StataCorp, College Station, TX).
Data analysis
Counts, percentages, medians, interquartile ranges (IQR), and ranges were calculated. Chi-square tests were used to assess for differences in characteristics of contacts between those who underwent HIV testing and those who did not. Univariable Kaplan-Meier plots were used to determine HIV testing probability. Log-rank tests were used to determine differences in survival curves. Cox proportional hazards regression analysis was used to assess contact characteristics associated with a shorter time to test for HIV once identified by the index client. Contacts not tested by the data collection date (August 27, 2018) were right censored at their last follow-up date. The proportionality of hazards violation was checked using Schoenfeld residuals, scaled Schoenfeld residuals, and an overall global test. Any violation necessitated using a multivariable model in which time-varying covariates (TVC) interacted with the natural logarithm of time to HIV test to adjust for the violations over time. Significant TVC interaction indicated a modification of hazards over time. Sub-county variations were adjusted for in the multivariable models. All the regression models accounted for facility-level clustering. We calculated hazard ratios (HR) with their respective 95% confidence intervals (CI) and p-values. All statistical tests were evaluated at 5% significance level. All analyses were done using Stata version 15.1.
Ethical approval
This study was approved by AMREF Ethics and Scientific Research Committee and was reviewed in accordance with the Centers for Disease Control and Prevention (CDC) human research protection procedures and was determined to be research. CDC investigators did not interact with human subjects or have access to identifiable data or specimens for research purposes.
Results
Sexual contacts’ characteristics and HIV testing
Of the 6,845 sexual contacts included in this analysis, 3,858 (56.4%) were men, and most (3,209 [46.9%]) were aged 25–34 years. Most sexual contacts (3,873 [56.6%]), were in married monogamous relationships. Slightly over half (3,781 [55.2%]) were non-spousal sexual contacts. Most were from Alego Usonga and Bondo sub-counties. Among the identified sexual contacts, 2,800 (40.9%) were tested for HIV; the rest were still being followed-up for HIV testing at the time of data analysis.
Of the 2800 contacts who were tested, a higher proportion of women (1,280 [45.7%]) were tested compared to those not tested (1,707 [42.2%]; p = 0.004). Significantly more sexual contacts in married monogamous relationships did not undergo testing (2,441 [60.3%]) compared to those who were tested, 1,432 (51.1%), whereas significantly more single contacts underwent testing (439 [15.7]) than those who did not (374 [9.2]; p<0.001). A significantly higher proportion of non-spouse sexual contacts were tested (1,832 [65.4%]) than those not tested (1,949 [48.2%]; p<0.001) as shown in Table 1.
Table 1. Characteristics of sexual contacts of HIV-positive index clients and HIV testing uptake in Siaya County, Kenya.
| Sexual Contacts Characteristics | Total (n = 6,845) | Not tested (n = 4,045) | Tested (n = 2,800) | P value |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| Sex | 0.004 | |||
| Female (n = 2,987) | 2,987 (43.6) | 1,707 (42.2) | 1,280 (45.7) | |
| Male (n = 3,858) | 3,858 (56.4) | 2,338 (57.8) | 1,520 (54.3) | |
| Age (years) | <0.001 | |||
| 18–24 (n = 1,085) | 1,085 (15.9) | 575 (14.2) | 510 (18.2) | |
| 25–34 (n = 3,209) | 3,209 (46.9) | 1,882 (46.5) | 1,327 (47.4) | |
| 35–44 (n = 1,691) | 1,691 (24.7) | 1,059 (26.2) | 632 (22.6) | |
