Abstract
The study aimed to explore whether cancer-related pain and opioids use are associated with the survival of cancer patients, and perform a cohort study and a meta-analysis to quantify the magnitude of any association.
A retrospective cohort study was performed to analyze the impact of pain level, and opioids use on cancer-specific survival (CSS) in advanced cancer patients. Patients and relevant medical records were selected from the registry of the Radiation and chemotherapy division of Ningbo First Hospital between June 2013 and October 2017. Hazard ratios (HRs) and 95% confidential intervals (CIs) for CSS by opioids use were calculated by univariate and multivariate Cox regression analyses. The systematic review included relevant studies published before October 2018. The combined HRs and 95% CIs for overall survival (OS) and progression-free survival (PFS) were calculated using random-effect models.
A total of consecutive 203 cancer patients were included in the cohort study. Kaplan–Meier curves indicate a negative association between CSS and cancer-related pain or opioids requirement, but less evidence of an association with the dose of opioids use. Multivariate models revealed that the pain level and opioids requirement were associated with shorter CSS, after adjusting for significant covariates. The results of the meta-analysis indicated that postoperative opioids use had a poor effect on PFS, and opioids use for cancer-related pain was associated with poor OS in cancer patients, while intraoperative opioids use was not associated with cancer survival.
We concluded that cancer-related pain and opioids requirements are associated with poor survival in advanced cancer patients, and postoperative opioids use and opioids use for cancer-related pain may have an adverse effect on the survival of cancer patients.
Keywords: cancer, opioids, pain, survival
1. Introduction
With the development of multiple anti-tumor treatment methods such as targeted therapy and immunotherapy, more and more patients with tumors can extend their survival, which lead to an increase in cancer survivors.[1] A major clinical challenge in treating cancer patients is the management of cancer pain. Cancer-related pain is significantly related to tumor stage. A systematic review reported that the prevalence of cancer pain rises with cancer progression and affects nearly 64% of patients with advanced cancer.[2] Cancer-related pain not only adversely affects the quality of life of patients with advanced cancer, but also induces cancer progression through several mechanisms.[3] Successfully controlling cancer-related pain is an indispensable and important work in clinical practice.
Cancer-related pain management mainly relies on the three-step analgesic principle recommended by World Health Organization (WHO).[4] The use of analgesics is gradually transitioning from non-opioids to weak opioids to strong opioids. Although non-opioid analgesics such as adjuvant analgesia, glucocorticoids, radiation therapy, and acupuncture play a role in pain relief, opioids are still the most effective treatment for severe advanced cancer pain.[5] However, in addition to social issues such as drug addiction, the use of opioids can also cause some adverse effects in pain patients, such as respiratory depression, constipation, nausea, and dizziness, which may contribute to increasing mortality in pain patients.[6] Additionally, evidence indicates that opioids may promote cancer progression by activating the mu-opioid receptor (MOR), by increasing angiogenesis or by inducing immunosuppression.[7] Despite the many adverse effects of opioid use, the priority is to control cancer pain. Until the new non-pharmacological analgesics and non-opioids approaches can replace opioids to treat severe advanced cancer pain, the adverse effects of opioids on tumor patients are still worth studying.
As for the concerns of the potential adverse effects related to opioids use and pain, the current evidence regarding the association among opioids use, pain, and cancer survival remains debatable.[8,9] To address this problem, we conduct the cohort analysis and meta-analysis evaluating the independent contribution of cancer-related pain and opioids use to the overall survival (OS), progression-free survival (PFS) and cancer-specific survival (CSS) of cancer patients.
2. Materials and methods
2.1. Cohort study
2.1.1. Patients
The study was approved by the ethic community of Ningbo First Hospital (No. R39, 20181220). Informed consent was signed at the time of admission. The consecutive advanced cancer patients who suffered cancer-related pain and were treated with opioids at the Radiation and chemotherapy division of Ningbo First Hospital between June 2013 and October 2017 were included as the opioid group, which was further divided into two subgroups of a low-dose group and a high-dose group. We also selected the consecutive contemporary inpatients who suffered advanced cancer but without opioids treatment as the non-opioid group. The patients in the non-opioid group are mainly patients with mild pain that can be controlled by non-steroidal anti-inflammatory drugs. These patients have no need for opioids. A total of 203 patients were enrolled in the analysis, with 97 in the opioid group (46 in the low-dose group, and 51 in the high-dose group) and 106 in the non-opioid group. Among these patients with advanced cancer included in this study, patients with nasopharyngeal carcinoma are mainly treated with radiation therapy, and other cancer patients are mainly treated with chemotherapy-based palliative treatment. Follow-up was conducted by telephone contact with the patients or their families.
2.2. Variables and Endpoint
Patient clinicopathological characteristics, visual analog score (VAS) for pain, and pharmacy data were obtained from patient records in Radiation and chemotherapy division. All oral and transdermal opioid prescriptions were collected. All opioids were concerted to Oxycontin equivalents per 12 h according to the transformation equation “Fentanyl patch 4.2 mg Q72h = MS Contin 30 mg Q12h = Oxycontin 15 mg Q12h”.[10] The dose of opioids less than median dose 20 mg Oxycontin per 12 h was deemed as low dose, otherwise as the high dose. Pain levels were measured by VAS and categorized as three levels: low (0–3), moderate (4–6), and severe (7–10). We used the patients’ maximum reported pain level before opioid treatment to analyze the effect of pain on the CSS. The primary endpoint of this study was CSS, defined as the time in months from first admission in Radiation and chemotherapy division to death due to cancer. For the patients alive, CSS was defined as the time between first admission and the data of the last follow-up.
2.3. Statistical analysis
After the normal distribution test of the clinicopathological characteristics data, the Chi-square test is used to compare differences in categorical variables, and analysis of variance (ANOVA) is used to compare differences in continuous variables. The CSS curves were constructed using the Kaplan–Meier methods, and log-rank test was used for pairwise comparison of survival. Univariate and multivariate Cox proportional hazards models were used to assess the effects of clinicopathological variables on CSS. The software SPSS 19.0 (SPSS Inc., Chicago, IL) was used for all analyses. A two-tailed P < .05 was considered significant in statistical tests.
