Abstract
Red blood cell distribution width (RDW) is a component of routine complete blood count, which reflects variability in the size of circulating erythrocytes. Recently, there have been many reports about RDW as a strong prognostic marker in various disease conditions in the adult population. However, only a few studies have been performed in children. This study aimed to investigate the association between RDW and pediatric intensive care unit (PICU) mortality in critically ill children. This study includes 960 patients admitted to the PICU from November 2012 to May 2018. We evaluated the associations between RDW and clinical parameters including PICU mortality outcomes. The median age of the study population was 15.5 (interquartile range, 4.8–54.5) months. The mean RDW was 15.6% ± 3.3%. The overall PICU mortality was 8.8%. As we categorized patients into 3 groups with respect to RDW values (Group 1: ≤14.5%; Group 2: 14.5%–16.5%; and Group 3: >16.5%) and compared clinical parameters, the higher RDW groups (Groups 2 and 3) showed more use of vasoactive-inotropic drugs, mechanical ventilator support, higher severity scores, including pediatric risk of mortality III, pediatric sequential organ failure assessment, pediatric logistic organ dysfunction-2 (PELOD-2), and pediatric multiple organ dysfunction syndrome scores, and higher PICU mortality than the lower RDW group (Group 1) (P < .05). Based on multivariate logistic regression analysis adjusted for age and sex, higher RDW value (≥14.5%) was an independent risk factor of PICU mortality. Moreover, adding RDW improved the performance of the PELOD-2 score in predicting PICU mortality (category-free net reclassification index 0.357, 95% confidence interval 0.153–0.562, P = .001). In conclusion, higher RDW value was significantly associated with worse clinical parameters including PICU mortality. RDW was an independent risk factor of PICU mortality and the addition of RDW significantly improved the performance of PELOD-2 score in predicting PICU mortality. Thus, RDW could be a promising prognostic factor with advantages of simple and easy measurement in critically ill pediatric patients.
Keywords: erythrocyte indices, intensive care units, mortality, pediatrics
1. Introduction
Red blood cell (RBC) distribution width (RDW), calculated by dividing the standard deviation of RBC volume by the mean corpuscular volume and multiplied by 100, is routinely reported as part of the complete blood count (CBC) using automated flow cytometry. RDW has been traditionally used as additional information in the differential diagnosis of the cause of anemia.[1]
RDW has been recently reported as a strong prognostic factor in several diseases of various organ systems, including the cardiovascular, respiratory, renal, neurologic, and gastrointestinal systems.[2–15] It also showed significant associations with ventilator-free days, postoperative outcome, intensive care unit (ICU) discharge outcome, out-of-hospital outcome, and all-cause mortality in critically ill patients.[16–20] However, most studies were conducted in adult patients. Only a few studies have investigated RDW in children, especially in the critically ill pediatric population.[21–23]
In these critically ill children, it is crucial to promptly and accurately assess the severity of illness and organ dysfunction and predict outcomes for prompt management. For this purpose, many studies have investigated proper prognostic factors including several scoring systems such as the pediatric risk of mortality (PRISM), pediatric sequential organ failure assessment (pSOFA), pediatric logistic organ dysfunction-2 (PELOD-2), and pediatric multiple organ dysfunction syndrome (pMODS).[24–27] However, considering the qualification of a good clinical parameter for predicting outcomes, which should be easily assessable, reproducible, widely accessible, and acceptable, these scoring systems could be slightly complex and inconvenient for use in practice.
Thus, this study aimed to evaluate the association between pediatric intensive care unit (PICU) mortality and RDW, which is easily measured as a component of CBC and investigate if RDW could be useful as a prognostic marker for predicting PICU mortality in critically ill pediatric patients.
2. Methods
2.1. Patients
The study protocol was approved by the institutional review board of the Asan Medical Center, Seoul, Korea. The requirement of parental consent was waived because of the retrospective nature of the study. All children who were consecutively admitted to the 14-bed multidisciplinary PICU of our tertiary academic children's hospital from November 2012 to May 2018 were eligible for enrollment. We limited our analysis to include patients under 18 years and in whom a CBC including RDW was obtained within 24 hours of PICU admission. Patients older than 18 years and with a history of hematopoietic stem cell transplantation and hemato-oncologic disorders were excluded.