| ≥45 (n = 860) | 860 (12.6) | 529 (13.1) | 331 (11.8) | |
| Marital Status | <0.001 | |||
| Married Monogamous (n = 3,873) | 3,873 (56.6) | 2,441 (60.3) | 1,432 (51.1) | |
| Married Polygamous (n = 264) | 264 (3.9) | 170 (4.2) | 94 (3.4) | |
| Single (n = 813) | 813 (11.9) | 374 (9.2) | 439 (15.7) | |
| Unknown (n = 1,855) | 1,855 (27.1) | 1,037 (25.6) | 818 (29.2) | |
| Widowed (n = 40) | 40 (0.6) | 23 (0.6) | 17 (0.6) | |
| Relationship to Index Client | <0.001 | |||
| Non-spouse Sexual Contact (n = 3,781) | 3,781 (55.2) | 1,949 (48.2) | 1,832 (65.4) | |
| Spouse (n = 3,064) | 3,064 (44.8) | 2,096 (51.8) | 968 (34.6) | |
| Sub-County | <0.001 | |||
| Alego Usonga (n = 1,830) | 1,830 (26.8) | 1,034 (25.6) | 796 (28.4) | |
| Bondo (n = 1,822) | 1,822 (26.6) | 1,065 (26.4) | 757 (27.0) | |
| Gem (n = 1,078) | 1,078 (15.8) | 659 (16.3) | 419 (15.0) | |
| Rarieda (n = 776) | 776 (11.3) | 403 (10.0) | 373 (13.3) | |
| Ugenya (n = 616) | 616 (9.0) | 424 (10.5) | 192 (6.9) | |
| Ugunja (n = 718) | 718 (10.5) | 455 (11.3) | 263 (9.4) |
Time to HIV testing and follow-up among identified sexual contacts
Median time to contact testing from notification of HIV exposure was 14.5 days (IQR, 2.5–62.0). Sexual contacts who were spouses and those in married monogamous relationships had the lowest median number of days to testing at 6.5 days (IQR, 0.5–35.5) and 7.5 days (IQR, 0.5–37.5), respectively. Men had a lower median time to test (14.5 days; IQR, 2.5–51.5) than women (16.5 days; IQR, 2.5–73.5). Among those not tested, the median follow-up duration was 173.5 days (IQR, 124.5–261.5). However, spouses not yet tested (194.5 days; IQR, 133.0–275.0) had longer follow-up durations than sexual contacts who were not spouses (159.5 days; IQR, 114.5–235.5) as shown in Table 2.
Table 2. Time to HIV testing and follow-up among identified sexual contacts of HIV-positive index clients in Siaya County, Kenya.
| Tested for HIV (n = 2,800) | Not Tested (n = 4,045) | |||
|---|---|---|---|---|
| Contact Characteristics | Median Days to HIV test (IQR) | Median Days on follow-up for HIV test for those not yet tested (IQR) | ||
| n | n | |||
| Overall Median | 2,800 | 14.5 (2.5–62.0) | 4,045 | 173.5 (124.5–261.5) |
| Contact Sex | ||||
| Female | 1,280 | 16.5 (2.5–73.5) | 1,707 | 175.5 (119.5–262.5) |
| Male | 1,520 | 14.5 (2.5–51.5) | 2,338 | 172.5 (125.5–259.5) |
| Contact Age, years | ||||
| 18–24 | 510 | 18.5 (3.5–70.5) | 575 | 186.5 (125.5–255.5) |
| 25–34 | 1,327 | 13.5 (2.5–54.5) | 1,882 | 175.5 (125.5–262.5) |
| 35–44 | 632 | 15.5 (2.5–56.5) | 1,059 | 168.5 (119.5–264.5) |
| ≥45 | 331 | 16.5 (2.5–78.5) | 529 | 165.5 (117.5–258.5) |
| Contact Marital Status | ||||
| Married Monogamous | 1,432 | 7.5 (0.5–37.5) | 2,441 | 185.5 (126.5–270.5) |
| Married Polygamous | 94 | 13.5 (2.5–46.5) | 170 | 182.5 (133.5–277.5) |
| Single | 439 | 20.5 (5.5–66.5) | 374 | 160.5 (116.5–252.5) |
| Unknown | 818 | 35.5 (8.5–98.5) | 1,037 | 161.5 (113.5–231.5) |
| Widowed | 17 | 19.5 (5.5–56.5) | 23 | 151.5 (111.5–236.5) |
| Relationship to Index Client | ||||
| Non-spouse Sexual Contact | 1,832 | 21.5 (5.5–74.5) | 1,949 | 159.5 (114.5–235.5) |
| Spouse | 968 | 6.5 (0.5–35.5) | 2,096 | 194.5 (133.0–275.0) |
| Sub-County | ||||
| Alego Usonga | 796 | 9.5 (1.5–35.5) | 1,034 | 199.5 (133.5–297.5) |
| Bondo | 757 | 12.5 (1.5–51.5) | 1,065 | 168.5 (125.5–257.5) |
| Gem | 419 | 24.5 (4.5–96.5) | 659 | 154.5 (108.5–238.5) |
| Rarieda | 373 | 25.5 (3.5–68.5) | 403 | 188.5 (119.5–264.5) |
| Ugenya | 192 | 16.5 (2.5–79.0) | 424 | 171.5 (112.5–222.5) |
| Ugunja | 263 | 39.5 (8.5–104.5) | 455 | 159.5 (117.5–237.5) |
Abbreviations: IQR, interquartile range.
