Abstract
Complete blood count (CBC)-derived parameters such as neutrophil-to-lymphocyte ratio (NLR), monocyte-to-lymphocyte ratio (MLR), eosinophil-to-lymphocyte (ELR) ratio, and platelet-to-lymphocyte ratio (PLR) are sensitive markers of occult inflammation and disease activity for systemic lupus erythematosus, rheumatoid arthritis, psoriasis, esophageal cancer, etc. We assessed NLR, PLR, MLR, and ELR as indicators of inflammation in achalasia patients.
This cross-sectional study included 103 achalasia patients and 500 healthy blood donor volunteers (HD). Demographic, clinical and laboratory information was collected. NLR, MLR, ELR and PLR were calculated. Peripheral Th22, Th17, Th2 and Th1 subsets were determined by flow cytometry. Correlation between hematologic indices and clinical questionnaires scores, HRM parameters and CD4+ T-cells were assessed. Hematologic parameters associated with the different achalasia subtypes were evaluated by logistic regression analysis.
Hemoglobin, leukocytes, lymphocytes, monocytes, and platelets counts were significantly lower in achalasia patients vs controls. NLR (P = .006) and ELR (P < .05) were higher in achalasia patients vs controls. NLR was significantly associated with achalasia in multivariate analysis (P < .001). Compared to HD, the achalasia group was 1.804 times more likely to have higher NLR (95% CI 1.287–2.59; P < .001). GERD-HRQL score had statistically significant correlations with PLR (Pearson's rho:0.318, P = .003), and ELR (Pearson's rho:0.216; P = .044). No correlation between CD4+ T-cells and hematologic indices were determined. NLR with a cut-off value of ≥2.20 and area under the curve of 0.581 yielded a specificity of 80% and sensitivity of 40%, for the diagnosis of achalasia.
NLR is increased in achalasia patients vs HD. Sensitivity and specificity achieved by NLR may contribute to a clinical and manometric evaluation. We suggest these indices as potential indicators of silent inflammation and disease activity.
Keywords: achalasia, biomarker, hematologic indices, neutrophil-to-lymphocyte ratio
Key points
Hematologic indices such as neutrophil-to-lymphocyte ratio have been shown to be markers of occult inflammation. Whether these indices are elevated in patients with achalasia has not been described.
Neutrophil-to-lymphocyte and eosinophil-to-lymphocyte ratio were higher in patients with achalasia compared to healthy controls.
Elevated hematologic indices support the pathophysiological role of the immune system and inflammation in achalasia. Some of these indices might be markers of disease activity.
1. Introduction
Achalasia is a primary esophageal motility disorder characterized by esophageal aperistalsis and an incomplete or absent relaxation of the lower esophageal sphincter (LES).[1] There are three distinct subtypes of achalasia which are defined with high-resolution manometry (HRM) parameters and are subclassified according to the Chicago Classification of esophageal motility disorders into: type I (classic achalasia with failed peristalsis), type II (with panesophageal pressurization), and type III (with premature spastic contractions).[2] It has an annual reported incidence of approximately 1/100,000 worldwide.[3] The pathogenesis of this motility disorder likely involves autoimmune and/or inflammatory processes.[4] Compared to the general population, patients with achalasia are three to four times more likely to suffer from an autoimmune disease,[5,6] and have a higher prevalence of serum autoantibodies against antigens present in the myenteric plexus.[7–10] Furthermore, there is a distinct pattern of inflammatory cells and cytokines present in both the LES and in peripheral blood samples.[9–11] Until today, there are no simple and clinically relevant biomarkers for patients with achalasia; therefore, associations of clinical outcomes with biomarkers obtained from routine tests, such as complete blood cell count (CBC), are worth investigating.
CBC-derived parameters such as neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR), among others, have recently shown to be highly sensitive markers of occult inflammation in autoimmune and inflammatory disorders.[12,13] These hematologic indices have also been widely used to determine the severity of inflammation and as predictors of poor outcomes in cardiovascular disease,[14] oncologic disease,[15,16] diabetes mellitus, hypertension, and autoinflammatory diseases.[17]
Nonetheless, these hematologic indices have not been analyzed in achalasia patients. For this reason, NLR, PLR, monocyte-to-lymphocyte ratio (MLR), and eosinophil-to-lymphocyte ratio (ELR) were determined in a cohort of patients with achalasia to evaluate the usefulness of these parameters as indicators and their clinical significance in silent inflammation and determining disease severity.
2. Methods
2.1. Design
This was an exploratory, observational, and cross-sectional study conducted in a tertiary referral care center (Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán), between January 2011 to June 2018. It included 603 participants, 103 consecutive patients with idiopathic achalasia and 500 healthy volunteers.
2.2. Patients
All patients were diagnosed by HRM, upper endoscopy and esophagogram. Patients ≥ 17 years old were enrolled in the study. Exclusion criteria included Chagas disease, esophageal stricture, esophageal scleroderma, gastric or esophageal cancer, peptic stricture, other esophageal motility disorders, ASA score of 4, hiatal hernia greater than 5 cm, contraindications to laparoscopic approach, pregnant patients or those who had hematologic disease, cancer, severe renal or liver disease, ongoing infection, patients on aspirin or steroid treatment. Patients’ clinical records were carefully reviewed according to a pre-established protocol. The following data was collected retrospectively for each study participant from the hospital's medical records: demographic features, type of achalasia, family history of autoimmunity, and current diagnosis of organ or systemic autoimmunity. When a comorbid autoimmune diagnosis was found, all relevant data (i.e., date of diagnosis, presenting symptoms, clinical and laboratory confirmatory test results, and treatment administered) were recorded. Finally, the presence of chronic inflammatory conditions (i.e., asthma, allergic rhinitis, gout, and rosacea) was recorded. CBC parameters used in the study were the latest laboratory findings recorded prior to surgical intervention.
For comparison, 500 healthy controls who volunteered at the blood bank were recruited for the study. All included controls had no previously known cardiovascular, metabolic, inflammatory or neoplastic disease. Demographic, clinical and laboratory information were also collected.