2.4. Systematic review and meta-analysis
2.4.1. Search strategy
This systematic review and meta-analysis were conducted based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).[11] The electronic databases including PubMed, PubMed Central (PMC), Ovid, EMBASE, and Web of Science were searched from inception to October 20, 2018, for articles investigating the effects of pain and opioids use on cancer survival. Our search strategy included terms for opioids use (opioid, anesthetic, or analgesia), cancer (cancer, tumor neoplasm, or carcinoma), and survival (survival, prognosis, death, or mortality). The references in identified articles were also reviewed manually for possible inclusions.
2.4.2. Selection and exclusion criteria
Eligible studies were enrolled in this meta-analysis in line with the following criteria:
-
1.
they included a cohort of cancer patients in which exposure to opioids treatment for cancer-related pain was measured and recorded;
-
2.
the outcomes of follow-up were in terms of PFS, recurrence-free survival (RFS), disease-free survival (DFS), OS or CSS;
-
3.
they included sufficient data to estimate the hazard ratio (HR) and its 95% CI according to the opioids use.
When multiple articles based on the same population, the most recent or complete one was enrolled. The exclusion criteria were the following:
-
1.
articles without adequate survival data for extracting HR and its 95% CI;
-
2.
case reports, reviews, letters to the editor, and summary of the meeting.
Titles and abstracts were screened to identify eligible studies, and then the full-text manuscripts were evaluated carefully. Any disagreement was resolved by mutual discussion.
2.4.3. Data extraction and quality assessment
Relevant data was extracted from all the eligible studies by two reviewers independently using a purpose-designed form. Any discrepancy was resolved via consensus. The following items were recorded: first author's name, publication year, country, study years, patient's age, gender, tumor type, tumor stage, sample size, basis for grouping, cutoff value, the time of opioids treatment, the formulation of opioids, period of follow-up, and assessments for outcomes. HRs and 95% CIs were also collected as applicable. If the survival outcomes in the eligible studies were presented by both univariate and multivariate Cox regression analyses, the results of multivariate Cox regression analyses were primarily selected. For studies in which HRs was not provided explicitly, we extracted the survival estimates from the original data or Kaplan–Meier curves using Tierney's methods.[12,13] Quality assessment for studies was performed by the Newcastle-Ottawa Scale (NOS).[14] A study achieving a score of six or more was regarded as a high-quality one.
2.5. Statistical analysis
The statistical analysis was conducted using software STATA 12.0 (Stata Inc., TX). Pooled HRs and 95% CIs were used to assess the effect of opioids use on cancer survival. Heterogeneity was evaluated using I-squared statistics.[15] A random-effect model was applied if I2 > 50%; otherwise, a fixed-effect model was used. To explore the possible sources of heterogeneity and further investigate the effect in different applications, subgroup analyses were adopted. Potential publication bias was evaluated by the Begg's and Egger's test. Finally, we also performed sensitivity analyses by removing each single study to assess the stability of the results.
3. Results
3.1. Cohort study
3.1.1. Association of opioids use and dose with clinicopathological characteristics
A total of 203 patients were included in the cohort study. Among them, 106 patients never required opioids. The opioids were required in 97 patients, which were further divided into the low-dose group (n = 46) and high-dose group (n = 51). Two patients in the non-opioids group were missing during the follow-up. The patients’ clinicopathological factors and their association with opioids requirement and dose were presented in Table 1. When age was analyzed as continuous variable, the opioids required group was younger than the non-opioid group, and high-dose group was younger than the low-dose group (P < .01). When age was converted as a categorical variable, there was no difference among the three groups. The pain level in the opioid required group was more severe than that in the non-opioid group, but there was no significant difference between the low-dose group and the high-dose group. OxyContin was the most common drug used in both the low-opioid group and the high-opioid group, and there was no significant difference in the opioid type between the two groups (P = .296). The gender and the cancer types did not differ among the three groups.
Table 1.
Association of opioids use with the clinicopathologic characteristics of 203 patients with advanced cancer.

3.1.2. Influence of pain and opioids requirement on the survival of cancer patients
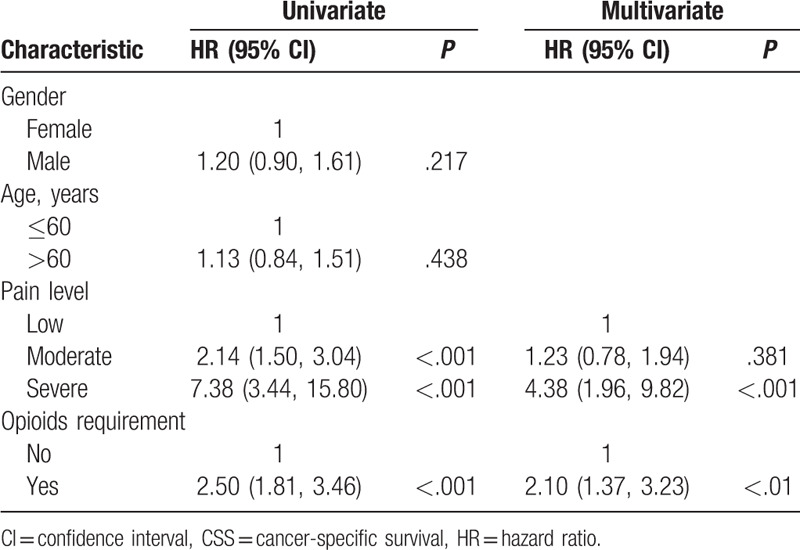
Kaplan–Meier survival curves for CSS based on the pain level and opioids requirement are shown in Figure 1A and B. It shows that the patients with low pain level had better CSS than those with moderate and severe pain (P < .001), and patients with moderate pain had better CSS compared with those suffering from severe pain (P < .01). Opioids requirement is significantly associated with shorter CSS (P < .001). Results of univariate and multivariate Cox regression of prognostic factors for CSS are presented in Table 2. In univariate Cox regression analysis, age and gender are not associated with survival, but more severe pain (P < .001) and opioids requirement (P < .001) predict short CSS. In multivariate Cox regression analysis that adjusted for the pain level and opioids requirement, the severe pain (P < .001) and opioids requirement (P < .01) are still related to worse CSS. Still, moderate pain is no longer associated with CSS (P = .381).
Figure 1.