2.2. Data collection
We retrospectively reviewed the electronic medical records and available PICU database of all enrolled patients and obtained data on demographics such as sex, age, weight, height, underlying diseases, reasons for PICU admission, duration of PICU stay, length of hospital stay, and PICU mortality. We also collected data on the use of vasoactive-inotropic drugs, mechanical ventilator (MV) support, laboratory tests, including routine CBC with RDW, chemistry, C-reactive protein (CRP) level, serum lactate level, and B-type natriuretic peptide (BNP) level. We evaluated the presence of anemia, which was defined according to the diagnostic cutoff values with respect to age and sex suggested by the World Health Organization (WHO). For evaluation of the severity of illness and organ dysfunction, the PRISM-III, pSOFA, PELOD-2, and pMODS scores were calculated using the worst documented values within the first 24 hours of admission to the PICU.
2.3. Primary exposure and outcomes
Our primary exposure of interest was RDW, which was obtained as a component of CBC within 24 hours of PICU admission. According to this RDW value, we apportioned the included patients into 3 groups (Group 1: ≤14.5%; Group 2: 14.5%–16.5%; and Group 3: >16.5%).
Our primary outcome was PICU mortality, which was defined as death during PICU stay. To evaluate the relationship between RDW and PICU mortality, we first performed a general descriptive analysis by comparing groups, and then, we performed univariate logistic regression analysis for all variables and an adjusted multivariate logistic regression analysis. In addition, we evaluated the performance of predicting PICU mortality using areas under the receiver-operating characteristic (AUROC) curves.
2.4. Statistical analysis
Data were analyzed using the Statistical Package for the Social Sciences (SPSS version 21.0 for Windows; SPSS Inc, Chicago, IL). Descriptive statistics were performed for patients with each group classified according to RDW values and mortality (survivors and non-survivors).Continuous data are expressed as mean ± standard deviation or median and interquartile range (IQR), as appropriate. Categorical variables are expressed as number and percentages.
We used the χ2 or 2-tailed Fisher exact test to compare qualitative variables and Student t test to compare continuous quantitative categorical variables between 2 groups. We performed trend analysis for the 2 variables using a linear-by-linear association test. For the 3 group comparison, we used 1-way analysis of variance. Risk factors for PICU mortality were evaluated using univariate and multivariate logistic regression analyses. The multivariate logistic regression analysis with backward elimination was conducted using variables yielding P values <.05 in the univariate analysis to identify independent variables associated with PICU mortality. Confounding factors were entered into the multivariate logistic regression analysis for adjustment. Model fits were assessed using the Hosmer–Lemeshow goodness-of-fit test. A nonsignificant value (P > .05) for the Hosmer–Lemeshow goodness-of-fit test suggested the absence of a biased fit. Results are expressed as the adjusted odds ratios (OR) and respective 95% confidence intervals (CIs).
Given the rarity of data on the relationship between RDW and PICU mortality, a realistic a priori sample size calculation was not feasible. Instead, we performed a post hoc power analysis to assess the likelihood of type II error and explored a range of OR that can be detectable with our study sample size.
The usefulness and discrimination capacity of the models for predicting PICU mortality were evaluated by determining the AUROC curves with a 95% CI. To evaluate whether RDW provided additional prognostic information to improve the performance for predicting PICU mortality or not, the integrated discrimination improvement index (IDI) and category-free net reclassification index (cNRI) were calculated using the method proposed by Pencina et al,[28] which was implemented in the R package PredictABEL (Suman Kundu, Yurii S. Aulchenko, A. Cecile J.W. Janssens). For all analyses, variables with a 2-sided P value <.05 were considered statistically significant.
3. Results
3.1. Study population
A total of 960 critically ill pediatric patients were included (Fig. 1). There were 495 boys and 465 girls; the median age and body weight of 15.5 (IQR, 4.8–54.5) months and 8.5 (IQR, 5.0–16.0) kg, respectively. The mean RDW was 15.6% ± 3.3%. The overall PICU mortality was 8.8% (Table 1).
Figure 1.