2.3. Laboratory information
All CBC analyses were performed with an automatic hematologic analyzer (Beckmancoulter DxH 800 Hematology Analyzer). Hemoglobin (Hb), white blood cell (WBC), neutrophils, lymphocytes, monocytes, eosinophils, and platelet counts were obtained prior to surgical treatment. NLR was obtained dividing neutrophil count by lymphocyte count; PLR was obtained by dividing platelet count by lymphocyte count; MLR was obtained by dividing monocyte count by lymphocyte count; and ELR was obtained by dividing eosinophil count by lymphocyte count. Blood samples were collected in dipotassium ethylenediaminetetraacetic acid tubes.
2.4. Peripheral blood samples
A venous blood sample was drawn from each subject to perform flow cytometry analysis and RNA isolation.
2.5. Flow Cytometry
Peripheral blood mononuclear cells (PBMCs) were obtained by gradient centrifugation on Lymphoprep (Axis-Shield PoC AS, Oslo, Norway). Cell pellet was resuspended in 1 mL RPMI at 1–2 × 106 cell/mL, and cells suspension was treated with 2 μL of a cell activation cocktail of phorbol-12myristate 13-acetate (40.5 μM) and ionomycin (669.3 μM) in DMSO (500X) and brefeldin A (BioLegend Inc., San Diego, CA) for 6 hours at 37°C in CO2 incubator.
PBMCs were incubated with 5 μL of Human TruStain FcXTM (BioLegend Inc.) per million cells in 100 mL PBS for 10 minutes and then they were labeled with 5 μL of antihuman CD3-FITC-labeled, antihuman CD4-PeCy5-labeled and antihuman CD161-APC–conjugated monoclonal antibodies (BD Biosciences, San Jose, CA); antihuman CD3-FITC–labeled, antihuman CD4-PeCy5–labeled and antihuman CD25-APC–conjugated monoclonal antibodies (BD Biosciences) in separated tubes during 20 min at 37°C in the dark. Cells were permeabilized with 200 μL of cytofix/cytoperm solution (BD Biosciences) at 4°C for 30 minutes. Intracellular staining was performed with an anti-human IL-22–PE–, IL-17A–PE–, IL-4–PE–, IFN-γ–PE–labeled mouse monoclonal antibodies (BD Biosciences) for 30 minutes at 4°C in the dark. An electronic gate was made for CD3+/CD4+/CD161- cells, CD3+/CD4+/CD161+ cells, and CD3+/CD4+/CD25-cells (Fig. 1). Results are expressed as the relative percentage of IL-22+, IL-17A+, IL-4+, and IFN-γ+ expressing cells in each gate (Fig. 2). As isotype control, IgG1-FITC/IgG1-PE/CD45-PeCy5 mouse IgG1 kappa (BD Tritest, BD Biosciences) was used to set the threshold and gates in the cytometer. We ran an unstained (autofluorescence control) and permeabilized PBMCs sample. Autofluorescence control was compared to single stained cell positive controls to confirm that the stained cells were on scale for each parameter. Besides, BD Calibrate 3 beads were used to adjust instrument settings, set fluorescence compensation, and check instrument sensitivity (BD calibrates, BD Biosciences). Fluorescence minus one (FMO) controls were stained in parallel using the panel of antibodies with sequential omission of one antibody, except for the anti-IL-22, anti-IL-17A, anti-IL-4, anti-IFN-γ, which was replaced by an isotype control rather than simply omitted. Finally, T subsets were analyzed by flow cytometry with an Accuri C6 (BD Biosciences). A total of 500,000 to 1,000,000 events were recorded for each sample and analyzed with the FlowJo X software (Tree Star, Inc.).
Figure 1.
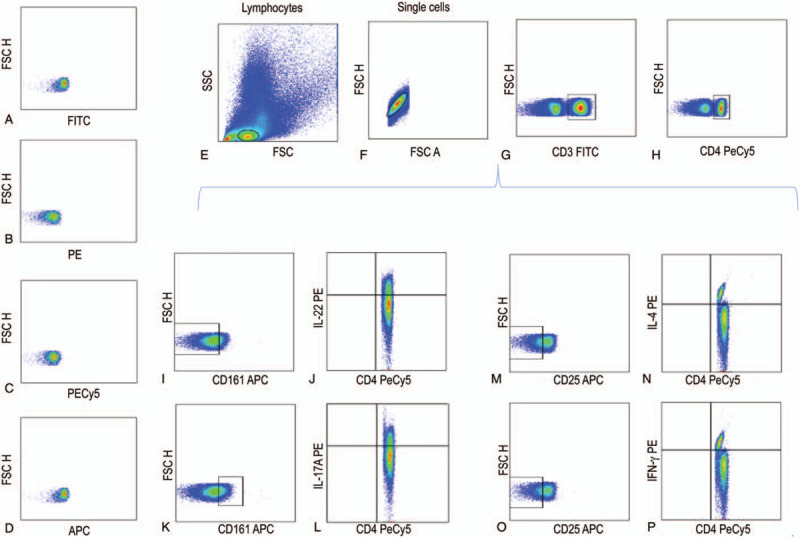
Representative gating strategy of each cell population of CD4 effector T cells. (A) IgG1 FITC isotype control, (B) IgG1 PE isotype control, (C) IgG1 PECy5 isotype control, (D) IgG1 APC isotype control, (E) Lymphocytes, (F) Single cells, (G) CD3+ cells, H) CD3+/CD4+ cells, (I) CD3+/CD4+/CD161- cells, (J) CD3+/CD4+/CD161-/IL-22+ cells, K) CD3+/CD4+/CD161+ cells, (L) CD3+/CD4+/CD161+/IL-17+ cells, (M) CD3+/CD4+/CD25- cells, (N) CD3+/CD4+/CD25-/IL-4+ cells, (O) CD3+/CD4+/CD25- cells, and (P) CD3+/CD4+/CD25-/IFN-γ+ cells.
