Abstract
Advanced gastric cancer has a poor prognosis because of advanced gastric cancer is prone to metastasis. It is urgent for us to find an indicator to predict the prognosis of gastric cancer in a timely fashion. Research has revealed that inflammation has an important role in predicting survival in some cancers. The purpose of this study was to evaluate the significance of neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) on the prognosis of metastatic gastric cancer (GC).
This was a retrospective review of 110 patients were at presentation diagnosed with stage IV metastatic GC and all patients received palliative chemotherapy between January 2012 and January 2016 at the Affiliated Hospital of Qingdao University. Pretreatment NLR and PLR, as well as clinicopathological characteristics were collected. Patients were divided into high and low groups according to the cutoff values for NLR and PLR. The Kaplan–Meier method was applied to estimate the overall survival (OS) and the Cox proportional hazards model to evaluate the related risk factors for OS. All tests were 2-tailed and a P < .05 was considered to indicate a statistically significant difference.
One hundred ten patients were enrolled. Eighty-four patients were men, 24 patients were women, 61 patients were ≥65 years of age, and 49 patients were <65 years of age. The Eastern Cooperative Oncology Group (ECOG) score of most patients (n = 107) ranged from 0 to 1. Ten patients were human epidermal growth factor receptor 2 (HER2)-positive. Seventy-one patients presented with an elevated carcinoembryonic antigen (CEA) level and 49 patients had an elevated Carcinoembryonic 199 (CA-199) level. Fifty-two patients received first-line chemotherapy only. Nineteen patients received third-line or greater chemotherapy. One hundred patients chose dual drug chemotherapy. The median duration of follow-up was 11.6 months. Based on the receiver operating characteristic (ROC) curve, the optimal cut-off value for NLR and PLR was 2.48 and 143.39. Patients with high NLR and high PLR had poor overall survival compared with those who had low NLR and low PLR (P < .001 and P = .013, respectively). In univariate analysis, old age (P = .013), liver metastasis (P = .001), >1 metastatic sites (P = .028), higher NLR (P = .000), and higher PLR (P = .014) were identified as poor prognostic factors associated with OS. Our multivariate analysis had indicated that high NLR (hazard ratio [HR]: 1.617, 95% CI: 1.032–2.525, P = .036) and peritoneal metastasis (HR: 1.547, 95% CI:1.009–2.454, P = .045) was independent prognostic factors for overall survival; however, the PLR was not shown to be an independent prognostic factor.
Our study suggested that the pretreatment NLR can be used as significant prognosis biomarker in metastatic gastric cancer patients receiving palliative chemotherapy.
Keywords: gastric cancer, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, prognostic
1. Introduction
Gastric cancer (GC) is one of the most common cancers worldwide. GC has a high incidence and high mortality, especially in East Asia.[1] Most patients with GC present in an advanced stage, and thus, have an overall poor survival.[2] Thus, there is an urgent need to test and verify a reliable indicator for prognosis and improve therapeutic strategies in patients with GC.
Riihimaki et al[3] first reported the occurrence of GC metastasis in Sweden. The most common sites of metastasis were the liver, peritoneum, lung, and bone.[3] In addition, different metastatic sites are associated with different survival periods.[3] Thus, it is necessary to determine the prognosis associated with metastatic sites of tumors.
Inflammation has always been closely related to the occurrence and development of many tumors, especially GC. First, Helicobacter pylori infection is the primary cause in most GC patients (i.e., chronic inflammation caused by H pylori results in tumorigenesis).[4] Second, patients with chronic atrophic gastritis have an increased risk of subsequent GC.[5] A large number of epidemiologic studies have shown that non-steroidal anti-inflammatory drugs (NSAIDs) are negatively correlated with the risk of GC, and may become one of the economic and feasible methods to prevent GC.[6–9] Inflammation can be directly and clearly reflected by peripheral blood parameters, including white blood cells, neutrophils, lymphocytes, and platelets. Studies indicate that the neutrophil-to-lymphocyte ratio (NLR) and the platelet-to-lymphocyte ratio (PLR) as the important prognostic indicators in a variety of tumors, including soft tissue sarcomas, colorectal cancer, and GC[10–12];however, no observations have focused on the influence of NLR and PLR on metastasis sites and an analysis of subgroups in peritoneal metastasis of GC.