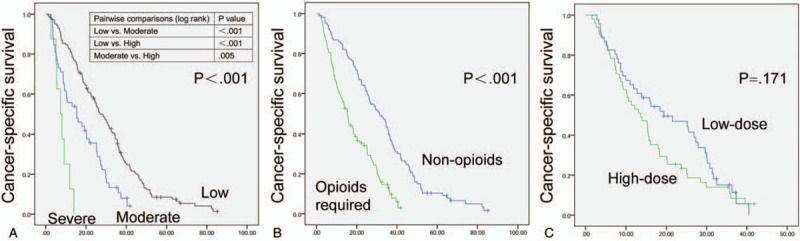
Kaplan–Meier survival curves depicting cancer-specific survival according to pain level (A), opioids requirement (B) and opioids dose (C). (A) Kaplan–Meier curves of high-dose and low-dose groups.
Table 2.
Univariable and multivariate Cox regression analyses predicting CSS in 203 advanced cancer patients.
3.1.3. Influence of opioids dose on the survival of cancer patients
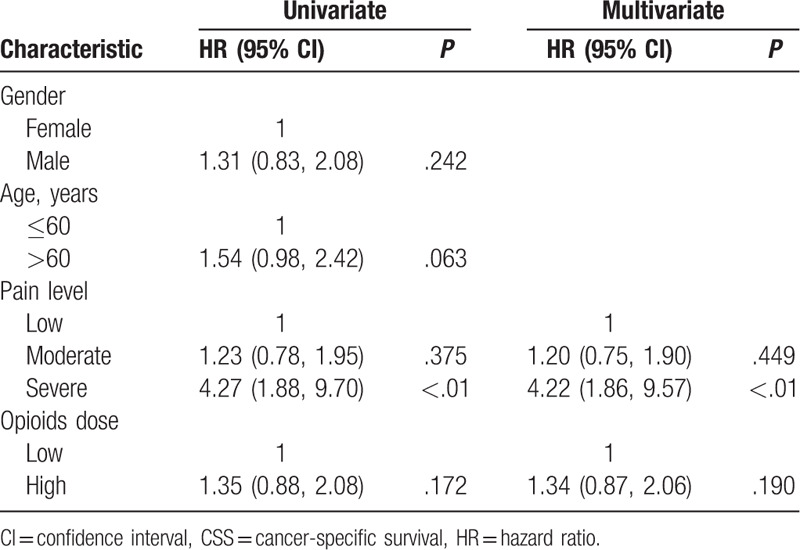
The opioids high and low dose groups were analyzed for exploring the effect of opioid dose on CSS. Kaplan–Meier survival curves for CSS based on the opioids dose are presented in Figure 1C. There was no significant separation between the low-dose group and the high-dose group (P = .171). The Cox regression analyses are shown in Table 3. Based on the univariate and multivariate Cox regression analyses, gender, age, moderate pain, and opioids dose are not related to the CSS. Still the patients with severe pain levels exhibited the poor prognosis (P < .01).
Table 3.
Univariable and multivariate Cox regression analyses predicting CSS in 97 opioids required patients.
3.2. Systematic review and meta-analysis
3.2.1. Search results and study characteristics
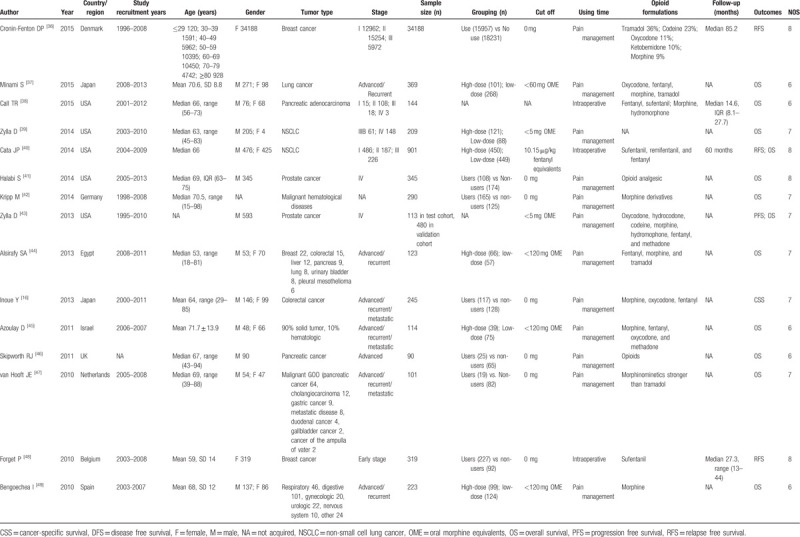
We searched 32,715 articles through the electronic databases and three articles from the references. After scanning the titles and abstracts, only 28 records were deemed eligible (Fig. 2). They were all cohort studies, which included one prospective cohort study[8] and 27 retrospective cohort studies. Seven studies reported outcomes for intraoperative opioids use; four studies reported postoperative opioids use, and 17 reported opioids use in the treatment of cancer-related pain. According to the NOS, the quality of these 28 articles ranged from 5 to 9, with a mean of 7.4. The main characteristics of the included studies were listed in Table 4 .
Figure 2.
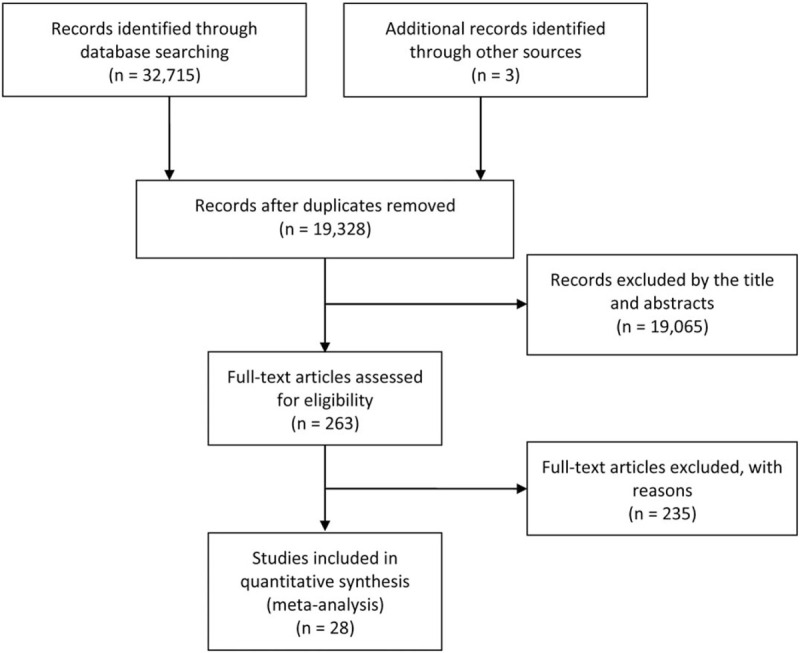
Flow diagram of the study selection process.