Figure 2.
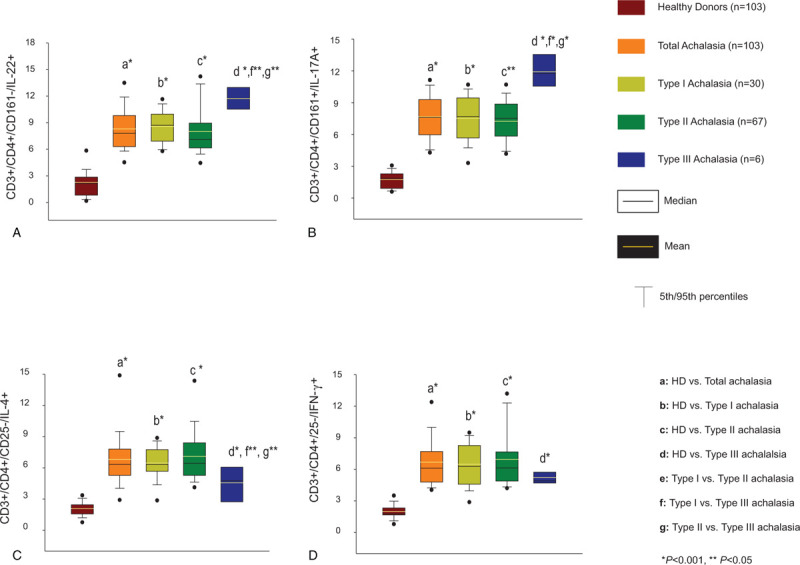
Percentages of circulating (A) CD4+/CD161-/IL-22+ cells, (B) CD4+/CD161+/IL-17A+ cells, (C) CD4+/CD25-/IL-4+ cells, and (D) CD4+/CD25-/IFN-γ+ cells. The results are expressed as the mean (horizontal yellow line), median (horizontal black line), and 5th/95th percentiles. HD, healthy donors.
2.6. Symptoms score evaluation
At the time of diagnosis, patients with achalasia were required to complete 3 international questionnaires (Eckardt symptom score, eating assessment tool (EAT-10) questionnaire, gastroesophageal reflux disease-health related quality of life (GERD-HRQL) questionnaire) aimed to assess the frequency and severity of esophageal symptoms (i.e., higher scores represent higher frequency/severity).[18] Data obtained from these questionnaires, as well as HRM-derived parameters, were used as surrogate markers of disease severity.
2.7. Antinuclear antibodies (ANA) testing
Only those patients in the idiopathic achalasia group newly diagnosed and without previous treatment donated a blood sample that was used for ANA assessment by indirect immunofluorescence with HEp-2 cells IgG isotype (Inova Diagnostics Inc, San Diego, CA). Positivity was assigned according to our local cut-off values (i.e., speckled: >1:160; nucleolar: >1:40; cytoplasmic: >1:40; mitochondrial: >1:160; and others: >1:40).[19]
2.8. Ethical considerations
The protocol was approved by the Ethical Medical Committee in our institution and it was according to the principles expressed in the Declaration of Helsinki, 1989. Only patients who gave a written informed consent were recruited for this study.
2.9. Statistical analysis
Continuous parameters are described as mean ± standard deviation (SD) and qualitative parameters as numbers and percentages. To determine differences between groups, Kruskal–Wallis test, Student t test, and Mann-Whitney U test were performed for continuous variables. Gender differences between groups were compared using χ2 test. Pearson correlation coefficient was used for assessment of correlation between hematologic indices and clinical questionnaires’ scores, as well as HRM parameters. Hematologic parameters associated with the different achalasia types were assessed by logistic regression analysis. Receiver operating characteristic curves (ROC) were plotted and areas under the curve (AUC) were calculated to assess differentiating performance of NLR, PLR, MLR, and ELR between achalasia patients and controls. One-way analysis of variance on ranks Kruskal–Wallis, if the Kruskal–Wallis test was significant, a post-hoc analysis (Dunn test) was performed for all pairwise multiple comparison procedures regarding cytometric analysis. A P value ≤.05 was considered statistically significant. Statistical analyses were performed with SPSS version 25.0 (SPSS, Chicago, IL).
3. Results
3.1. Demographic and clinical characteristics
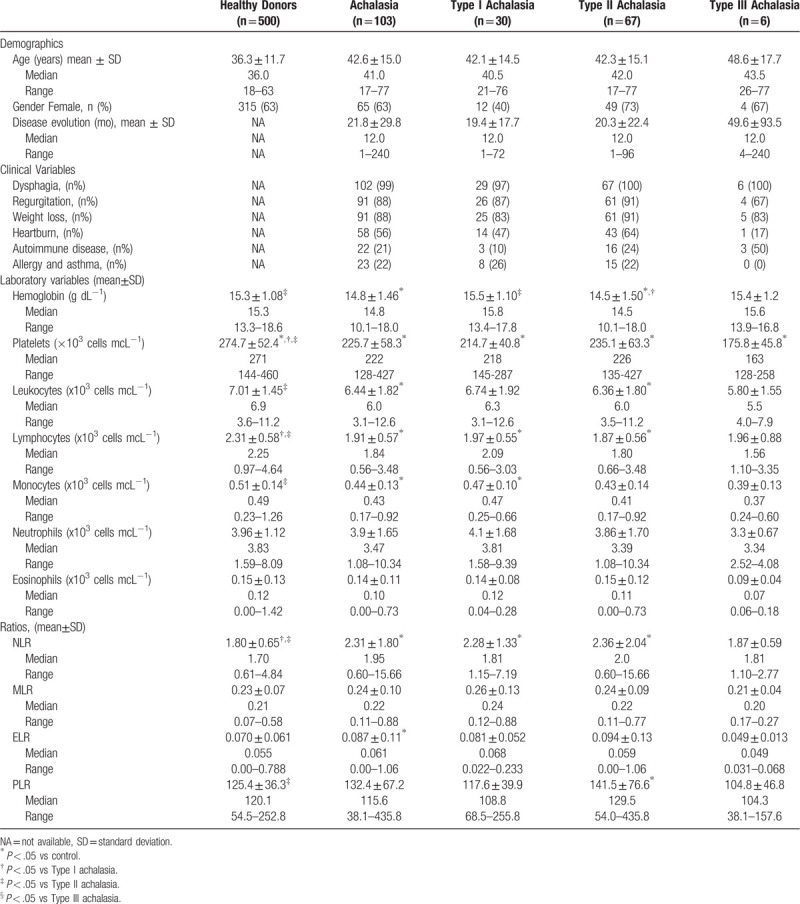
Age, gender, and laboratory data for 103 achalasia patients and 500 healthy controls are shown in Table 1. There was no significant difference in gender, with both groups having 63% of females. Clinical manifestations in patients with achalasia included dysphagia (99%), regurgitation (88%), and weight loss (88%). Prevalence of autoimmune comorbidity was 21% in achalasia patients, and 22% had a history of allergy or asthma. The median duration of disease at the time of assessment was 12 (range: 1–240) months.