Therefore, in this study we retrospectively investigated the influence of the preoperative NLR and PLR on overall survival (OS) in GC, especially the different effects of NLR and PLR on peritoneal subgroups and the correlation with metastasis sites.
2. Methods
2.1. Patients
This was a retrospective review of 110 patients were diagnosed with stage IV metastatic GC and all patients received palliative chemotherapy between January 2012 and January 2016 at the Affiliated Hospital of Qingdao University. Tumor staging was assigned according to the American Joint Committee on Cancer (AJCC [version 7]) for gastric cancer. Gastric carcinomas were assessed histologically and classified using the World Health Organization (WHO) criteria for gastric cancer. The inclusion criteria were as follows: histologically-proven gastric adenocarcinoma; no infections, leukemia, and inflammation-related diseases; no previous anti-neoplastic treatment; no primary tumors in other sites; patients with adequate medical records; at least 1 measurable or evaluable lesion not eligible for radical resection; and all patients received standard chemotherapy, including combination chemotherapy (oxaliplatin and fluorouracil), combination chemotherapy (taxus and fluorouracil), and the single drug, fluorouracil. The exclusion criteria were as follows: serious liver and kidney function damage; blood system diseases; evidence of infection; double cancers; and irregular follow-up evaluations.
The protocol for the research project has been approved by the Ethics Committee of the Affiliated Hospital of Qingdao University and conforms to the provisions of the Declaration of Helsinki in 1995 (as revised in Edinburgh [2000]). Written informed consent was obtained from all patients before chemotherapy. Patient records were anonymized and de-identified prior to analysis.
2.2. Data collection
The clinical pathologic characteristics recorded one day before starting first-line palliative chemotherapy include sex, age, Eastern Cooperative Oncology Group performance status (ECOG PS), primary site, metastasis sites, and baseline evaluation of primary and metastatic tumor lesions according to the Response Evaluation Criteria in Solid Tumors (RECIST [version 1.0]) criteria. The location of metastasis was based on imaging examinations at the time of the most recent chemotherapy or the results of the most recent chemotherapy puncture, such as neck lymph node puncture before chemotherapy, a consideration of the stomach source in combination with the immune tissue, liver metastasis, number of metastatic sites, carcinoembryonic antigen (CEA) level, left supraclavicular lymph nodes, H pylori infection, peritoneal metastasis, human epidermal growth factor receptor 2 (HER2), HER2 status (The detection process for HER2 status was as follows: immunohistochemistry 0/1+ indicates negative; 3+ indicates positive; and 2+ indicates hybridization with amplification positive and without amplification negative); carcinoembryonic 199 (CA-199), neutrophil count, lymphocyte count, and platelet count. The NLR was defined as the neutrophil count divided by the lymphocyte count. The PLR was defined as the platelet count divided by the lymphocyte count. The number of patients receiving chemotherapy in each line and the number of patients in different chemotherapy regimens.
As the primary end point, the OS time was defined as the time from the date the patient received first-line chemotherapy to the date of death or the last follow-up evaluation.
2.3. Statistical analysis
The receiver operating characteristic (ROC) curve was applied and the largest Youden index was calculated to determine the optimal cut-off points for the NLR and PLR. The Kaplan–Meier method was performed to analyze OS and the log-rank test was used to compare the differences. A chi-square test or Fisher exact probability test was used to assess the correlation of clinicopathologic characteristics with NLR and PLR. Univariate and multivariate Cox regression analyses were applied to examine the independent predictors for OS. All analyses were performed using the SPSS 24.0 statistical software program (SPSS Inc., Chicago, IL). All tests were 2-tailed and a P < .05 was considered to indicate a statistically significant difference.