Probability of HIV testing
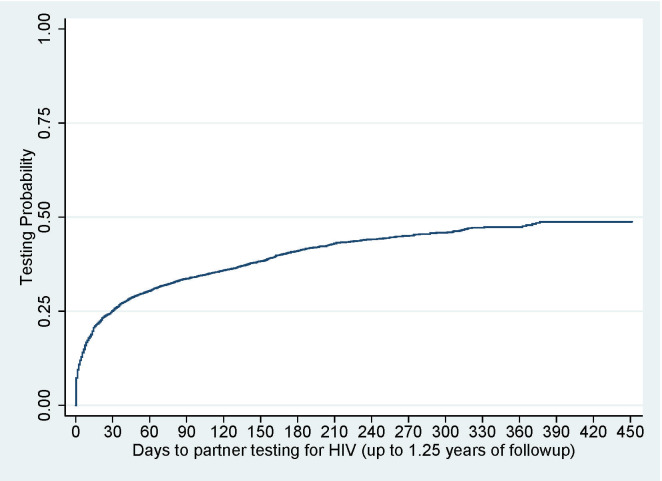
The overall probability of HIV testing among sexual contacts was 48.8% (95% CI: 46.8%–50.8%) over 1.25 years of follow-up (Fig 1).
Fig 1. Overall probability of HIV testing among identified sexual contacts.
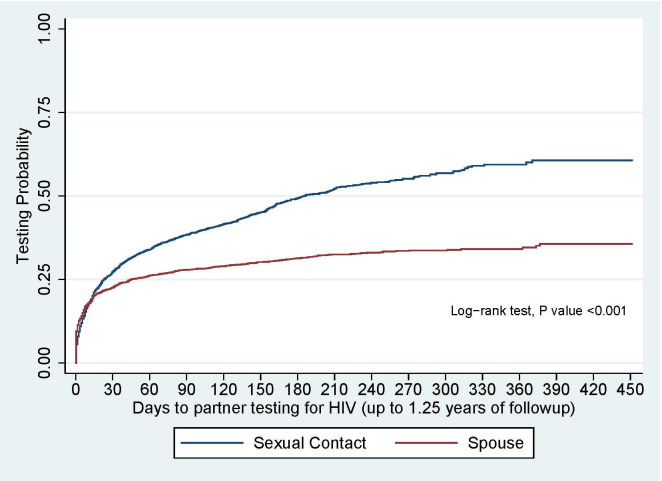
There was strong evidence to show that non-spouse sexual contacts had a significantly shorter time to test than spouses (p<0.001; Fig 2).
Fig 2. Contact time to test for HIV by relationship to index.