Table 4.
Main characteristics of studies included in the meta-analysis.
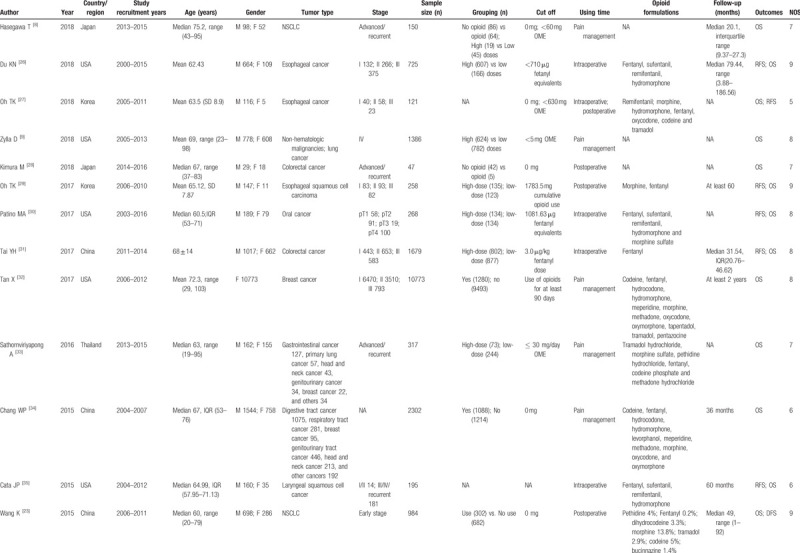
Table 4 (Continued).
Main characteristics of studies included in the meta-analysis.
3.2.2. Meta-analysis results
In our analysis, we merged PFS, DFS, and RFS together considering the similarities among them. Since only one study[16] reported CSS and most studies used OS, it was difficult to conduct a meta-analysis by listing the single study separately. The purpose of this study was to explore the impact of experimental factors on the long-term survival of cancer patients, and CSS could more accurately describe the effects of experimental factors on the patient's long-term survival than OS, so we boldly merged CSS and OS together.[17] A total of 26 articles with 28 studies evaluated OS, and 11 articles with 12 studies evaluated PFS. The random-effects models were used to pool the HRs and 95 CIs because of obvious statistical heterogeneity. Compared with the patients with no opioids use or low-dose opioid use, the opioids use, or high-dose opioids use groups were associated with an inferior PFS (HR = 1.086, 95%CI 1.011–1.166, P = .024) and OS (HR = 1.006, 95%CI 1.001–1.012, P = .015) (Fig. 3A and B).
Figure 3.
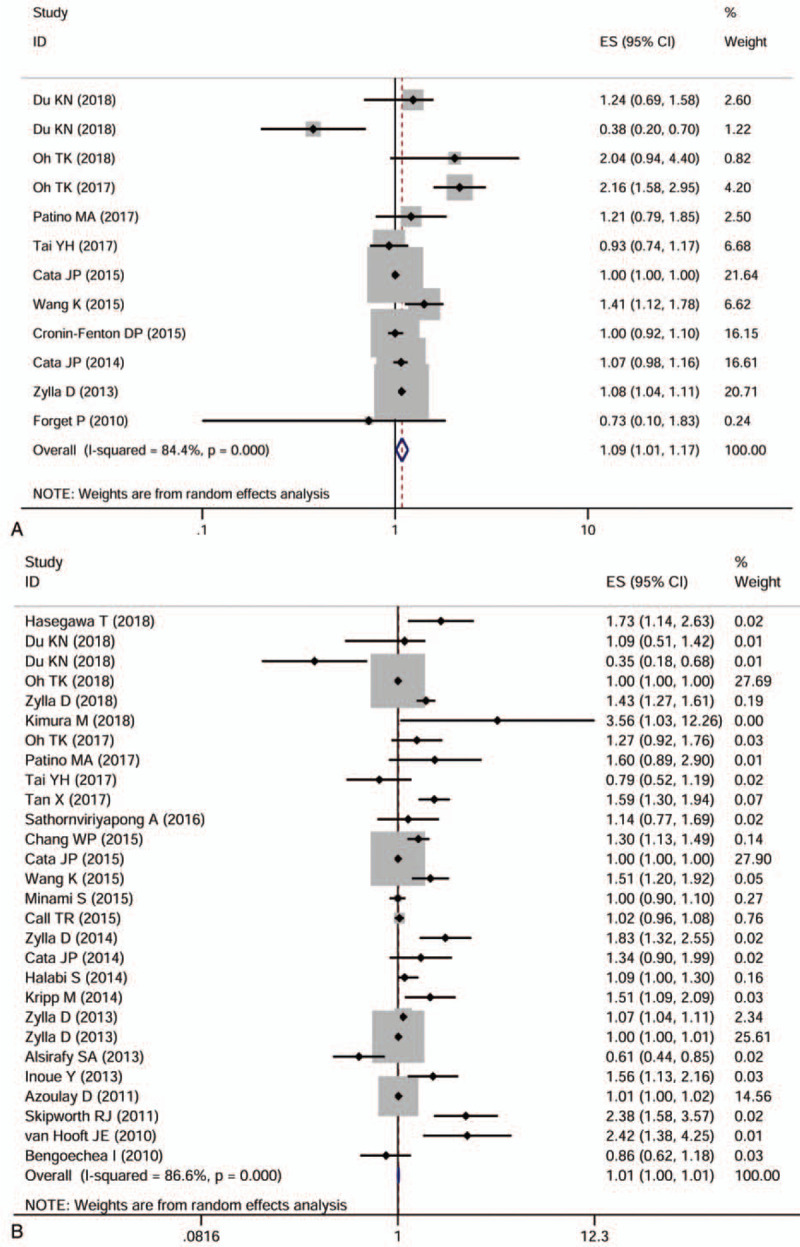
Forest plots of studies evaluating the effect of opioids use on cancer progression-free survival (A) and overall survival (B).