3. Results
3.1. Clinicopathologic characteristics of patients
In Table 1, the majority of patients were men (76.36%) and 61 patients (55.45%) were ≥65 years of age. Among all the cases, 41 patients (37.27%) were diagnosed with gastric antrum cancer and 37 patients (33.64%) had gastric body cancer. The ECOG score of most patients (107 [97.27%]) ranged from 0 to 1 point. Approximately one-half of the patients (53) had liver metastasis and 31 patients (28.18%) had left supraclavicular lymph nodes. Greater than one-half of the patients (102 [93.64%]) had >1 metastatic site. Only 10 patients (9.09%) were HER2-positive. Seventy-one patients (64.55%) presented with an elevated CEA level and 49 patients (44.55%) had an elevated CA-199 level.
Table 1.
Association of the patients’ characteristics with the neutrophil-to-lymphocyte and platelet-to-lymphocyte ratio.
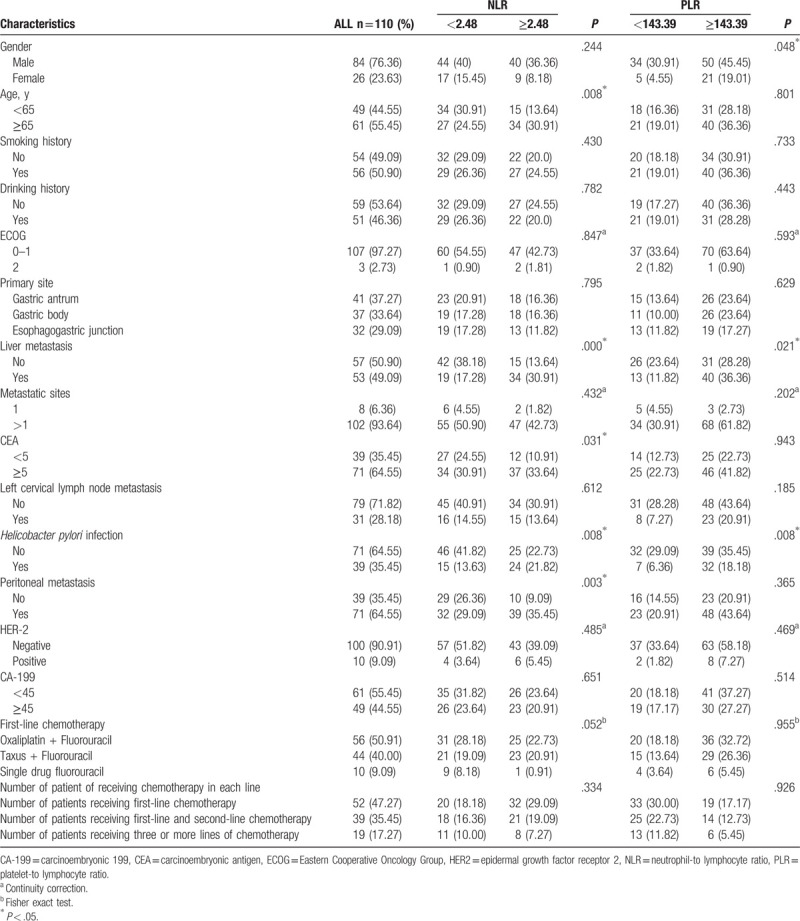
The area under the curve (AUC) of NLR was 0.845 and the optimal cut-off value based on the ROC curve was 2.48 (P = .005). The AUC of PLR was 0.675 and the optimal cut-off value based on the ROC curve was 143.39 (P = .002). In our study we did not count the NLR of the healthy population, but we refer to Professor Tao's study and think that the NLR of the healthy population is 0.62 to 1.64, and the PLR of the healthy population is 45.81 to 108.33.[11] As summarized in Table 1, an increased NLR level was significantly associated with age (P = .008), liver metastases (P < .001), H pylori infection (P = .008), peritoneal metastasis (P = .003). The PLR was associated with liver metastasis (P = .021), sex (P = .048), and with H pylori infection (P = .008), which was not associated with age, HER-2, CA-199 level, metastatic sites, and left cervical lymph node metastases. However, the choice of a chemotherapy plan and the number of chemotherapy plans received by patients are not related to the NLR and PLR levels.