Cox proportional hazards regression analysis of time to HIV testing
On univariable analysis, shorter time to testing was associated with younger sexual contacts aged 18–24 years (HR, 1.32 [95% CI: 1.15–1.51]) or 25–34 years (HR, 1.14 [95% CI: 1.04–1.24]), women (HR, 1.1 [95% CI: 1–1.12]), and single contacts (HR, 1.58 [95% CI: 1.25–2.0]). Shorter time to testing also was associated with being a non-spouse sexual contact (HR, 1.68 [95% CI: 1.44–1.96]) and living in Alego Usonga (HR, 1.49 [95% CI: 1.07–2.08]), Bondo (HR, 1.42 [95% CI: 1.02–1.97), or Rarieda sub-counties (HR, 1.65 [95% CI: 1.24–2.18).
On multivariable analysis, younger contacts aged 18–24 years (adjusted hazards ratio [aHR], 1.32 [95% CI: 1.01–1.73]) or 25–34 years (aHR, 1.18 [95% CI: 1.01–1.39]) were associated with shorter time to HIV testing. Age, contact marital status, relationship to index client, and sub-county violated the proportional hazards assumption. Results from the model including the TVC component indicated that married polygamous contacts (aHR, 1.12 [95% CI: 1.01–1.25]) and single contacts (aHR, 1.17 [95% CI: 1.08–1.27]) were associated with a shorter time to HIV testing compared to married monogamous contacts. Similarly, non-spouse sexual contacts were associated with a shorter time to HIV testing compared to spouses (aHR, 1.23 [95% CI: 1.15–1.32]; Table 3).
Table 3. Cox proportional hazards regression analysis of time to HIV testing among sexual contacts of HIV-positive index clients in Siaya County, Kenya.
| Time to Testing | Univariable | Multivariable (Main Model) | Multivariable (Time-Varying Covariate)* | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P value | aHR (95% CI) | P value | aHR (95% CI) | P value | |
| Age, years§ | ||||||
| 18–24 | 1.32 (1.15–1.51) | <0.001 | 1.32 (1.01–1.73) | 0.04 | 0.94 (0.87–1.02) | 0.153 |
| 25–34 | 1.14 (1.04–1.24) | 0.005 | 1.18 (1.01–1.39) | 0.038 | 0.96 (0.9–1.01) | 0.126 |
| 35–44 | Ref | Ref | Ref | |||
| ≥45 | 1.03 (0.89–1.18) | 0.727 | 0.96 (0.79–1.18) | 0.727 | 1.02 (0.95–1.11) | 0.54 |
| Sex | ||||||
| Female | 1.10 (1–1.2) | 0.04 | 0.95 (0.81–1.1) | 0.496 | - | - |
| Male | Ref | Ref | ||||
| Marital Status§^ | ||||||
| Married Monogamous | Ref | Ref | Ref | |||
| Married Polygamous | 0.93 (0.73–1.17) | 0.527 | 0.78 (0.54–1.12) | 0.174 | 1.12 (1.01–1.25) | 0.039 |
| Single | 1.58 (1.25–2) | <0.001 | 0.71 (0.51–1) | 0.05 | 1.17 (1.08–1.27) | <0.001 |
| Unknown | 1.18 (0.98–1.41) | 0.079 | 0.43 (0.3–0.64) | <0.001 | 1.25 (1.14–1.38) | <0.001 |
| Widowed | 1.18 (0.77–1.81) | 0.456 | 0.71 (0.29–1.74) | 0.459 | 1.08 (0.82–1.42) | 0.588 |
| Relationship to Index Client§^ | ||||||
| Non-spouse Sexual Contact | 1.68 (1.44–1.96) | <0.001 | 1.12 (0.84–1.48) | 0.444 | 1.23 (1.15–1.32) | <0.001 |
| Spouse | Ref | Ref | Ref | |||
| Sub-County§ | ||||||
| Alego Usonga | 1.49 (1.07–2.08) | 0.019 | 1.90 (1.14–3.17) | 0.014 | 0.90 (0.77–1.05) | 0.186 |
| Bondo | 1.42 (1.02–1.97) | 0.037 | 1.47 (0.92–2.35) | 0.11 | 0.95 (0.82–1.09) | 0.449 |
| Gem | 1.28 (0.98–1.67) | 0.067 | 1.06 (0.73–1.54) | 0.755 | 1.09 (0.93–1.28) | 0.27 |
| Rarieda | 1.65 (1.24–2.18) | 0.001 | 1.39 (0.9–2.15) | 0.14 | 1.07 (0.91–1.25) | 0.425 |
| Ugenya | Ref | Ref | Ref | |||
| Ugunja | 1.15 (0.82–1.61) | 0.417 | 0.71 (0.4–1.26) | 0.242 | 1.20 (1.01–1.42) | 0.038 |
*Time-varying covariate interacted with natural logarithm of time
§Violated proportional hazards assumptions and was included as a time-varying covariate in Cox model
^Significant time-varying covariate interaction
Abbreviations: HR, hazards ratio; CI, confidence interval; aHR, adjusted hazards ratio.