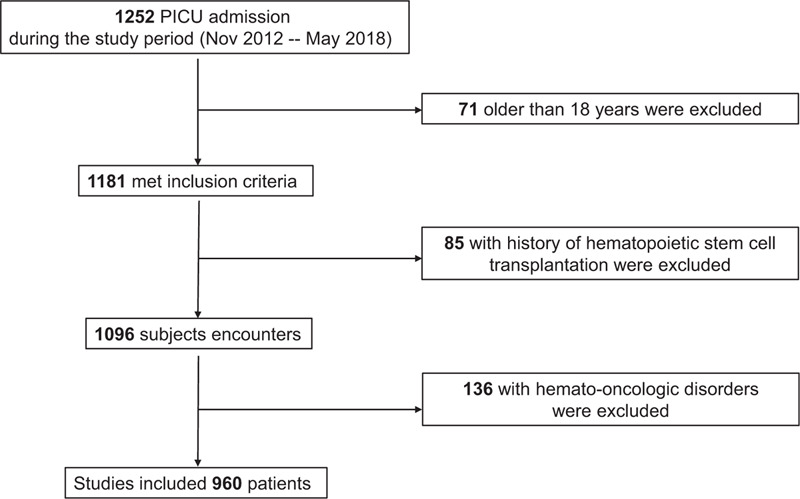
Flow diagram for patient inclusion and exclusion.
Table 1.
Comparison of variables between three groups in the study cohort.
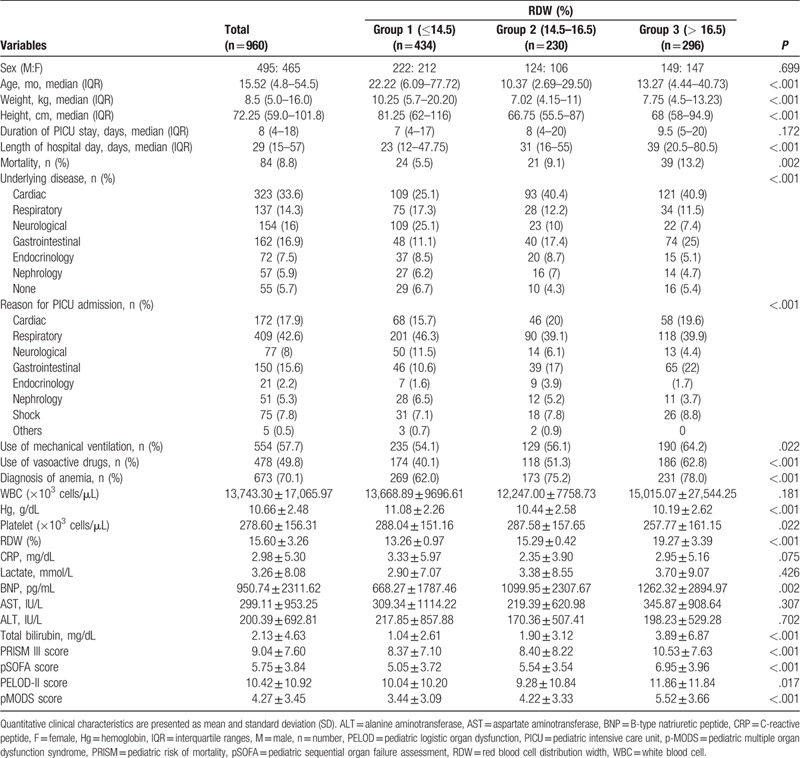
On comparison of the 3 groups categorized based on RDW, the highest RDW group (Group 3) showed a lower hemoglobin (Hg) and platelet (plt) count and higher total bilirubin, BNP, and severity of illness and organ failure scores, including PRISM-III, pSOFA, PELOD-2, and pMODS scores. The proportions of patients requiring vasoactive-inotropic drugs and MV support and nonsurvivors was higher in Group 3 than in the other 2 groups, which showed a statistically significant increasing tendency in the linear-by-linear association test (P < .001, P = .009, and P < .001, respectively).