3.2. Correlation analysis between NLR change and OS
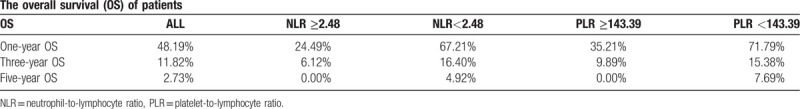
Table 2 shows that the 1, 3, and 5-year OS of all 110 patients included were 48.19%, 11.82%, and 2.73%, respectively. We calculated the OS of each group. For patients with an increased NLR, the 1, 3, and 5-year OS were 24.49%, 6.12%, and 0.00%, respectively. For patients with a lower NLR, the 1, 3, and 5-year OS were 67.21%, 16.40%, and 4.92%, respectively. For patients with an increased PLR, the 1, 3, and 5-year OS were 35.21%, 9.89%, and 0.00%, respectively. For patients with a lower PLR, the 1, 3, and 5-year OS were 71.79%, 15.38%, and 7.69%, respectively.
Table 2.
The overall survival (OS) of patients.
3.3. NLR was identified as an independent prognostic factor associated with OS
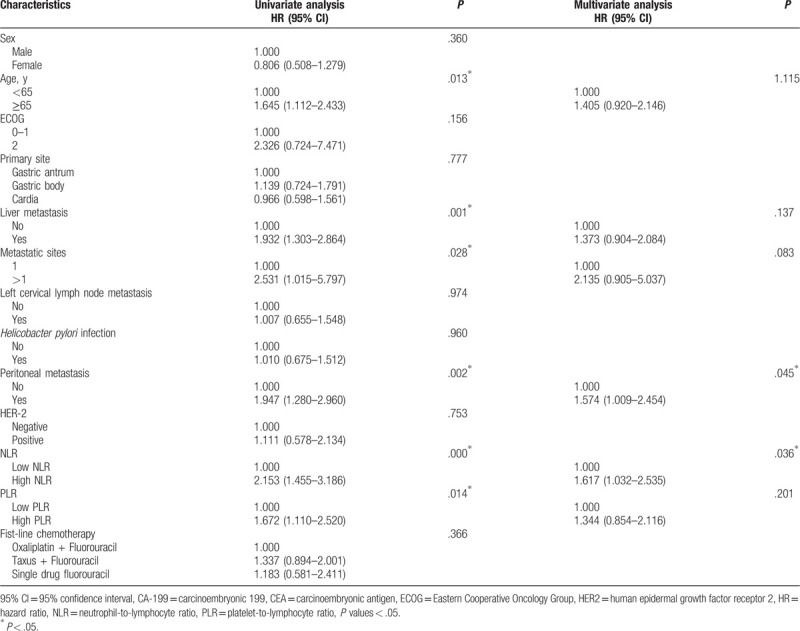
Based on univariate analysis, old age (P = .013), liver metastases (P = .001), >1 metastatic sites (P = .028), higher NLR (P < .001), peritoneal metastasis (P = .002), and higher PLR (P = .014) were identified as poor prognostic factors associated with OS (Table 3). Based on multivariate analysis with these selected parameters using a Cox regression model, only NLR (HR: 1.617, 95% CI: 1.032–2.525, P = .036) and peritoneal metastasis (HR: 1.547, 95% CI: 1.009–2.454, P = .045) was identified as an independent prognostic factor associated with OS.
Table 3.
Univariate and multivariate analyses of factors for the prediction of overall survival.
3.4. OS of total patients and different subgroups in the high NLR and low NLR group
In Figs. 1 and 2, the median OS for all patients was 11.50 months. We showed that the median OS in the high NLR group was 8.37 months. The median OS was 17.47 months in the low NLR group (P < .001). In the high and low PLR groups, the median OS was 9.60 months and 15.10 months, respectively (P = .013).
Figure 1.

Kaplan–Meier survival curves of overall survival according to the neutrophil-to-lymphocyte ratio (NLR).
Figure 2.