Discussion
In our study, overall median time to testing of identified sexual contacts was 14.5 days, but other studies have presented varying time-to-test results. A cluster randomized controlled trial in Kenya reported a 67% testing rate of sexual partners within 6 weeks of enrolment of index clients. Those enrolled in the delayed group had only 13% testing in the period after 6 weeks [13]. Another randomized controlled trial in Malawi reported an overall return to clinic rate of 35%, and time to presentation to clinic of partners was associated with the notification approach. The 7-day median time to presentation was lowest (3 days) among locatable partners notified through passive referral [22]. Overall likelihood of testing in our study was 48.8% over 1.25 years is representative of this implementation setting but was higher than the Malawi trial and lower than the Kenya trial.
Sexual contacts who were married in polygamous relationships, who were single, or who were not married to the index client had a shorter time to testing for HIV. Other studies have suggested the importance of marital status in successfully referring contacts for HIV testing. A 2015 cross-sectional study in Tanzania demonstrated a higher rate of testing among sexual partners who were married [23]. Although similar to our findings, in our study, testing proportions were significantly higher among contacts in married polygamous relationships and among non-spouse contacts. The study in Tanzania showed a lower likelihood of referral and testing among sexual contacts who were casual partners or boyfriend/girlfriend compared to those who were married [23]. While this study does not report coverage, it was notable that a shorter time to test was found among non-spouse sexual contacts.
Younger sexual contacts had a shorter time to test than older contacts. A study evaluating data from 55 health departments in the U.S. suggested a higher likelihood of testing among younger sexual contacts aged 13–24 years compared with older partners aged 35–44 years [24]. Similarly, a cluster randomized controlled trial in Kenya reported a higher likelihood of younger sexual contacts opting for immediate as opposed to delayed assisted partner services [13]. While these studies did not evaluate time to test as an outcome, it was notable that while younger contacts had a shorter time to test in the model, the effect was not significant over time.
Early referral of sexual contacts is essential for access to HIV-testing services. Experiences in Malawi suggest that sexual contacts who are located and notified of their exposure were likely to seek testing services. Delays in notification decrease access to testing [25]. In our study, follow-up of identified sexual contacts could exceed 25 weeks, which delayed access to HIV prevention and treatment services and indicates the difficulty of reaching all sexual contacts in resource-constrained settings [25]. Notably, we found that non-spouse sexual contacts had shorter follow-up periods overall. In spite of these findings, the literature suggests that any service that encourages partner notification within implementation settings is beneficial in supporting access to HIV services [17].
Our study has several limitations. This study used routine implementation data; the available data and data collection tools were not specifically designed to investigate our research question and may have been incomplete and or have inaccuracies, which could bias our findings. The analysis methods used included robust multivariable regression methods adjusting for patient characteristics and facility level clustering to mitigate some of the bias. The study also was limited to health facilities within Siaya County, which limits the generalizability of our findings to other counties. The study included all identified sexual contacts eligible for HIV testing at CHS-supported health facilities, reducing participant-related biases. To the best of our knowledge, no other studies have investigated time to HIV testing among contacts identified through partner services in implementation settings in Kenya.