3.2. Comparison between survivors and non-survivors
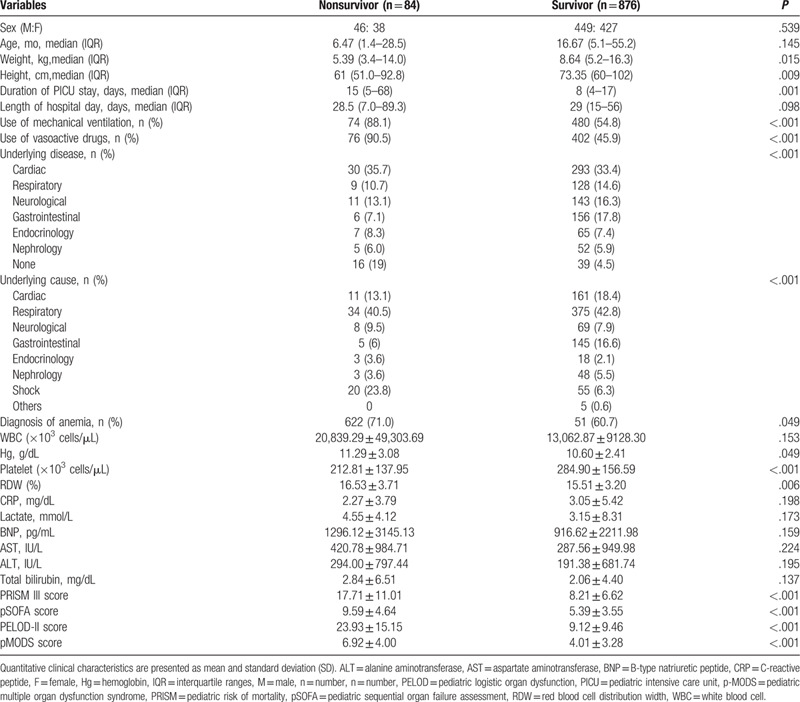
There were significant differences between survivors and nonsurvivors. Nonsurvivors required more vasoactive-inotropic drugs and MV support. They showed a lower plt count, higher Hg, RDW, and severity of illness and organ failure scores (PRISM-III, pSOFA, PELOD-2, and pMODS scores) than survivors (P < .05) (Table 2).
Table 2.
Comparison of variables between survivors and non-survivor in the study cohort.
3.3. Evaluation of the association between RDW and PICU mortality
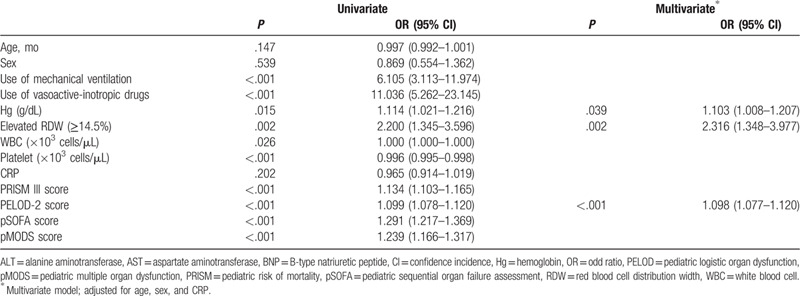
The univariate logistic regression analysis revealed that the requirement for MV support, use of vasoactive-inotropic drugs, Hg, white blood cell count, plt count, RDW, and severity of illness and organ failure scores, including PRISM-III, pSOFA, PELOD-2, pMODS scores, were significantly associated with PICU mortality. The multivariate analysis adjusted for age, sex, and CRP revealed that the PELOD-2 score, Hg, and elevated RDW (>14.5%) were independent risk factors for PICU mortality (Table 3).
Table 3.
Univariate and Multivariate logistic regression analysis of the pediatric intensive care unit mortality.
Power analysis was conducted based on the previous study in which the estimated PICU mortality was 11% and 20% in the lower and higher RDW groups (RDW: ≤15.7 vs >15.7% OR = 2.0), respectively.[29] Considering that the considered cutoff value is 14.5%, we investigated a minimum detectable OR. The sample size used in our analysis (n = 960) can detect a minimum of OR = 1.34 with at least 80% power.
3.4. Evaluation of the usefulness of RDW as a prognostic factor
Discrimination capacity of PELOD-2 for predicting PICU mortality, which was assessed and determined using AUROC, was 0.803 (95% CI 0.751–0.855). Adding the RDW slightly improved the performance (AUROC 0.821, 95% CI 0.773–0.868). In terms of cNRI, the performance of RDW added model was significantly better (cNRI 0.357, 95% CI 0.153–0.562, P = .001). However, IDI failed to show a significant improvement in discrimination (IDI: 0008 [95% CI 0.004–0.021], P = .196) (Fig. 2).
Figure 2.