The included studies reported opioids used for different therapeutic purposes and in various types of cancer. To explore the possible correlation between opioids use and cancer survival based on three main features, including therapeutic purpose, therapeutic dose, and cancer type, we performed a series of subgroup analyses. The first subgroup analysis was performed based on therapeutic purposes. The results indicated that intraoperative opioids use (HR = 1.009, 95%CI 0.913–1.116, P = .857) and opioids used for cancer pain management (HR = 1.051, 95%CI 0.979–1.130, P = .171) had no effect on PFS, while postoperative opioids use (HR = 1.760, 95%CI 1.264–2.450, P = .001) was associated with poor PFS (Fig. 4A). As for the OS, the intraoperative opioids use (HR = 1.006, 95%CI 0.923–1.097, P = .888) and postoperative opioids use (HR = 1.300, 95%CI 0.948–1.781, P = .103) had no effect on the OS. Still, opioids use for cancer pain treatment (HR = 1.100, 95%CI 1.061–1.141, P < .001) had poor effect on the OS (Fig.4 B).
Figure 4.
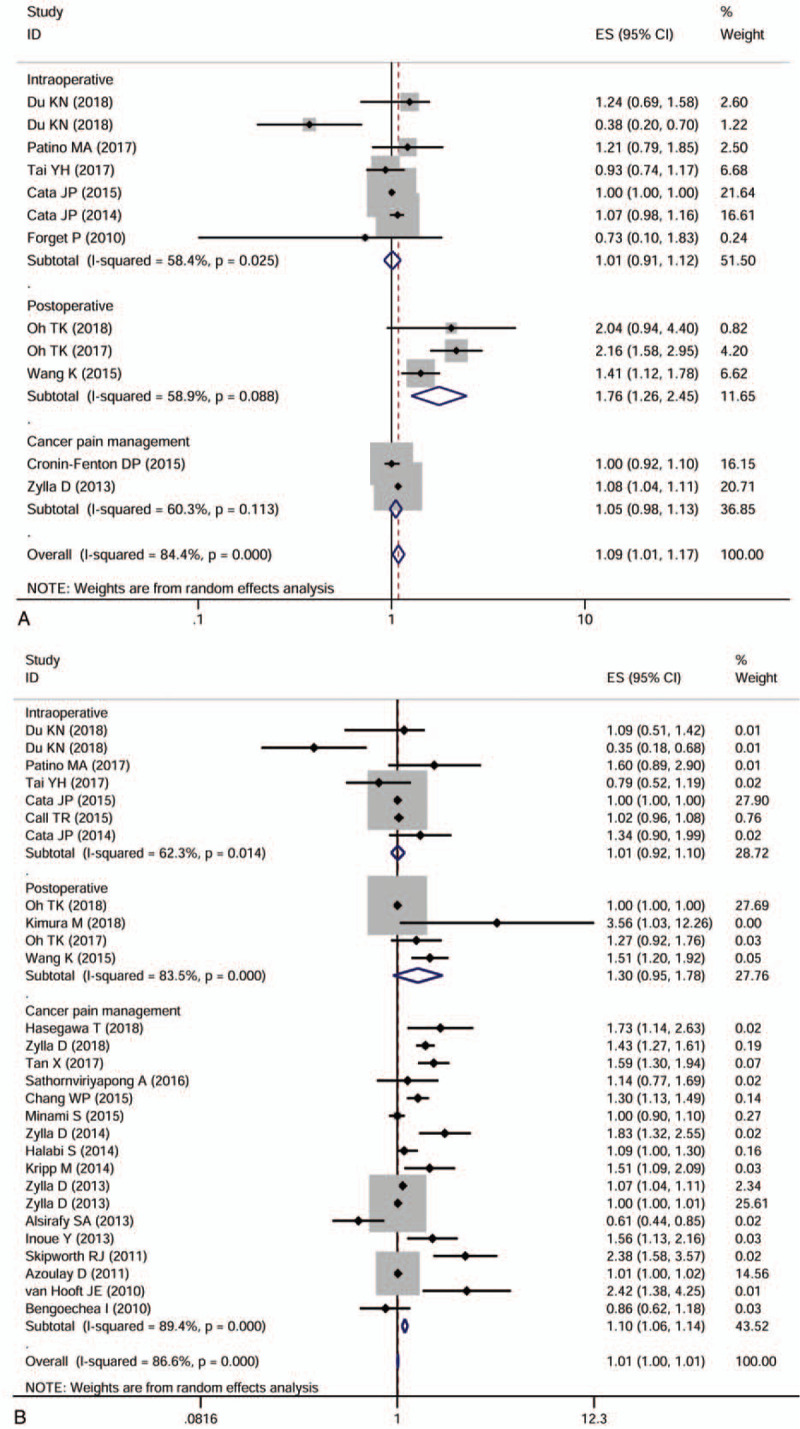
Forest plots of subgroup analyses evaluating the effect of opioids use on cancer progression-free survival (A) and overall survival (B) according to different therapeutic purposes.
In the opioids used for cancer pain treatment subgroup, we analyzed the effect of opioids on PFS and OS stratified by therapeutic dose or cancer type. High-dose opioids use (HR = 1.080, 95%CI 1.045–1.116, P < .001) was associated with poor PFS, whereas the requirement of opioids had no effect on PFS (Fig. 5A). Opioids use in prostate cancer (HR = 1.080, 95%CI 1.045–1.116, P < .001) had a bad effect on PFS, whereas that in breast cancer did not (Fig. 5B). With respect to OS, opioids required (HR = 1.532, 95%CI 1.253–1.873, P < .001) and high-dose opioids use (HR = 1.053, 95%CI 1.019–1.088, P = .002) were all related to poor OS (Fig. 5C). Opioids use was associated with poor OS in breast cancer (HR = 1.590, 95%CI 1.302–1.942, P < .001), malignant hematological diseases (HR = 1.509, 95%CI 1.092–2.085, P = .013), colorectal cancer (HR = 1.564, 95%CI 1.134–2.158, P = .006) and pancreatic cancer (HR = 2.378, 95%CI 1.583–3.572, P < .001), but not in lung cancer, prostate cancer, and mixed cancer types (Fig. 5D).