Conclusions
Median time to HIV testing was about 2 weeks. Time to HIV testing was shorter among sexual contacts in married polygamous relationships, single sexual contacts, and among non-spouse sexual contacts. Overall, just under half of all sexual contacts were projected to access HIV testing over 1.25 years of follow-up. Understanding contact characteristics associated with time to HIV testing could help inform differentiated interventions aimed at improving testing service outcomes, to facilitate early diagnosis, same day treatment, and prevention in Western Kenya and sub-Saharan Africa at large.
Acknowledgments
We would like to acknowledge clients who received HIV-testing services at all CHS-supported facilities in Siaya County, HIV-testing providers for testing and following up with clients, health records officers who diligently collected these data, and all CHS staff for administrative support. In addition, we acknowledge support from the Siaya County government and the CDC offices in Kenya and Atlanta for their input during manuscript review.
Data Availability
Data cannot be shared publicly because it contains individual level identifiable information. Data are available from the Siaya County Department of Health (contact via Chief Officer of Health, Siaya County through the lead author) for researchers who meet the criteria for access to confidential data. The email for the Siaya County Chief Officer of Health is omondi.samuel@gmail.com.
Funding Statement
This study has been supported by the President's Emergency Plan for AIDS Relief (PEPFAR) through Centers for Disease Control and Prevention (CDC under the terms of cooperative agreement 1NU2GGH001946) to PW. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hogben M, Behel S. Assisted partner services for HIV case-finding. Lancet HIV. 2017;4(2):e55–6. 10.1016/S2352-3018(16)30211-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Myers RS, Feldacker C, Cesár F, Paredes Z, Augusto G, Muluana C, et al. Acceptability and Effectiveness of Assisted Human Immunodeficiency Virus Partner Services in Mozambique: Results from a Pilot Program in a Public, Urban Clinic. Sex Transm Dis. 2016;43(11):690–5. 10.1097/OLQ.0000000000000529 [DOI] [PubMed] [Google Scholar]
- 3.Payne C, Nakyanjo N, Ddaaki W, Hutchinson N, Burke V, Nalugoda F, et al. HIV Partner Notification Values and Preferences in Rakai, Uganda: A Qualitative Study. Ann Glob Heal [Internet]. 2017;83(1):162 Available from: 10.1016/j.aogh.2017.03.363 [DOI] [Google Scholar]
- 4.WHO Guidelines on Self testing and PNS.pdf.
- 5.Bernstein KT, Stephens SC, Moss N, Scheer S, Parisi MK, Philip SS. Partner Services as Targeted HIV Screening—Changing the Paradigm. Public Health Rep. 2014;129(1_suppl1):50–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guthrie BL, de Bruyne G, Farquhar C. HIV-1-Discordant Couples in Sub-Saharan Africa: Explanations and Implications for High Rates of Discordancy. Curr HIV Res. 2007;5(4):416–29. 10.2174/157016207781023992 [DOI] [PubMed] [Google Scholar]
- 7.Matoga M, Mmodzi P, Massa C, Bula A, Hosseinipour M, Chasela C. Health System Factors Influencing Partner Notification for STIs and HIV in Lilongwe Malawi. A Pre-intervention Phase Assessment for a Quality Improvement Project. J Infect Dis Med. 2018;03(01). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hosseinipour MC, Rosenberg NE. HIV partner notification: Possible and Essential. Sex Transm Dis. 2013;40(12):915–6. 10.1097/OLQ.0000000000000060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma M, Ying R, Tarr G, Barnabas R, Division ID, Hutchinson F. A sustematic review and meta-analysis of community and facility-based approaches to address gaps in HIV testing and linkage in sub-Saharan Africa. Nature. 2015;528(7580):1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. Guidelines on HIV Self-testing and Partner notification [Internet]. 2016. Available from: http://who.int [PubMed]