Kaplan–Meier survival curves of overall survival according to the platelet-to-lymphocyte ratio (PLR).
Figure 3 presents the Kaplan–Meier curves of some significant variables of OS by age, with or without liver metastases, CEA level, with or without peritoneal metastases, with or without H pylori infection according to the NLR optimal cut-off value. NLR has meaning both in patients <65 years and ≥65 years of age. In the <65 years age group, the median OS was 18.1 months in the low NLR group, while the median OS was 10.03 months in the high NLR group (P = .036). In patients with a high CEA level, the median OS was 0.705 months and 2.04 months in the high and low NLR subgroups, respectively (P < .001). In patients with H pylori infections, the median OS was 33.6 months in the low NLR subgroup and the median OS was 6.7 months in the high NLR subgroup (P = .012). In patients without H pylori infections, the median OS was 9.6 months in high NLR subgroup, while the median OS was 15.1 months in the low NLR subgroup (P = .006). Among patients with peritoneal metastases, the median OS was 13.03 months in the low NLR subgroup and the median OS was 8.38 months in the high NLR subgroup (P = .001). In patients with liver metastases, the median OS was 6.7 months in the high NLR subgroup compared with 17.47 months in the low NLR subgroup (P = .002).
Figure 3.
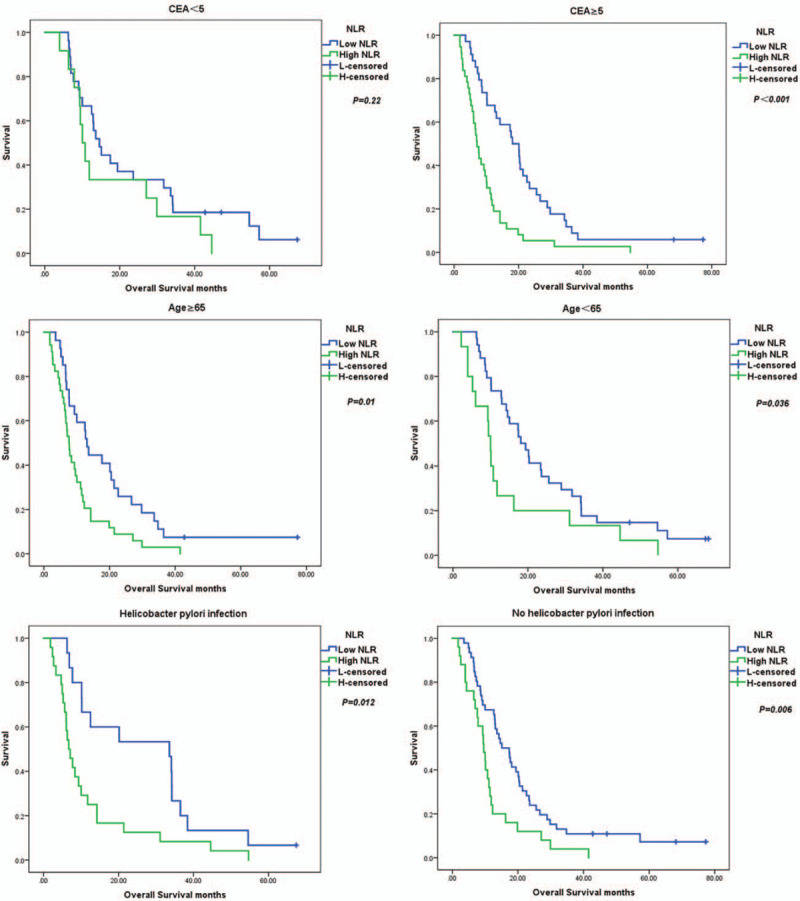
Kaplan–Meier survival curves for significant variables based on univariate analysis.
Figure 3 (Continued).