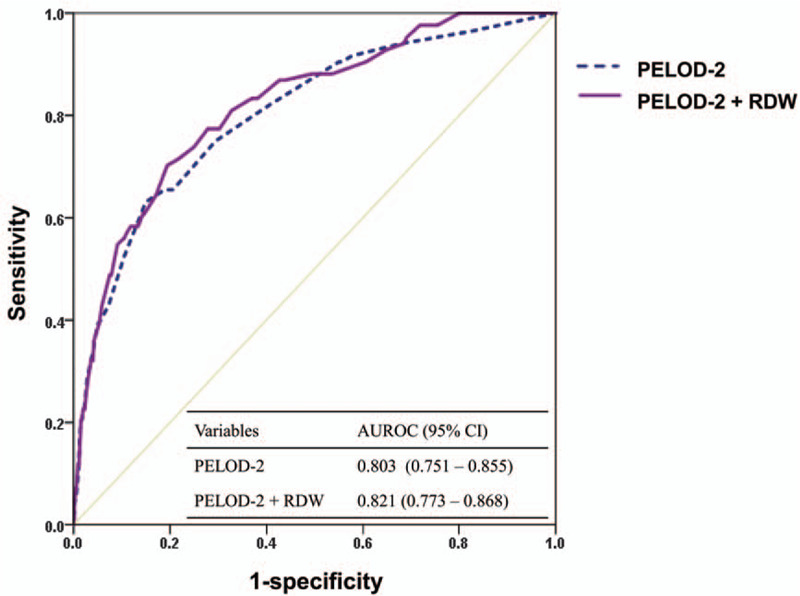
Area under the receiver-operating characteristic curves of the pediatric logistic organ dysfunction-2 (PELOD-2) score and addition of red blood cell distribution width (RDW) to the PELOD-2 score for predicting pediatric intensive care unit (PICU) mortality.
4. Discussion
This study showed that compared with the other 2 groups categorized based on RDW, the higher RDW group was associated with higher PICU mortality and other worse clinical outcomes, such as more requirements of vasoactive-inotropic drugs, MV support, and higher severity of illness and organ failure scores. Furthermore, adding RDW to the PELOD-2 score significantly improved the performance for PICU morality prediction.
Although several previous studies reported an association of RDW with various clinical parameters in adults, there have only been a few studies that included children. Recently, it was shown that a higher RDW at admission was associated with a higher pediatric index of mortality (PIM) 2 score, higher need for MV support, lower 28-day ventilator-free days, and higher hospital mortality.[23,30] However, to the best of our knowledge, this is the first study to extensively evaluate the associations between RDW and various clinical parameters and outcomes, including the use of vasoactive-inotropic drugs, MV support, and several severity scores, in critically ill pediatric patients. As RDW is a simple and routinely reported measurement without additional effort or costs, it is advantageous as a prognostic factor. Furthermore, although it has been controversial whether or not adding RDW improves the discrimination and performance for outcome prediction of some previously used scores or models, this study showed a significant improvement in the performance of prediction of PICU mortality assessed using the cNRI test,[7,13,30,31] which could prove its validity.
However, despite several reports on the associations between RDW and various clinical conditions, the exact pathophysiologic mechanisms have not been elucidated. High RDW can result from any disease process that causes ineffective RBC production and releasing more premature RBCs into the blood circulation. This could be attributable to inflammatory and related process. There are growing evidences of the understanding of crosstalk between the inflammatory and hematologic systems. It was well established that inflammatory cytokines interfere with the maturation of RBCs in the bone marrow through multiple mechanisms, such as the inhibition of the production of or response to erythropoietin, which impairs iron metabolism and shortens RBC survival, in turn contributing to high RDW.[1,11,32,33] A high RDW has been shown to be associated with elevated inflammatory markers, such as the CRP level, erythrocyte sedimentation rate, and interleukin-6. RDW was reported to have a strong, graded association with inflammatory biomarkers.[2] A proinflammatory cytokine, tumor necrosis factor-α, promotes hypoferremia and enhances erythrophagocytosis. Other cytokines, such as interleukin-6 and interleukin-1β, have been shown to directly and negatively affect the survival of RBCs in the circulation, promote deformity of the RBC membrane, and suppress erythrocyte maturation. These inflammatory mediators can thus lead to newer and larger reticulocytes entering the peripheral circulation and increasing RDW, indicating that RDW also reflects the severity of inflammation.[2,13,23,33–35] Therefore, RDW has been suggested as the final result of multiple pathologic processes associated with inflammation.
Another explanation was oxidative stress. Although the erythrocytes usually have an excellent antioxidant capacity and serve as the primary “oxidative sink,” they could be prone to oxidative damage.[36] Oxidative stress plays a role in RBC homeostasis such as fragility, maturation, and lifespan that reduces RBC survival. It also affects many biological processes, including apoptosis and inflammatory reactions, which could change the size of RBC, consequently, increasing RDW.[37–40]
In addition, there were several other suggestions for possible pathophysiology for high RDW, such as poor nutritional status, activation of renin-angiotensin system, and impaired renal function.[34,41,42]
As this study included critically ill pediatric patients, all the aforementioned explanations could be applied. However, the exact pathophysiology still largely remains unknown, which requires further large-scale multicenter clinical studies with a specified subgroup analysis.