Figure 5.
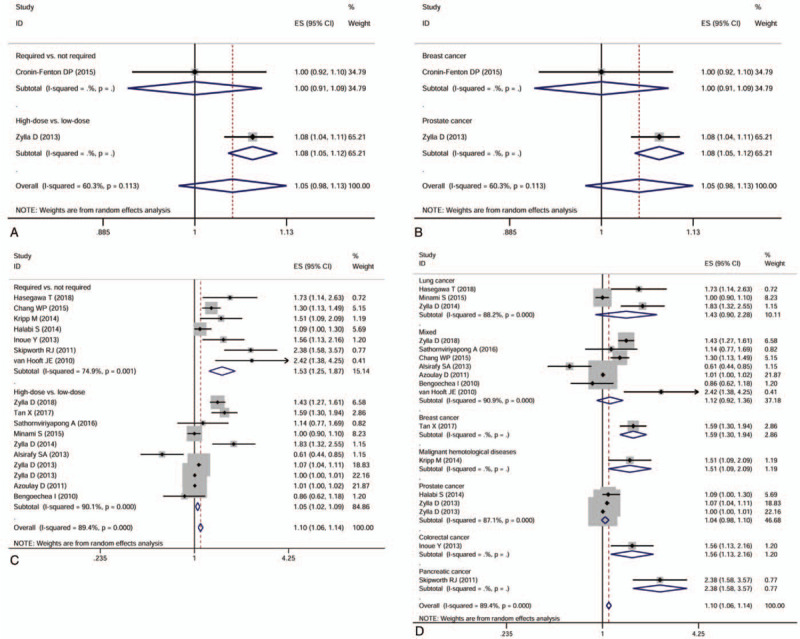
Forest plots of subgroup analyses evaluating the effect of opioids use on cancer progression-free survival based on therapeutic dose (A) and cancer types (B), and the effect of opioids use on overall survival based on therapeutic dose (C) and cancer types (D).
3.2.3. Publication bias and Sensitivity analysis
Publication bias for PFS and OS were not significant based on the Begg's and Egger's test. Sensitivity analysis performed by removing omitting each single study sequentially indicated that the synthetic estimates of the effect of opioids use on PFS and OS did not vary significantly, which meant that the results of this meta-analysis were robust.
4. Discussion
In this study, we found that moderate to severe pain and the requirement of opioids were associated with reduced CSS in a variety of cancer patients. Cancer-related pain is one of the most morbidities experienced by patients with advanced cancer. Recent studies reported that pain was an important predictor of clinical outcome in patients with cancer.[15,18,19] Armstrong et al[19] considered that pain could be a statistically significant prognostic factor for OS based on the TAX 327 trial. Roviello et al[15] identified pain as a predictive factor for OS in men with prostate cancer. The results in the present study are consistent with previous researches on other cancers. Increased pain and opioid demand were mostly due to disease progression (e.g., bone metastases, extensive pleural invasion, and vital organs compression), which were associated with significant morbidity and mortality.[18,20] Pain itself also affects the survival of patients, because pain can make it hard for patients to eat, sleep, or even continue cancer treatments.[20] Accurate assessment of pain and coverage of all patients in studies using pain as a variable is important. Pain assessment in most previous studies and in this study has used patient self-reporting methods. For non-verbal patients who suffer pain, the Critical Care Pain Observation Tool (CPOT) and Behavioral Pain Scale (BPS) behavioral pain scales can play a major role in pain assessment.[21]
In addition to pain, opioids may also affect cancer patients’ survival through respiratory depression, delirium, addiction, or directly acting on tumor cells.[7,8,22] The patients who suffered severe pain were more likely to require opioids treatment. Based on the comparison between the non-opioid group and opioid group, we couldn’t distinguish this poor effect owing to opioids use or pain because there were more patients with moderate to severe pain in the opioid demand group. To explore the effect of opioids use on cancer survival, we compared the high-dose group with the low-dose group. The high-dose group survived worse than the low-dose group, but the statistical difference was not obvious. These results were not consistent with the previous results, which reported that opioids use, especially at high-dose, was associated with short survival in cancer patients.[9,23] The inconsistency may be due to our small sample size, as the sample size of each subgroup after subgrouping is reduced.
In terms of the purpose of opioid use, we found that intraoperative opioids use was not associated with cancer survival. In contrast, postoperative opioids use and opioids use for cancer pain treatment had a bad effect on cancer survival. Bimonte summarized in his review that different outcomes might be due to different concentrations and/or duration of use of opioids.[22] Intraoperative analgesia tends to use a large dose of intravenous short-acting analgesics; Postoperative analgesia with optimal analgesia and minimum dose as a principle is more likely to continue to use short-acting analgesic drugs for a certain period time; while the analgesic effect of cancer pain is often based on oral long-acting analgesics. At present, oxycodone has an increasing trend in the application of cancer pain. In addition to the total dose of opioids, drug type, frequency, single-dose, and duration of medication may be the factor that affects the outcomes.[22]
The strength of the study lies in conducting a meta-analysis with a large population to assist the cohort study for exploring the effect of pain and opioids use on cancer survival. There are also some limitations to this study. First, our cohort study and the studies included in the meta-analysis are observational studies, so there is the potential residual confounding that we could not control. Second, the follow-up period in some included studies was relatively short (median 20 months), which may not be sufficient to assess the effect of opioids use fully. Considering the short life span of advanced cancer patients, the 2-year follow-up may be enough to draw statistical conclusions. Third, the patients included in this study were mainly palliative care patients with relatively poor physical conditions, and patient self-reporting assessment of pain levels for such patients may have some deviations. Using patient self-reporting assessment methods in combination with CPOT and BPS tools to evaluate pain values in future studies may lead to more accurate results.[24,25] Fourth, the dose and formulation of opioids in different studies varied, so it is hard for us to determine the dose and opioids form at work accurately. In our cohort study, the main form of opioids is Oxycontin. As for the studies included in the meta-analysis, the dose of different opioids form was converted to OME, which may play a clinical reference role in opioids dose and formulations. Based on the above limitations, the results should be interpreted with caution.