Table 1.
Demographic, clinical and laboratory variables of achalasia cohort.
3.2. Percentage of circulating CD4+ T cell subpopulations
To determine the effector CD4 T-cell subpopulations, PBMCs were immunophenotyped and analyzed by flow cytometry. Relative percentage of circulating Th22, Th17, Th2 and Th1 cells from achalasia patients were conspicuously higher when compared with healthy individuals (Fig. 2). Moreover, type III achalasia patients had higher levels of Th22 and Th17 compared to type II and type I achalasia (Fig. 2; P < .05), while they had lower levels of Th2 vs type II and type I achalasia (Fig. 2; P < .05).
3.3. Complete blood count, NLR, PLR, MLR, and ELR levels in patients with achalasia
CBC results were within normal limits in both groups. Hemoglobin, platelet count, leukocyte count, total lymphocytes, and monocytes were significantly lower in achalasia patients compared to controls (P = .003, P < .001, P = .003, P < .001, and P < .001; respectively).
NLR (2.31 ± 1.80 vs 1.80 ± 0.65; P = .006), and ELR (0.087 ± 0.11 vs 0.070 ± 0.061; P = .031) were significantly increased in achalasia patients compared with controls (Table 1). Moreover, NLR showed statistically significant difference between type I and type II achalasia versus control group (2.28 ± 1.3 and 2.36 ± 2.04 vs 1.80 ± 0.65; P = .038, P < .001, respectively), while PLR among type II achalasia and control group (141.5 ± 76.6 vs 125.4 ± 36.3; P = .021). Nonetheless, there was no significant statistical difference when comparing MLR (0.26 ± 0.13 vs 0.24 ± 0.09 vs 0.21 ± 0.04; P = .52), and ELR (0.081 ± 0.052 vs 0.094 ± 0.13 vs 0.049 ± 0.013; P = .61) between type I, type II, and type III achalasia, respectively.
3.4. Multivariate analysis of NLR, PLR, MLR, and ELR with achalasia
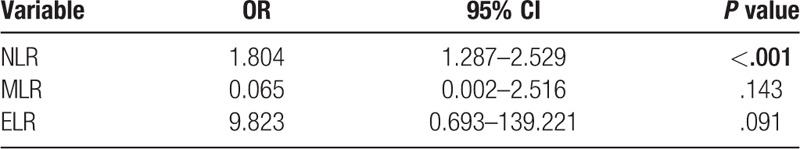
In univariate analysis, these hematologic indices were associated with achalasia at the 10% level of significance, except for PLR (P = .137), which was excluded from multivariate analysis. The multivariate analysis with binary logistic regression showed a significant association between NLR and achalasia. NLR was a predictor of the presence of achalasia (odds ratio [OR] = 1.804, 95% CI [CI] = 1.287–2.529, P < .001). These associations remained statistically significant after adjusting for age and gender (Table 2).
Table 2.
Logistic regression analysis between achalasia and controls.
3.5. Predictive value of NLR and ELR for diagnosing achalasia
ROC was created to determine the cut-off and usefulness of NLR as a diagnostic tool to predict the presence of achalasia. In the ROC analysis of NLR, the AUC was 0.581 (95% CI = 0.515–0.646, P = .01). ROC analysis suggested that the optimal cutoff value for predicting the presence of achalasia was ≥2.20 for NLR, which maximized the Youden's index, yielding 40% sensitivity and 80% specificity (Fig. 3). The AUC for the ROC analysis of ELR was not statistically significant.
Figure 3.
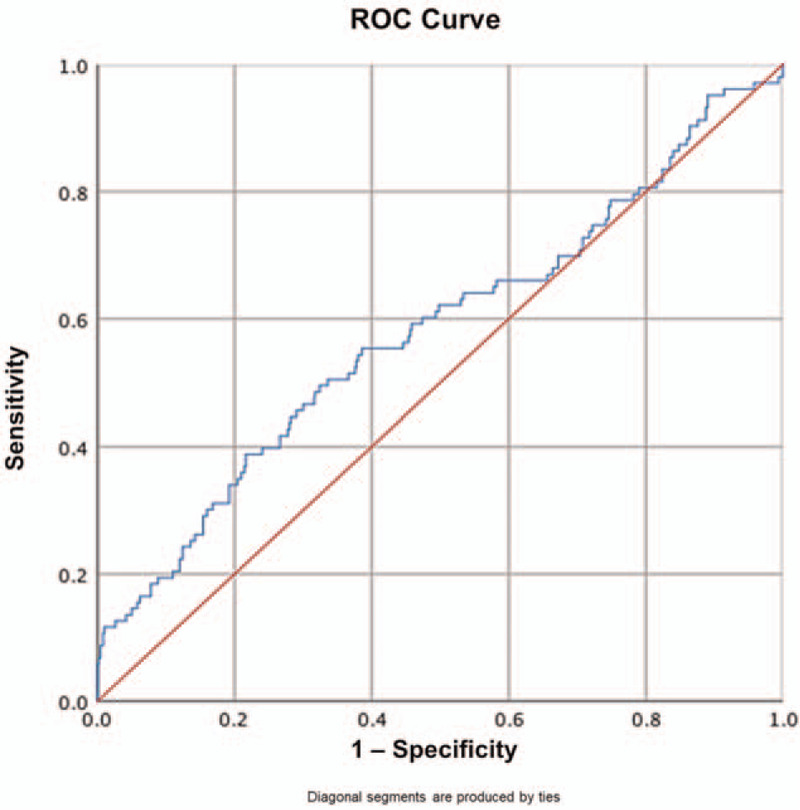
Receiver-operator curve to determine NLR cut-off value for predicting the presence of achalasia. NLR = Neutrophil-to-lymphocyte ratio.