This study had several strengths. The study included a relatively significant number of critically ill pediatric patients. Post hoc power analysis demonstrated that the sample size used in our analysis (n = 960) can detect a minimum of OR = 1.34 with at least 80% power. We excluded patients with hemato-oncologic disorders that could affect RDW and reduced potential confounding factors. We extensively evaluated and showed significant associations between RDW and several clinical parameters including the use of vasoactive-inotropic drugs, MV support, and severity scores of illness and organ failure scores (PRISM-III, pSOFA, PELOD-2, and pMODS scores) as well as PICU mortality. These are important parameters in critically ill pediatric patients, which could be helpful in managing these patients. Therefore, we suggested RDW as a promising prognostic marker with advantages of simple measurement, good cost-effectiveness, and being well-correlated with other risk factors for worse clinical outcomes in these patients.
However, this study had several limitations. First, it was a retrospective observational study conducted at a single tertiary academic hospital and only included critically ill pediatric patients who were admitted to the PICU. Thus, there could be some limitations in generalizability of the results to other patients in different settings. Second, there could be other confounders to be adjusted for several potential confounding parameters. Third, we analyzed the RDW measurement only at the time of admission to PICU, which could be insufficient to obtain information of these patients’ conditions associated with worse clinical outcomes. Fourth, by considering the effect of anemia and inflammation on RDW, we included Hg and CRP as clinical markers in the multivariate logistic regression analysis. However, we did not collect data on patients’ iron status, erythropoietin levels, or other precise inflammatory cytokines, which could provide more helpful information.
5. Conclusions
RDW in critically ill children is strongly associated with PICU mortality and poor clinical parameters, including higher severity scores, use of vasoactive-inotropic drugs, and MV support. It was an independent risk factor for PICU mortality with additional effect to improve the performance of PELOD-2 for predicting PICU mortality. Its measurement is simple, inexpensive, and convenient as included in routine CBC; thus, it could be a promising additional prognostic factor in these patients. Further large-scale studies, including clinical, epidemiologic, and molecular biologic studies, are required to identify the exact causative pathophysiologic mechanisms of the association between RDW and worse clinical outcomes.
Author contributions
Da Hyun Kim: Investigation, Formal analysis, Writing – Original Draft. Eun Ju Ha: Investigation, Formal analysis. Seong Jong Park: Conceptualization, Methodology. Won Kyoung Jhang: Conceptualization, Methodology, Formal analysis, Writing – Original Draft, Writing – Review & Editing, Supervision.
Footnotes
Abbreviations: ANOVA = analysis of variance, AUROC = areas under the receiver operating characteristic, BNP = B-type natriuretic protein, CBC = complete blood count, CI = confidence intervals, Cnri = category-free net reclassification index, CRP = C-reactive protein, Hg = hemoglobin, ICU = intensive care unit, IDI = integrated discrimination improvement index, IQR = interquartile range, MV = mechanical ventilator, OR = odds ratios, PELOD = pediatric logistic organ dysfunction, PICU = pediatric intensive care unit, PIM = pediatric index of mortality, plt = platelet, pMODS = pediatric multiple organ dysfunction syndrome, PRISM = pediatric risk of mortality, pSOFA = pediatric sequential organ failure assessment, RBC = red blood cell, RDW = red blood cell distribution width, WHO = world health organization.
How to cite this article: Kim DH, Ha EJ, Park SJ, Jhang WK. Evaluation of the usefulness of red blood cell distribution width in critically ill pediatric patients. Medicine. 2020;99:36(e22075).
The authors report no conflicts of interest.
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sector.