In conclusion, combining the results of this cohort study and the meta-analysis suggest that cancer-related pain and opioids requirements are associated with poor survival in advanced cancer patients, and postoperative opioids use and opioids use for cancer-related pain may have a negative effect on survival of cancer patients. Although opioids may promote cancer progression in some cases, controlling severe pain symptoms remains a priority. The development of other analgesic modes such as non-pharmacological and non-opioids modes, and improvements in opioids should address this clinical problem. Further prospective studies are needed to clarify the effect of pain and opioids use on cancer survival.
Author contributions
Conceptualization: Jungang Zheng, Zihui Lu, Changshun Huang.
Data curation: Weifei Wang, Xinger Qian, Jun Wang.
Formal analysis: Saihong Cai.
Investigation: Saihong Cai, Jun Wang.
Methodology: Jing He, Linhai Zhu, Changshun Huang.
Supervision: Jungang Zheng, Zihui Lu, Changshun Huang.
Validation: Weifei Wang, Haidong Zhou, Linhai Zhu, Xinger Qian, Zihui Lu, Changshun Huang.
Writing – original draft: Jing He, Haidong Zhou.
Footnotes
Abbreviations: ANOVA = analysis of variance, CIs = confidential intervals, CSS = cancer-specific survival, DFS = disease-free survival, HRs = Hazard ratios, MOR = mu-opioid receptor, NOS = Newcastle-Ottawa Scale, OME = oral morphine equivalents, OS = overall survival, PFS = progression-free survival, RFS = recurrence-free survival, VAS = visual analogue score.
How to cite this article: Zheng J, He J, Wang W, Zhou H, Cai S, Zhu L, Qian X, Wang J, Lu Z, Huang C. The impact of pain and opioids use on survival in cancer patients: Results from a population-based cohort study and a meta-analysis. Medicine. 2020;99:9(e19306).
JZ and JH contributed equally to this work.
This study was supported by the Natural science foundation project of Ningbo city, Zhejiang province (project 2019A610186 and 2017A610244), and the Zhejiang medical and health science and technology program (project 2019KY157, 2019KY572, and 2020KY249).
The authors have no conflicts of interest to disclose.
References
- [1].Miller KD, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin 2019;69:363–85. [DOI] [PubMed] [Google Scholar]
- [2].van den Beuken-van Everdingen MH, de Rijke JM, Kessels AG, et al. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol 2007;18:1437–49. [DOI] [PubMed] [Google Scholar]
- [3].Herndon JE, 2nd, Fleishman S, Kornblith AB, et al. Is quality of life predictive of the survival of patients with advanced nonsmall cell lung carcinoma? Cancer 1999;85:333–40. [DOI] [PubMed] [Google Scholar]
- [4].WHO Guidelines for the Pharmacological and Radiotherapeutic Management of Cancer Pain in Adults and Adolescents. Geneva, Switzerland: World Health Organization; 2018. [PubMed] [Google Scholar]
- [5].Pergolizzi JV, Gharibo C, Ho KY. Treatment considerations for cancer pain: a global perspective. Pain Pract 2015;15:778–92. [DOI] [PubMed] [Google Scholar]
- [6].Baldini A, Von Korff M, Lin EH. A review of potential adverse effects of long-term opioid therapy: a practitioner's guide. Prim Care Companion CNS Disord 2012;14: doi: 10.4088/PCC.11m01326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Singleton PA, Moss J, Karp DD, et al. The mu opioid receptor: a new target for cancer therapy? Cancer 2015;121:2681–8. [DOI] [PubMed] [Google Scholar]
- [8].Hasegawa T, Oguri T, Osawa T, et al. Opioid dose and survival of patients with incurable nonsmall cell lung cancer: a prospective cohort study. J Palliat Med 2018;21:1436–41. [DOI] [PubMed] [Google Scholar]
- [9].Zylla D, Steele G, Shapiro A, et al. Impact of opioid use on health care utilization and survival in patients with newly diagnosed stage IV malignancies. Suppor Care Cancer 2018;26:2259–66. [DOI] [PubMed] [Google Scholar]
- [10].Gaguski ME, Karcheski T. Equianalgesic dosing: principles of practice for the care team. Clin J Oncol Nurs 2013;17:80–3. [DOI] [PubMed] [Google Scholar]
- [11].2010;Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statementJT Int J Surg (Lond, Engl). 8:336–41. [DOI] [PubMed] [Google Scholar]
- [12].Altman DG, Bland JM. How to obtain the confidence interval from a P value. BMJ (Clin Res Ed) 2011;343:d2090. [DOI] [PubMed] [Google Scholar]
- [13].Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [15].Roviello G, Gallicchio R, Bozza G, et al. Pain predicts overall survival in men with metastatic castration-resistant prostate cancer treated with radium-223. OncoTargets Ther 2019;12:9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Inoue Y, Iwata T, Okugawa Y, et al. Prognostic significance of opioid use in the active treatment of advanced colorectal cancer. Mol Clin Oncol 2013;1:59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Saad ED, Buyse M. Overall survival: patient outcome, therapeutic objective, clinical trial end point, or public health measure? J Clin Oncol 2012;30:1750–4. [DOI] [PubMed] [Google Scholar]
- [18].Halabi S, Vogelzang NJ, Kornblith AB, et al. Pain predicts overall survival in men with metastatic castration-refractory prostate cancer. J Clin Oncol 2008;26:2544–9. [DOI] [PubMed] [Google Scholar]
- [19].Armstrong AJ, Garrett-Mayer ES, Yang YC, et al. A contemporary prognostic nomogram for men with hormone-refractory metastatic prostate cancer: a TAX327 study analysis. Clin Cancer Res 2007;13:6396–403. [DOI] [PubMed] [Google Scholar]
- [20].Pain management for patients with, cancer. CA Cancer J Clin 2018;68:197–8. [DOI] [PubMed] [Google Scholar]
- [21].Kotfis K, Zegan-Baranska M, Szydlowski L, et al. Methods of pain assessment in adult intensive care unit patients—Polish version of the CPOT (Critical Care Pain Observation Tool) and BPS (Behavioral Pain Scale). Anaesthesiol Intensive Ther 2017;49:66–72. [DOI] [PubMed] [Google Scholar]