3.6. Correlation of NLR with circulating CD4+ T Cell subpopulations
After performing a bivariate (Pearson) correlation analysis, there was no statistically significant correlation between NLR and Th22 (P = .989), Th17 (P = .165), Th2 (P = .980), or Th1 (P = .988) cells in patients with achalasia.
3.7. Correlation of antinuclear antibodies with NLR, PLR, MLR, and ELR
There was no significant difference in any hematologic index when comparing ANA-positive and ANA-negative achalasia patients (Table 3).
Table 3.
Subgroup comparisons of hematologic indices.
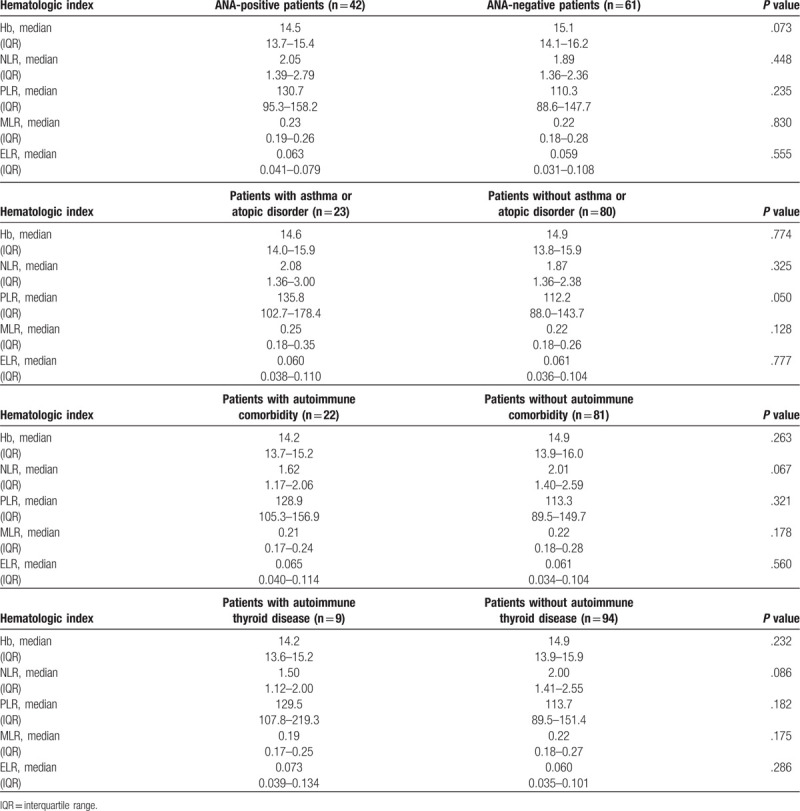
3.8. Correlation of NLR, PLR, MLR, and ELR with achalasia/atopic disorders and achalasia/autoimmune disease
Achalasia patients with history of asthma or atopic disorders had significantly higher PLR (135.8 vs 112.2, P = .05) compared to patients without inflammatory diseases (Table 3).
There was no significant difference in any hematologic index when comparing patients with autoimmune comorbidity and patients without an autoimmune disorder. Neither there was difference when comparing those with thyroid versus without thyroid involvement (Table 3).
3.9. Correlation of NLR, PLR, MLR, and ELR with disease severity
GERD-HRQL score had statistically significant correlations with both PLR (Pearson rho: 0.318, P = .003), and ELR (Pearson rho: 0.216; P = .044). In addition, MLR correlated weakly with EGJ resting pressure (Pearson rho: –0.225, P = .038). There were no other statistically significant correlations between hematologic indices and disease severity markers, such as questionnaires’ scores and HRM parameters (Table 4).
Table 4.
Correlation between disease severity markers and NLR, MLR, BLR, and PLR.