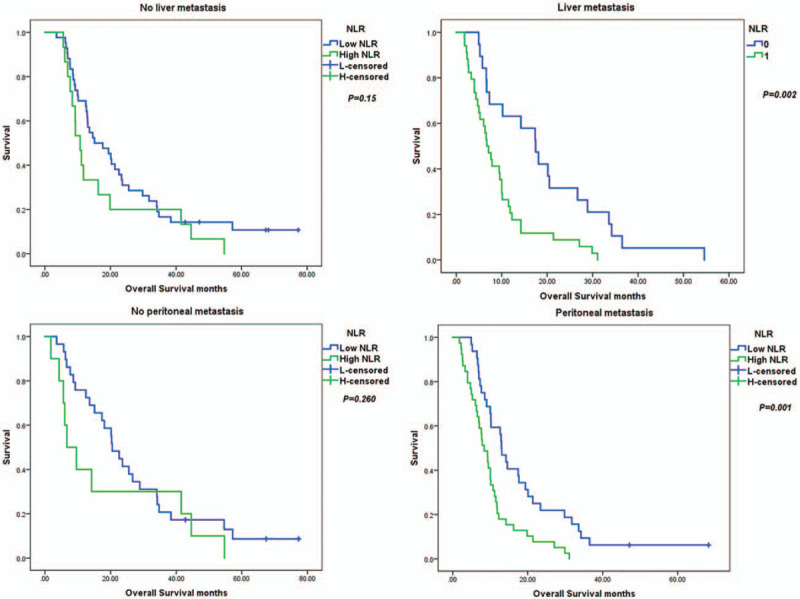
Kaplan–Meier survival curves for significant variables based on univariate analysis.
4. Discussion
Although therapeutic efficacy for GC have improved significantly in recent years, most GC patients still have a poor outcome and a low survival rate.[13] Until recently, the prognosis of patients with GC has mainly depended on TNM staging of tumors, but outcomes are not limited to tumor stage. Therefore, it is important to identify an indicator to precisely predict prognosis.
In agreement with previous studies, we showed that a high NLR was associated with demographic and clinicopathologic characteristics, such as age, CEA, H pylori infection, liver metastasis, and peritoneal metastasis, while a high PLR was associated with sex, H pylori infection and liver metastasis. Both a high NLR and PLR were associated with a poor OS based on univariate analysis, but only the NLR was an independent prognostic factor for OS based on multivariate analysis. Furthermore, the optimum value of NLR also had an effect on the stratified survival of some significant variables. Moreover, the NLR and PLR were not affected by the number of chemotherapy regimens, which also showed that NLR and PLR can predict the prognosis of patients with early-stage disease and adopt a reasonable treatment plan. Unlike previous studies, we focused on an analysis of the effect of NLR on survival time for each subgroup, the subgroup with an elevated CEA, the subgroup with liver metastasis, and the subgroup with peritoneal metastasis; the NLR had a statistically significant impact on survival.
Inflammation and tumorigenesis are inextricably linked.[14] It is recognized that inflammation is a milestone for cancer progression and microenvironment. Substantial research has focused on inflammatory indicators changes, including neutrophil, lymphocyte, and platelet counts. Jin et al[15] reported that early GC patients with an elevated NLR produce an abundance of cytokines by activated neutrophils, and the activated neutrophils elevate CD10 and CD35, which have adverse effects on the development of GC. Shaul and Fridlender[16] noted that neutrophils serve as a link between circulating tumor cells and metastatic sites. In addition, it is possible that the increased number of neutrophils arrest the anti-tumor immune responses of cancer.[15–17]
Lymphocytes are associated with a favorable prognosis in some tumors, including subgroups, such as CD8+ and CD3+ T cells. A growing number of observations have shown that T lymphocytes can eliminate cancer cells and arrest tumor progression by inducing an anti-tumor response. One piece of evidence involves tumor-infiltrating lymphocytes (TILs), which lead cell-mediated apoptosis by releasing tumor antigens into the microenvironment.[18–20] With respect to PLR, previous studies have shown that some platelet receptors, such as GP1b/IX/V and P-selectin, have been associated with cancer growth, and can facilitate tumor progression by enhancing angiogenesis via the cytokine, vascular endothelial growth factor (VEGF).[21,22] Thus, high NLR and PLR levels may contribute to adverse anti-tumor function.