Funding: This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
References
- [1].Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med 2005;352:1011–23.. [DOI] [PubMed] [Google Scholar]
- [2].Jo YH, Kim K, Lee JH, et al. Red cell distribution width is a prognostic factor in severe sepsis and septic shock. Am J Emerg Med 2013;31:545–8.. [DOI] [PubMed] [Google Scholar]
- [3].Felker GM, Allen LA, Pocock SJ, et al. Red cell distribution width as a novel prognostic marker in heart failure: data from the CHARM program and the Duke Databank. J Am Coll Cardiol 2007;50:40–7.. [DOI] [PubMed] [Google Scholar]
- [4].Cakal B, Akoz AG, Ustundag Y, et al. Red cell distribution width for assessment of activity of inflammatory bowel disease. Dig Dis Sci 2009;54:842–7.. [DOI] [PubMed] [Google Scholar]
- [5].Hampole CV, Mehrotra AK, Thenappan T, et al. Usefulness of red cell distribution width as a prognostic marker in pulmonary hypertension. Am J Cardiol 2009;104:868–72.. [DOI] [PubMed] [Google Scholar]
- [6].Allen LA, Felker GM, Mehra MR, et al. Validation and potential mechanisms of red cell distribution width as a prognostic marker in heart failure. J Card Fail 2010;16:230–8.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Oh HJ, Park JT, Kim JK, et al. Red blood cell distribution width is an independent predictor of mortality in acute kidney injury patients treated with continuous renal replacement therapy. Nephrol Dial Transplant 2012;27:589–94.. [DOI] [PubMed] [Google Scholar]
- [8].Pascual-Figal DA, Bonaque JC, Redondo B, et al. Red blood cell distribution width predicts long-term outcome regardless of anaemia status in acute heart failure patients. Eur J Heart Fail 2009;11:840–6.. [DOI] [PubMed] [Google Scholar]
- [9].Ye Z, Smith C, Kullo IJ. Usefulness of red cell distribution width to predict mortality in patients with peripheral artery disease. Am J Cardiol 2011;107:1241–5.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Senol K, Saylam B, Kocaay F, et al. Red cell distribution width as a predictor of mortality in acute pancreatitis. Am J Emerg Med 2013;31:687–9.. [DOI] [PubMed] [Google Scholar]
- [11].Jelkmann WE, Fandrey J, Frede S, et al. Inhibition of erythropoietin production by cytokines. Implications for the anemia involved in inflammatory states. Ann N Y Acad Sci 1994;718:300–9.. discussion 309-311. [PubMed] [Google Scholar]
- [12].Yesil A, Senates E, Bayoglu IV, et al. Red cell distribution width: a novel marker of activity in inflammatory bowel disease. Gut Liver 2011;5:460–7.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sadaka F, O’Brien J, Prakash S. Red cell distribution width and outcome in patients with septic shock. J Intensive Care Med 2013;28:307–13.. [DOI] [PubMed] [Google Scholar]
- [14].Ku NS, Kim HW, Oh HJ, et al. Red blood cell distribution width is an independent predictor of mortality in patients with gram-negative bacteremia. Shock 2012;38:123–7.. [DOI] [PubMed] [Google Scholar]
- [15].Wang B, Lu H, Gong Y, et al. The association between red blood cell distribution width and mortality in critically ill patients with acute kidney injury. Biomed Res Int 2018;2018:9658216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wang B, Aihemaiti G, Cheng B, et al. Red blood cell distribution width is associated with all-cause mortality in critically ill patients with cardiogenic shock. Med Sci Monit 2019;25:7005–15.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Fernandez R, Cano S, Catalan I, et al. High red blood cell distribution width as a marker of hospital mortality after ICU discharge: a cohort study. J Intensive Care 2018;6:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Havens JM, Seshadri AJ, Salim A, et al. Red cell distribution width predicts out of hospital outcomes in critically ill emergency general surgery patients. Trauma Surg Acute Care Open 2018;3:e000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Otero TMN, Yeh DD, Bajwa EK, et al. Elevated red cell distribution width is associated with decreased ventilator-free days in critically ill patients. J Intensive Care Med 2018;33:241–7.. [DOI] [PubMed] [Google Scholar]