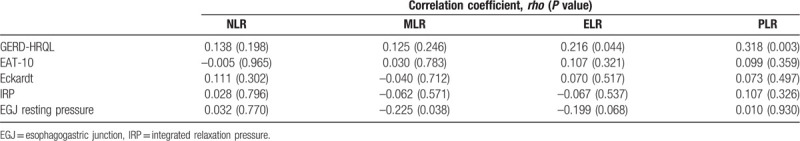
4. Discussion
Chronic inflammation is triggered by overproduction of acute-phase reactants, reactive nitrogen and oxygen intermediates, inflammatory cytokines, autoantibodies, and immune complex deposition. The inflammation leads to changes on one or more cellular lineages of the hematopoietic system. Thus, CBC-derived parameters and their relation to certain diseases have recently received attention from researchers. One of these CBC parameters is the NLR, where neutrophils are a critical component of the innate immune response, while lymphocytes of the adaptive system. It has been described that neutrophils participate in the process of inflammation and antigen presentation, regulating the activity of other cell types and destroying tissue in inflammatory bowel disease[20] and autoimmune disorders.[21,22]The increased in NLR during inflammation can be due to a drop in lymphocyte count, an increase in neutrophil count or both.[23] This theoretical evidence justifies the use of NLR in quantifying inflammation. Therefore, NLR is considered to be a marker of inflammation and, due to its simplicity, reproducibility and low cost, has been studied in many medical conditions. An elevated NLR is an indicator of poor prognosis in multiple myeloma, where a relative lymphocytopenia and neutrophil leukocytosis is in favor of pro-tumor inflammatory response.[24,25] Increased NLR is also, a predictive parameter of an inflammatory situation, an indicator associated with occult inflammation in certain conditions[26–28] or an indicator of disease activity in some autoimmune diseases.[21,29,30] In fact, some studies have reported an increased NLR and ELR levels in patients with psoriasis, systemic lupus erythematosus, esophageal cancer, euthyroid chronic autoimmune thyroiditis, rheumatoid arthritis, etc.,[15,21,23,28–32] which are in line with our study where we demonstrated that NLR and ELR were significantly increased in patients with achalasia. This finding suggests that NLR could be a indicator of underlying silent inflammation in patients with achalasia. Additionally, this indicates that an increased proportion of neutrophil count may play a role in achalasia. Moreover, NLR was a predictor of the presence of achalasia (OR = 1.804, 95% CI = 1.287–2.529, P < .001). Despite this, the full extent to which NLR contributes to the clinical manifestations remains to be elucidated, since NLR values did not correlate with disease symptom severity. ROC analysis of our data suggested that a cut-off value of 2.20 for NLR would maximize specificity (80%) and sensitivity (40%) of the test. This cut-off value is within the described range in the meta-analysis reported by Yodying.[15]
There was no statistically significant correlation between NLR and the different CD4+ T cell subpopulations. This data suggests an independent implication of the innate and adaptive immune system in the pathophysiology of achalasia.[4,7–9] It is important to highlight that type II achalasia had an increment of Th17 (potentially pro-inflammatory cell subset) and a decrease of Th2 cell percentage (potentially anti-inflammatory cell subset) when compared with type I achalasia. This supports that type II achalasia is an active pathologic process in an earlier stage of the disease. NLR being an indicator of occult inflammation, whose evaluation is simple, inexpensive and rapid, and its value can be easily calculated. In contrast, the application of more sophisticated and expensive laboratory techniques (i.e., flow cytometry) allow to further characterize the specific subsets of inflammatory cells belonging adaptive immune response in achalasia patients and in the future probably to design specific therapies address to modulate a particular subset. It is important to highlight that in healthy populations, NLR is increased in the elderly.[33] However, in the multivariate analysis, NLR remained a risk factor for achalasia independent of age and gender.
There were not statistically significant differences on the hematological indices when comparing ANAs positive with ANAs negative patients. Moreover, there were not statistically significant differences on hematological indices when comparing achalasia patients without autoimmune comorbidities with a subgroup of patients with achalasia and another autoimmune comorbidity. The above can be attributed to the inflammatory autoimmune etiopathogenesis of the disease. However, a subgroup of patients with achalasia who had a history of asthma or atopic disorders had higher PLR values than patients who did not have such comorbidities.
Platelets also have a critical role in inflammation. They facilitate the secretion of chemokines and inflammatory cytokines and interact with both the classical and alternative pathways of complement. Platelets play an active integral role in innate and adaptive immunity.[34] In disease state or sterile inflammation, platelet microparticles released from thrombosis sites activate adaptive immune cells leading to antibody synthesis and alter lymphocytes activities, therefore, an immune response is stimulated. PLR is also an inflammatory index in some diseases, for example, it is increased in patients with rheumatoid arthritis and systemic lupus erythematosus.[21] Moreover, achalasia patients have shown a correlation among GERD-HRQL score PLR and ELR, and MLR with EGJ resting pressure.
Eosinophils have been traditionally associated with allergic inflammation.[35] However, eosinophils have recently been shown to play a role in regulating innate and adaptive immunity through production of cytokines.[36] More importantly, eosinophils might contribute to the initiation of immune responses in the gastrointestinal tract. Specifically, eosinophils appear to target Th1 cells,[37] and might interact with neighboring dendritic cells within the tissue.[38] Whether this local activity is reflected systemically in eosinophil counts has not been described. Nonetheless, ELR might be useful in predicting patients at risk of recurrence in nasal polyposis and polyp intensity[39,40] and in assessing the severity of allergic rhinitis in pediatric patients.[41] This hematologic index has been reported to be elevated in some systemic autoimmune diseases.[29] Our findings reflect this, since ELR values were higher among achalasia patients. Furthermore, it may also be a marker of disease activity, since it had a positive correlation with the esophageal symptom's questionnaire GERD-HRQL.
The current study has some limitations. First, this was a cross-sectional study. Thus, we could not assess if there was any change in the hematological indices through the course of the disease. Second, our study was limited by its retrospective design, which could lead to selection bias. Third, the patients in our study were recruited from a single center.
Certainly, we acknowledge all the aforementioned factors as limitations of our study. However, we consider our manuscript of relevance particularly for the scant information regarding achalasia and its hematological indices that certainly deserve to be studied.
To our knowledge, this is the first study to evaluate the usefulness of NLR, PLR, MLR, and ELR in differentiating between achalasia patients and healthy controls.
In conclusion, NLR is definitely increased in patients with achalasia compared to healthy controls, and other indices such as ELR and PLR might be indicators of disease activity as measured by the GERD-HRQL questionnaire. We proposed NLR as a potential indicator of silent inflammation. The sensitivity and specificity achieved are not enough to replace a well conducted clinical evaluation and HRM, which remains the gold standard for diagnosing this entity. Notwithstanding the above, these findings contribute to the growing evidence that supports the pathophysiological role of the innate and adaptive immune responses and inflammation in achalasia. The utility of these hematologic indices to discriminate different diseases with esophageal manifestations (i.e., dysphagia) or whether these indices resemble the local inflammatory phenotype seen under histological analysis remains to be elucidated.
Author contributions
Conceptualization: Fidel López-Verdugo, Janette Furuzawa-Carballeda, Gonzalo Torres-Villalobos.
Data curation: Fidel López-Verdugo, Janette Furuzawa-Carballeda, Fernanda Romero-Hernández, Enrique Coss-Adame, Miguel A Valdovinos, Gonzalo Torres-Villalobos.