- [22].Bimonte S, Barbieri A, Palma G, et al. The role of morphine in animal models of human cancer: does morphine promote or inhibit the tumor growth? BioMed Res Int 2013;2013:258141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wang K, Qu X, Wang Y, et al. Effect of mu agonists on long-term survival and recurrence in nonsmall cell lung cancer patients. Medicine 2015;94:e1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kotfis K, Strzelbicka M, Zegan-Baranska M, et al. Validation of the behavioral pain scale to assess pain intensity in adult, intubated postcardiac surgery patients: a cohort observational study—POL-BPS. Medicine 2018;97:e12443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kotfis K, Zegan-Baranska M, Strzelbicka M, et al. Validation of the Polish version of the Critical Care Pain Observation Tool (CPOT) to assess pain intensity in adult, intubated intensive care unit patients: the POL-CPOT study. Arch Med Sci 2018;14:880–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Du KN, Feng L, Newhouse A, et al. Effects of intraoperative opioid use on recurrence-free and overall survival in patients with esophageal adenocarcinoma and squamous cell carcinoma. Anesth Analg 2018;127:210–6. [DOI] [PubMed] [Google Scholar]
- [27].Oh TK, Kim K, Jheon SH, et al. Long-term oncologic outcomes, opioid use, and complications after esophageal cancer surgery. J Clin Med 2018;7: doi: 10.3390/jcm7020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kimura M, Iwai M, Usami E, et al. Prognostic factors in patients with advanced and recurrent colorectal cancer receiving last-line chemotherapy. Die Pharmazie 2018;73:115–9. [DOI] [PubMed] [Google Scholar]
- [29].Oh TK, Jeon JH, Lee JM, et al. Association of high-dose postoperative opioids with recurrence risk in esophageal squamous cell carcinoma: reinterpreting ERAS protocols for long-term oncologic surgery outcomes. Dis Esophagus 2017;30:1–8. [DOI] [PubMed] [Google Scholar]
- [30].Patino MA, Ramirez RE, Perez CA, et al. The impact of intraoperative opioid use on survival after oral cancer surgery. Oral Oncol 2017;74:1–7. [DOI] [PubMed] [Google Scholar]
- [31].Tai YH, Wu HL, Chang WK, et al. Intraoperative fentanyl consumption does not impact cancer recurrence or overall survival after curative colorectal cancer resection. Sci Rep 2017;7:10816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Tan X, Camacho TF, LeBaron VT, et al. Opioid use among female breast cancer patients using different adjuvant endocrine therapy regimens. Breast Cancer Res Treat 2017;165:455–65. [DOI] [PubMed] [Google Scholar]
- [33].Sathornviriyapong A, Nagaviroj K, Anothaisintawee T. The association between different opioid doses and the survival of advanced cancer patients receiving palliative care. BMC Palliat Care 2016;15:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Chang WP, Lin CC. Use of opioid analgesics or sleeping medication and survival of cancer patients. Eur J Oncol Nurs 2015;19:199–206. [DOI] [PubMed] [Google Scholar]
- [35].Cata JP, Zafereo M, Villarreal J, et al. Intraoperative opioids use for laryngeal squamous cell carcinoma surgery and recurrence: a retrospective study. J Clin Anesth 2015;27:672–9. [DOI] [PubMed] [Google Scholar]
- [36].Cronin-Fenton DP, Heide-Jorgensen U, Ahern TP, et al. Opioids and breast cancer recurrence: a Danish population-based cohort study. Cancer 2015;121:3507–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Minami S, Fujimoto K, Ogata Y, et al. Opioids have no negative effect on the survival time of patients with advanced lung cancer in an acute care hospital. Support Care Cancer 2015;23:2245–54. [DOI] [PubMed] [Google Scholar]
- [38].Call TR, Pace NL, Thorup DB, et al. Factors associated with improved survival after resection of pancreatic adenocarcinoma: a multivariable model. Anesthesiology 2015;122:317–24. [DOI] [PubMed] [Google Scholar]
- [39].Zylla D, Kuskowski MA, Gupta K, et al. Association of opioid requirement and cancer pain with survival in advanced non-small cell lung cancer. Br J Anaesth 2014;113: Suppl 1: i109–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Cata JP, Keerty V, Keerty D, et al. A retrospective analysis of the effect of intraoperative opioid dose on cancer recurrence after non-small cell lung cancer resection. Cancer Med 2014;3:900–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Halabi S, Lin CY, Kelly WK, et al. Updated prognostic model for predicting overall survival in first-line chemotherapy for patients with metastatic castration-resistant prostate cancer. J Clin Oncol 2014;32:671–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kripp M, Willer A, Schmidt C, et al. Patients with malignant hematological disorders treated on a palliative care unit: prognostic impact of clinical factors. Ann Hematol 2014;93:317–25. [DOI] [PubMed] [Google Scholar]
- [43].Zylla D, Gourley BL, Vang D, et al. Opioid requirement, opioid receptor expression, and clinical outcomes in patients with advanced prostate cancer. Cancer 2013;119:4103–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Alsirafy SA, Galal KM, Abou-Elela EN, et al. The use of opioids at the end-of-life and the survival of Egyptian palliative care patients with advanced cancer. Ann Palliat Med 2013;2:173–7. [DOI] [PubMed] [Google Scholar]
- [45].Azoulay D, Jacobs JM, Cialic R, et al. Opioids, survival, and advanced cancer in the hospice setting. J Am Med Dir Assoc 2011;12:129–34. [DOI] [PubMed] [Google Scholar]
- [46].Skipworth RJ, Moses AG, Sangster K, et al. Interaction of gonadal status with systemic inflammation and opioid use in determining nutritional status and prognosis in advanced pancreatic cancer. Support Care Cancer 2011;19:391–401. [DOI] [PubMed] [Google Scholar]
- [47].van Hooft JE, Dijkgraaf MG, Timmer R, et al. Independent predictors of survival in patients with incurable malignant gastric outlet obstruction: a multicenter prospective observational study. Scand J Gastroenterol 2010;45:1217–22. [DOI] [PubMed] [Google Scholar]
- [48].Forget P, Vandenhende J, Berliere M, et al. Do intraoperative analgesics influence breast cancer recurrence after mastectomy? A retrospective analysis. Anesth Analg 2010;110:1630–5. [DOI] [PubMed] [Google Scholar]
- [49].Bengoechea I, Gutierrez SG, Vrotsou K, et al. Opioid use at the end of life and survival in a Hospital at Home unit. J Palliat Med 2010;13:1079–83. [DOI] [PubMed] [Google Scholar]