There is ample evidence supporting the prognostic value of NLR and PLR in some solid tumors. Mori et al[12] recruited 100 patients with stage II to III GC and reported that a high NLR indicated a poor OS and RFS. Diema et al.[23] examined the role of pretreatment NLR in 52 patients with non-small cell lung cancer (NSCLC) treated with nivolumab and reported that a high NLR and PLR was significantly associated with a shorter OS and PFS. In a study involving 2536 patients with colon or rectal cancer conducted by Rashtak,[24] a high NLR (>3) was independently associated with non-metastatic colon cancer patients. Wang et al[25] retrospectively analyzed 273 patients with metastatic GC who received first-line chemotherapy and reported that a lower PLR was associated with a longer OS based on univariate and multivariate analyses. Nieswandt et al[26] suggested that high platelet and low lymphocyte counts are associated with tumor progression by protecting tumor cells from lysis by natural killer cells.
5. Conclusion
Both preoperative high NLR and high PLR collected from routine blood tests are related to a poor OS, but only NLR was an independent prognostic marker in patients with metastatic GC. This minimally invasive method can help clinicians obtain survival information at an early date. In the future, we will expand the number of cases to determine the underlying mechanism and validate the prognostic value.
Acknowledgments
Written informed consent was obtained from every eligible patient or their family members. This study was conducted in accordance with the Declaration of Helsinki.
Author contributions
Conceptualization: Guanghui Zhao, Wensheng Qiu.
Data curation: Shasha Wang, Xiaoxu Song, Yaoyue Qi.
Formal analysis: Shasha Wang, Jing Guo.
Supervision: Wensheng Qiu, Jing Lv.
Writing – original draft: Guanghui Zhao, Ning Liu.
Writing – review & editing: Guanghui Zhao, Ning Liu.
Footnotes
Abbreviations: CI = confidence interval, ECOG PS = Eastern Cooperative Oncology Group performance status, GC = gastric cancer, HER2 = human epidermal growth factor receptor 2, mGC = metastatic gastric cancer, NLR = neutrophil to lymphocyte ratio, OS = overall survival, ROC = receiver operating characteristic, TNM = tumor-node-metastasis.
How to cite this article: Zhao G, Liu N, Wang S, Guo J, Song X, Qi Y, Qiu W, Lv J. Prognostic significance of the neutrophil-to-lymphocyte and platelet-to-lymphocyte ratio in patients with metastatic gastric cancer. Medicine. 2020;99:10(e19405).
This study was supported by the National Natural Science Foundation of China (no. 81472338).
The authors have no conflicts of interest to disclose.
References
- [1].Rawla P, Barsouk A. Epidemiology of gastric cancer: global trends, risk factors and prevention. Prz Gastroenterol 2019;14:26–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Song Z, Wu Y, Yang J, et al. Progress in the treatment of advanced gastric cancer. Tumour Biol 2017;39:1010428317714626. [DOI] [PubMed] [Google Scholar]
- [3].Riihimaki M, Hemminki A, Sundquist K, et al. Metastatic spread in patients with gastric cancer. Oncotarget 2016;7:52307–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Piazuelo MB, Riechelmann RP, Wilson KT, et al. Resolution of gastric cancer-promoting inflammation: a novel strategy for anti-cancer therapy. Curr Top Microbiol Immunol 2019;421:319–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bang CS, Lee JJ, Baik GH. Prediction of chronic atrophic gastritis and gastric neoplasms by serum pepsinogen assay: a systematic review and meta-analysis of diagnostic test accuracy. J Clin Med 2019;8:pii: E657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Huang XZ, Chen Y, Wu J, et al. Aspirin and non-steroidal anti-inflammatory drugs use reduce gastric cancer risk: a dose-response meta-analysis. Oncotarget 2017;8:4781–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kong P, Wu R, Liu X, et al. The effects of anti-inflammatory drug treatment in gastric cancer prevention: an update of a meta-analysis. J Cancer 2016;7:2247–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Epplein