Formal analysis: Fidel López-Verdugo, Janette Furuzawa-Carballeda, Gonzalo Torres-Villalobos.
Investigation: Fidel López-Verdugo, Janette Furuzawa-Carballeda, Fernanda Romero-Hernández, Enrique Coss-Adame, Miguel A Valdovinos, Angel Priego-Ranero, Héctor Olvera-Prado, Sofía Narváez-Chavez, Gonzalo Torres-Villalobos.
Methodology: Fidel López-Verdugo, Janette Furuzawa-Carballeda, Fernanda Romero-Hernández, Enrique Coss-Adame, Miguel A Valdovinos, Gonzalo Torres-Villalobos.
Supervision: Janette Furuzawa-Carballeda.
Writing – original draft: Fidel López-Verdugo, Janette Furuzawa-Carballeda, Gonzalo Torres-Villalobos.
Writing – review & editing: Fernanda Romero-Hernández, Enrique Coss-Adame, Miguel A Valdovinos, Angel Priego-Ranero, Héctor Olvera-Prado, Sofía Narváez-Chavez, José Peralta-Figueroa.
Janette Furuzawa-Carballeda orcid: 0000-0001-5804-7221.
Footnotes
Abbreviations: 95% CI = 95% confidence interval, ANA = antinuclear antibodies, AUC = area under the curve, CBC = complete blood count, EAT-10 = eating assessment tool, EGJ = esophagogastric junction, ELR = eosinophil-to-lymphocyte ratio, GERD-HRQL = gastroesophageal reflux disease-health related quality of life, Hb = hemoglobin, HRM = high resolution manometry, LES = lower esophageal sphincter, MLR = monocyte-to-lymphocyte ratio, NLR = neutrophil-to-lymphocyte ratio, OR = odds ratio, PLR = platelet-to-lymphocyte ratio, ROC = receiver-operator curve, WBC = white blood cells.
How to cite this article: López-Verdugo F, Furuzawa-Carballeda J, Romero-Hernández F, Coss-Adame E, Valdovinos MA, Priego-Ranero A, Olvera-Prado H, Narváez-Chavez S, Peralta-Figueroa J, Torres-Villalobos G. Hematological indices as indicators of silent inflammation in achalasia patients: A cross-sectional study. Medicine. 2020;99:9(e19326).
FLV and JFC contributed equally to this study.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Schlottmann F, Patti MG. Esophageal achalasia: current diagnosis and treatment. Expert Rev Gastroenterol Hepatol 2018;8:1–1. [DOI] [PubMed] [Google Scholar]
- [2].Kahrilas PJ, Bredenoord AJ, Fox M, et al. The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil 2015;27:160–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Vaezi MF, Felix VN, Penagini R, et al. Achalasia: from diagnosis to management. Ann N Y Acad Sci 2016;1381:34–44. [DOI] [PubMed] [Google Scholar]
- [4].Furuzawa-Carballeda J, Torres-Landa S, Valdovinos MÁ, et al. New insights into the pathophysiology of achalasia and implications for future treatment. World J Gastroenterol 2016;22:7892–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Booy JD, Takata J, Tomlinson G, et al. The prevalence of autoimmune disease in patients with esophageal achalasia. Dis Esophagus 2012;25:209–13. [DOI] [PubMed] [Google Scholar]
- [6].Romero-Hernández F, Furuzawa-Carballeda J, Hernández-Molina G, et al. Autoimmune comorbidity in achalasia patients. J Gastroenterol Hepatol 2018;33:203–8. [DOI] [PubMed] [Google Scholar]
- [7].Kraichely RE, Farrugia G, Pittock SJ, et al. Neural autoantibody profile of primary achalasia. Dig Dis Sci 2010;55:307–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kallel-Sellami M, Karoui S, Romdhane H, et al. Circulating antimyenteric autoantibodies in Tunisian patients with idiopathic achalasia. Dis Esophagus 2013;26:782–7. [DOI] [PubMed] [Google Scholar]
- [9].Furuzawa-Carballeda J, Aguilar-León D, Gamboa-Domínguez A, et al. Achalasia—An autoimmune inflammatory disease: a cross-sectional study. J Immunol Res 2015;2015:729217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Patel DA, Lappas BM, Vaezi MF. An Overview of Achalasia and Its Subtypes. Gastroenterol Hepatol (N Y) 2017;13:411–21. [PMC free article] [PubMed] [Google Scholar]
- [11].Sodikoff JB, Lo AA, Shetuni BB, et al. Histopathologic patterns among achalasia subtypes. Neurogastroenterol Motil 2016;28:139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wu Y, Chen Y, Yang X, et al. Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) were associated with disease activity in patients with systemic lupus erythematosus. Int Immunopharmacol 2016;36:94–9. [DOI] [PubMed] [Google Scholar]
- [13].Hao X, Li D, Wu D, et al. The Relationship between Hematological Indices and Autoimmune Rheumatic Diseases (ARDs), a Meta-Analysis. Sci Rep 2017;7:10833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Dentali F, Nigro O, Squizzato A, et al. Impact of neutrophils to lymphocytes ratio on major clinical outcomes in patients with acute coronary syndromes: A systematic review and meta-analysis of the literature. Int J Cardiol 2018;266:31–7. [DOI] [PubMed] [Google Scholar]
- [15].Yodying H, Matsuda A, Miyashita M, et al. Prognostic significance of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in oncologic outcomes of esophageal cancer: a systematic review and meta-analysis. Ann Surg Oncol 2016;23:646–54. [DOI] [PubMed] [Google Scholar]
- [16].Temur I, Kucukgoz Gulec U, Paydas S, et al. Prognostic value of pre-operative neutrophil/lymphocyte ratio, monocyte count, mean platelet volume, and platelet/lymphocyte ratio in endometrial cancer. Eur J Obstet Gynecol Reprod Biol 2018;226:25–9. [DOI] [PubMed] [Google Scholar]