- [20].Bazick HS, Chang D, Mahadevappa K, et al. Red cell distribution width and all-cause mortality in critically ill patients. Crit Care Med 2011;39:1913–21.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Schepens T, De Dooy JJ, Verbrugghe W, et al. Red cell distribution width (RDW) as a biomarker for respiratory failure in a pediatric ICU. J Inflamm (Lond) 2017;14:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Said AS, Spinella PC, Hartman ME, et al. RBC distribution width: biomarker for red cell dysfunction and critical illness outcome? Pediatr Crit Care Med 2017;18:134–42.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Perlstein TS, Weuve J, Pfeffer MA, et al. Red blood cell distribution width and mortality risk in a community-based prospective cohort. Arch Intern Med 2009;169:588–94.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Pollack MM, Patel KM, Ruttimann UE. PRISM III: an updated Pediatric Risk of Mortality score. Crit Care Med 1996;24:743–52.. [DOI] [PubMed] [Google Scholar]
- [25].Matics TJ, Sanchez-Pinto LN. Adaptation and validation of a pediatric sequential organ failure assessment score and evaluation of the Sepsis-3 definitions in critically ill children. JAMA Pediatr 2017;171:e172352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Leteurtre S, Duhamel A, Salleron J, et al. PELOD-2: an update of the PEdiatric logistic organ dysfunction score. Crit Care Med 2013;41:1761–73.. [DOI] [PubMed] [Google Scholar]
- [27].Graciano AL, Balko JA, Rahn DS, et al. The Pediatric Multiple Organ Dysfunction Score (P-MODS): development and validation of an objective scale to measure the severity of multiple organ dysfunction in critically ill children. Crit Care Med 2005;33:1484–91.. [DOI] [PubMed] [Google Scholar]
- [28].Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, et al. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 2008;27:157–72.. discussion 207-112. [DOI] [PubMed] [Google Scholar]
- [29].Hashemi SM, Khanbabaee G, Salarian S, et al. Association between red cell distribution width and mortality in pediatric patients admitted to intensive care units. Iran J Blood Cancer 2017;9:54–8.. [Google Scholar]
- [30].Loveday S, Sinclair L, Badrick T. Does the addition of RDW improve current ICU scoring systems? Clin Biochem 2015;48:569–74.. [DOI] [PubMed] [Google Scholar]
- [31].Hunziker S, Celi LA, Lee J, et al. Red cell distribution width improves the simplified acute physiology score for risk prediction in unselected critically ill patients. Crit Care 2012;16:R89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].de Gonzalo-Calvo D, de Luxan-Delgado B, Rodriguez-Gonzalez S, et al. Interleukin 6, soluble tumor necrosis factor receptor I and red blood cell distribution width as biological markers of functional dependence in an elderly population: a translational approach. Cytokine 2012;58:193–8.. [DOI] [PubMed] [Google Scholar]
- [33].Fujita B, Strodthoff D, Fritzenwanger M, et al. Altered red blood cell distribution width in overweight adolescents and its association with markers of inflammation. Pediatr Obes 2013;8:385–91.. [DOI] [PubMed] [Google Scholar]
- [34].Forhecz Z, Gombos T, Borgulya G, et al. Red cell distribution width in heart failure: prediction of clinical events and relationship with markers of ineffective erythropoiesis, inflammation, renal function, and nutritional state. Am Heart J 2009;158:659–66.. [DOI] [PubMed] [Google Scholar]
- [35].Pierce CN, Larson DF. Inflammatory cytokine inhibition of erythropoiesis in patients implanted with a mechanical circulatory assist device. Perfusion 2005;20:83–90.. [DOI] [PubMed] [Google Scholar]
- [36].Kiefer CR, Snyder LM. Oxidation and erythrocyte senescence. Curr Opin Hematol 2000;7:113–6.. [DOI] [PubMed] [Google Scholar]
- [37].Patel KV, Ferrucci L, Ershler WB, et al. Red blood cell distribution width and the risk of death in middle-aged and older adults. Arch Intern Med 2009;169:515–23.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Friedman JS, Lopez MF, Fleming MD, et al. SOD2-deficiency anemia: protein oxidation and altered protein expression reveal targets of damage, stress response, and antioxidant responsiveness. Blood 2004;104:2565–73.. [DOI] [PubMed] [Google Scholar]
- [39].Semba RD, Patel KV, Ferrucci L, et al. Serum antioxidants and inflammation predict red cell distribution width in older women: the Women's Health and Aging Study I. Clin Nutr 2010;29:600–4.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Marinkovic D, Zhang X, Yalcin S, et al. Foxo3 is required for the regulation of oxidative stress in erythropoiesis. J Clin Invest 2007;117:2133–44.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Montagnana M, Danese E. Red cell distribution width and cancer. Ann Transl Med 2016;4:399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Cavusoglu E, Chopra V, Gupta A, et al. Relation between red blood cell distribution width (RDW) and all-cause mortality at two years in an unselected population referred for coronary angiography. Int J Cardiol 2010;141:141–6.. [DOI] [PubMed] [Google Scholar]