M, Nomura AM, Wilkens LR, et al. Nonsteroidal antiinflammatory drugs and risk of gastric adenocarcinoma: the multiethnic cohort study. Am J Epidemiol 2009;170:507–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ye X, Fu J, Yang Y, et al. Frequency-risk and duration-risk relationships between aspirin use and gastric cancer: a systematic review and meta-analysis. PLoS One 2013;8:e71522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chan JY, Zhang Z, Chew W, et al. Biological significance and prognostic relevance of peripheral blood neutrophil-to-lymphocyte ratio in soft tissue sarcoma. Sci Rep 2018;8:11959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Tao Y, Ding L, Yang GG, et al. Predictive impact of the inflammation-based indices in colorectal cancer patients with adjuvant chemotherapy. Cancer Med 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mori M, Shuto K, Kosugi C, et al. An increase in the neutrophil-to-lymphocyte ratio during adjuvant chemotherapy indicates a poor prognosis in patients with stage ii or iii gastric cancer. BMC Cancer 2018;18:1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kim YJ, Chung WC, Youn GJ, et al. The predictive factors of gastric cancer recurrence after the completion of adjuvant chemotherapy in advanced gastric cancer. Rev Esp Enferm Dig 2019;111:537–42. [DOI] [PubMed] [Google Scholar]
- [14].Diakos CI, Charles KA, McMillan DC, et al. Cancer-related inflammation and treatment effectiveness. Lancet Oncol 2014;15:e493–503. [DOI] [PubMed] [Google Scholar]
- [15].Jin H, Zhang G, Liu X, et al. Blood neutrophil-lymphocyte ratio predicts survival for stages iii-iv gastric cancer treated with neoadjuvant chemotherapy. World J Surg Oncol 2013;11:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Shaul ME, Fridlender ZG. Neutrophils as active regulators of the immune system in the tumor microenvironment. J Leukoc Biol 2017;102:343–9. [DOI] [PubMed] [Google Scholar]
- [17].Swierczak A, Mouchemore KA, Hamilton JA, et al. Neutrophils: important contributors to tumor progression and metastasis. Cancer Metastasis Rev 2015;34:735–51. [DOI] [PubMed] [Google Scholar]
- [18].Lee JS, Won HS, Sun S, et al. Prognostic role of tumor-infiltrating lymphocytes in gastric cancer: a systematic review and meta-analysis. Medicine (Baltimore) 2018;97:e11769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Yu PC, Long D, Liao CC, et al. Association between density of tumor-infiltrating lymphocytes and prognoses of patients with gastric cancer. Medicine (Baltimore) 2018;97:e11387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kang BW, Kim JG, Lee IH, et al. Clinical significance of tumor-infiltrating lymphocytes for gastric cancer in the era of immunology. World J Gastrointest Oncol 2017;9:293–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bambace NM, Holmes CE. The platelet contribution to cancer progression. J Thromb Haemost 2011;9:237–49. [DOI] [PubMed] [Google Scholar]
- [22].Zhang LX, Wei ZJ, Xu AM, et al. Can the neutrophil-lymphocyte ratio and platelet-lymphocyte ratio be beneficial in predicting lymph node metastasis and promising prognostic markers of gastric cancer patients? Tumor maker retrospective study. Int J Surg 2018;56:320–7. [DOI] [PubMed] [Google Scholar]
- [23].Diem S, Schmid S, Krapf M, et al. Neutrophil-to-Lymphocyte ratio (NLR) and Platelet-toLymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung cancer 2017;111:176–81. [DOI] [PubMed] [Google Scholar]
- [24].Rashtak S, Ruan X, Druliner BR, et al. Peripheral Neutrophil to Lymphocyte Ratio Improves Prognostication in Colon Cancer. Clinical colorectal cancer 2017;16:115–23. e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wang J, Qu JL, Li Z, et al. Pretreatment platelet-to-lymphocyte ratio is associated with the response to first-line chemotherapy and survival in patients with metastatic gastric cancer. J Clin Lab Anal 2018;32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Nieswandt B, Hafner M, Echtenacher B, et al. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res 1999;59:1295–300. [PubMed] [Google Scholar]