- [17].Celikbilek M, Dogan S, Ozbakir O, et al. Neutrophil lymphocyte ratio as a predictor of disease severity in ulcerative colitis. J Clin Lab Anal 2018;27:72–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Torres-Villalobos G, Coss-Adame E, Furuzawa-Carballeda J, et al. Dor vs Toupet fundoplication after laparoscopic Heller myotomy: long-term randomized controlled trial evaluated by high-resolution manometry. J Gastrointest Surg 2018;22:13–22. [DOI] [PubMed] [Google Scholar]
- [19].Barahona-Garrido J, Camacho-Escobedo J, García-Martínez CI, et al. Antinuclear antibodies: a marker associated with steroid dependence in patients with ulcerative colitis. Inflamm Bowel Dis 2009;15:1039–43. [DOI] [PubMed] [Google Scholar]
- [20].Fournier BM, Parkos CA. The role of neutrophils during intestinal inflammation. Mucosal Immunol 2012;5:354–66. [DOI] [PubMed] [Google Scholar]
- [21].Hao X, Li D, Wu D, et al. The relationship between hematological indices and autoimmune rheumatic diseases (ARDs), a meta-analysis. Scientific Rep 2017;7:10833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Németh T, Mócsai A. The role of neutrophils in autoimmune diseases. Immunol Lett 2012;143:9–19. [DOI] [PubMed] [Google Scholar]
- [23].Kim DS, Shin D, Lee MS, et al. Assessments of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in Korean patients with psoriasis vulgaris and psoriatic arthritis. J Dermatol 2016;43:305–10. [DOI] [PubMed] [Google Scholar]
- [24].Shi L, Qin X, Wang H, et al. Elevated neutrophil-to-lymphocyte ratio and monocyte-to-lymphocyte ratio and decreased platelet-to-lymphocyte ratio are associated with poor prognosis in multiple myeloma. Oncotarget 2017;8:18792–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Dwivedi P, Greis KD. Granulocyte colony-stimulating factor receptor signaling in severe congenital neutropenia, chronic neutrophilic leukemia, and related malignancies. Exp Hematol 2017;46:9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Imtiaz F, Shafique K, Mirza SS, et al. Neutrophil lymphocyte ratio as a measure of systemic inflammation in prevalent chronic diseases in Asian population. Int Arch Med 2012;5:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Tamhane UU, Aneja S, Montgomery D, et al. Association between admission neutrophil to lymphocyte ratio and outcomes in patients with acute coronary syndrome. Am J Cardiol 2008;102:653–7. [DOI] [PubMed] [Google Scholar]
- [28].Aktas G, Sit M, Dikbas O, et al. Elevated neutrophil-to-lymphocyte ratio in the diagnosis of Hashimoto's thyroiditis. Rev Assoc Med Bras 2017;63:1065–8. [DOI] [PubMed] [Google Scholar]
- [29].Yang Z, Zhang Z, Lin F, et al. Comparisons of neutrophil-, monocyte-, eosinophil-, and basophil- lymphocyte ratios among various systemic autoimmune rheumatic diseases. APMIS 2017;125:863–71. [DOI] [PubMed] [Google Scholar]
- [30].Chandrashekara S, Mukhtar Ahmad M, Renuka P, et al. Characterization of neutrophil-to-lymphocyte ratio as a measure of inflammation in rheumatoid arthritis. Int J Rheum Dis 2017;20:1457–67. [DOI] [PubMed] [Google Scholar]
- [31].Keskin H, Kaya Y, Cadirci K, et al. Elevated neutrophil-lymphocyte ratio in patients with euthyroid chronic autoimmune thyreotidis. Endocr Regul 2016;50:148–53. [DOI] [PubMed] [Google Scholar]
- [32].Erre GL, Paliogiannis P, Castagna F, et al. Meta-analysis of neutrophil-to-lymphocyte and platelet-to-lymphocyte ratio in rheumatoid arthritis. Eur J Clin Invest 2018;13:e13037. [DOI] [PubMed] [Google Scholar]
- [33].Li J, Chen QY, Luo XH, et al. Neutrophil-to-lymphocyte ratio positively correlates to age in healthy population. J Clin Lab Anal 2015;29:437–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Thomas MR, Storey RF. The role of platelets in inflammation. Thromb Haemost 2015;114:449–58. [DOI] [PubMed] [Google Scholar]
- [35].Fulkerson PC, Rothenberg ME. Targeting eosinophils in allergy, inflammation and beyond. Nat Rev Drug Discov 2013;12:117–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Geering B, Stoeckle C, Conus S, et al. Living and dying for inflammation: neutrophils, eosinophils, basophils. Trends Immunol 2013;34:398–409. [DOI] [PubMed] [Google Scholar]
- [37].Arnold IC, Artola-Borán M, Tallón de Lara P, et al. Eosinophils suppress Th1 responses and restrict bacterially induced gastrointestinal inflammation. J Exp Med 2018;215:2055–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Chu DK, Jimenez-Saiz R, Verschoor CP, et al. Indigenous enteric eosinophils control DCs to initiate a primary Th2 immune response in vivo. J Exp Med 2014;211:1657–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Brescia G, Pedruzzi B, Barion U, et al. Are neutrophil-, eosinophil-, and basophil-to-lymphocyte ratios useful markers for pinpointing patients at higher risk of recurrent sinonasal polyps? Am J Otolaryngol 2016;37:339–45. [DOI] [PubMed] [Google Scholar]
- [40].Kara A, Guven M, Yilmaz MS, et al. Are neutrophil, platelet and eosinophil-to-lymphocyte ratio and red blood cell distribution width can be used for nasal polyposis? Eur Arch Otorhinolaryngol 2018;275:409–13. [DOI] [PubMed] [Google Scholar]
- [41].Yenigun A, Sezen S, Calim OF, et al. Evaluation of the eosinophil-to-lymphocyte ratio in pediatric patients with allergic rhinitis. Am J Rhinol Allergy 2016;30:e21–5. [DOI] [PubMed] [Google Scholar]