- 11.Rutstein SE, Brown LB, Biddle AK, Wheeler SB, Kamanga G, Mmodzi P, et al. Cost-effectiveness of provider-based HIV partner notification in urban Malawi. Health Policy Plan. 2014;29(1):115–26. 10.1093/heapol/czs140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green N, Hoenigl M, Chaillon A, Anderson CM, Pond SLK, Smith DM, et al. Partner services in adults with acute and early HIV infection. AIDS. 2017;31(2):287–93. 10.1097/QAD.0000000000001308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cherutich P, Golden MR, Wamuti B, Richardson BA, Ásbjörnsdóttir KH, Otieno FA, et al. Assisted partner services for HIV in Kenya: a cluster randomised controlled trial. Lancet HIV. 2017;4(2):e74–82. 10.1016/S2352-3018(16)30214-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goyette MS, Mutiti PM, Bukusi D, Wamuti BM, Otieno FA, Cherutich P, et al. HIV assisted partner services among those with and without a history of intimate partner violence in Kenya. J Acquir Immune Defic Syndr. 2018;78(1):16–9. 10.1097/QAI.0000000000001638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma M, Smith JA, Farquhar C, Ying R, Cherutich P, Golden M, et al. Assisted partner notification services are cost-effective for decreasing HIV burden in western Kenya. AIDS. 2018;32(2):233–41. 10.1097/QAD.0000000000001697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henley C, Forgwei G, Welty T, Golden M, Adimora A, Shields R, et al. Scale-Up and Case-Finding Effectiveness of an HIV Partner Services Program in Cameroon. Sex Transm Dis. 2013;40(12):909–14. 10.1097/OLQ.0000000000000032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dalal S, Johnson C, Fonner V, Kennedy CE, Siegfried N, Figueroa C, et al. Improving HIV test uptake and case finding with assisted partner notification services. AIDS. 2017;31(13):1867–76. 10.1097/QAD.0000000000001555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bocour A, Renaud TC, Udeagu C-CN, Shepard C. HIV partner services are associated with timely linkage to HIV medical care. AIDS. 2013;27(18):2959–61. 10.1097/QAD.0000000000000016 [DOI] [PubMed] [Google Scholar]
- 19.National AIDS and STI Control Programme (NASCOP). KENPHIA 2018 preliminary report. Vol. 1. 2020.
- 20.Ministry of Health. Kenya HIV Estimates report 2018 [Internet]. 2018. Available from: http://www.nacc.or.ke
- 21.KNBS. 2019 Kenya Population and Housing Census Volume 1: Population by County and Sub-County [Internet]. Vol. I, 2019 Kenya Population and Housing Census. 2019. 49 p. Available from: https://www.knbs.or.ke/?wpdmpro=2019-kenya-population-and-housing-census-volume-i-population-by-county-and-sub-county
- 22.Brown LB, Miller WC, Kamanga G, Nyirenda N, Mmodzi P, Pettifor A, et al. HIV partner notification is effective and feasible in sub-Saharan Africa: Opportunities for HIV treatment and prevention. J Acquir Immune Defic Syndr [Internet]. 2011;56(5):437–42. Available from: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed10&NEWS=N&AN=2011167281 10.1097/qai.0b013e318202bf7d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Plotkin M, Kahabuka C, Christensen A, Ochola D, Betron M, Njozi M, et al. Outcomes and Experiences of Men and Women with Partner Notification for HIV Testing in Tanzania: Results from a Mixed Method Study. AIDS Behav. 2018;22(1):102–16. 10.1007/s10461-017-1936-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song W, Mesfin MS, Michele R, Zhang H, Gilford JW. HIV testing and positivity patterns of partners of HIV-diagnosed people in partner services programs, United States, 2013–2014. Public Health Rep. 2017;132(4):455–62. 10.1177/0033354917710943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown LB, Miller WC, Kamanga G, Kaufman JS, Pettifor A, Dominik RC, et al. Predicting partner hiv testing and counseling following a partner notification intervention. AIDS Behav. 2012;16(5):1148–55. 10.1007/s10461-011-0094-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data cannot be shared publicly because it contains individual level identifiable information. Data are available from the Siaya County Department of Health (contact via Chief Officer of Health, Siaya County through the lead author) for researchers who meet the criteria for access to confidential data. The email for the Siaya County Chief Officer of Health is omondi.samuel@gmail.com.