Abstract
Stroke is a serious disease that can lead to disability and death in adults, and the prediction of functional outcome is important in the management of acute ischemic stroke (AIS). Blood biomarker is a promising technique, for the measurement is fast, cheap and convenient. Visinin-like protein-1 (VILIP-1) is a classic stroke biomarker, thus we tried to investigate the predictive value of VILIP-1 for early functional outcomes of AIS.
A total of 70 AIS patients were enrolled in our study. Venous blood samples of all patients were taken at day 3 after admission to the stroke unit, and levels of serum VILIP-1 were analyzed by the use of the enzyme-linked immunosorbent assay. All subjects underwent diffusion weighted imaging (DWI) of the brain MRI scanning at 72 hours after stroke onset, and infarct volumes were calculated. Initial neurological status was evaluated by the National Institutes of Health Stroke Scale (NIHSS) on admission. The short-term functional outcome was graded by the modified Rankin Scale (mRS) at discharge from the hospital. Baseline data between the favorable outcome group and poor outcome group were compared, and univariate and multivariable logistic regression analysis were used to identify risk factors of early functional outcome of AIS.
The multivariate logistic regression analysis showed age, initial NIHSS scores and levels of VILIP had a strong association with poor clinical outcomes.
Levels of serum VILIP-1 are associated with short-term functional outcomes in patients with AIS.
Keywords: ischemic stroke, outcome, visinin-like protein-1
1. Introduction
Stroke is one of the leading causes of adult disability and mortality in the world.[1] AIS is the most common type of stroke. It imposes a significant burden in terms of costs, restricted social functioning, tremendous mental stress on patients and their families. In addition, the risk of stroke recurrence is rather high worldwide.[2] In the past decades, scientists have made major advances in the pathophysiology, clinical diagnosis and management of ischemic stroke. For instance, blood biomarkers of stroke for diagnosis, predicting prognosis and therapeutic monitoring gained particular attention for its feasibility and cost-effectiveness compared with conventional physician's clinical experiences and brain imaging technology.[3] Blood markers of neuronal injury, astroglial injury, inflammatory mediators, and thrombotic/hemostatic proteins have been widely studied as the candidate markers for stroke.[4]
VILIP-1 is a classic stroke biomarker.[5] As a member of neuronal calcium sensors (NCSs), VILIP-1 plays multiple roles in normal neurons, including the regulation of neuronal ion channels, membrane traffic, learning, neuronal growth, and survival, yet in the pathogenesis of stroke, VILIP-1 is a neurotoxic factor for neurons under the condition of the disruption of calcium homeostasis caused by stroke.[6]
Early in 2006, Laterza et al found VILIP-1 in cerebrospinal fluid (CSF) of a rat model of stroke and in plasma of patients after stroke.[7] Then, the potential of VILIP-1 for the diagnosis of ischemic stroke was confirmed in a pilot study.[8] But the researches concerning the relationship of VILIP-1 and outcomes of stroke remained rare, except the latest study on the correlation of cognitive dysfunction after stroke with the levels of VILIP-1 by student t test.[9] Another research showed levels of VILIP-1 was not relevant to the long-term outcomes of stroke in multivariate logic regression analysis, probably due to the inappropriate sampling time for the slow release kinetics of VILIP-1 from damaged tissue to peripheral blood (that is, the release was prominent for several days after stroke onset).[4]
Thus, in the present study, we try to investigate the association between levels of serum VILIP-1 and functional outcomes after acute ischemic stroke (AIS).
2. Materials and methods
2.1. Subjects
Among the 135 AIS patients who were admitted to our hospital between January 2015 and June 2016, we included patients who met the following inclusion criteria:
-
(1)
age from 18 to 80 years;
-
(2)
stroke confirmed by brain CT or MRI;
-
(3)
the first onset of stroke;
-
(4)
NIHSS scores ranging between 4 and 22.
Exclusion criteria:
-
(1)
intravenous, intra-arterial thrombolytic therapy or any other interventional treatment;
-
(2)
patients with intracranial hemorrhage, cerebral injury or brain tumor;
-
(3)
patients with the history of cancer;
-
(4)
patients with neurodegenerative diseases;
-
(5)
patients with psychotic disorder;
-
(6)
patients quitting the trial.
At last, a total of 70 cases were included in our study. Patients were further classified into 4 subtypes on the basis of the diagnosis criteria of the Trial of Org 10172 in Acute Stroke Treatment (TOAST). All patients provided informed consent and Tangxian People's Hospital approved the study protocol.
2.2. Neurobiochemical examinations and neuroradiological examinations
Peripheral venous blood samples were collected from patients at day 3 after admission to the stroke unit. Separated serum was stored at –80°C for later analysis. The levels of serum VILIP-1 were determined by the ELISA kit of VSNL1 (Jianglai Biotech, Shanghai, China).
All subjects underwent DWI of the brain MRI scanning at 72 hours after stroke onset. Experienced radiologists blinded to clinical information concerning the patients manually outlined regions abnormal on DWI. Areas of increased signal intensity on DWI were considered as the infarct region. The total infarct volumes were automatically produced by MRIcro version 1.40 on the basis of the section thickness and overall outlined lesion areas.
2.3. Neurological and functional assessments
The initial neurological impairment was scaled by NIHSS score on admission.
The short-term functional outcome was graded by mRS score at discharge. The favorable outcome group was defined as patients with mRS scores of 0 to 2 (n = 43), and the poor functional outcome group was defined as patients with mRS scores ≥3 (n = 27).
2.4. Statistical analysis
All data were analyzed using SPSS version 22.0. The variables were presented as means ± SD or number (%). The student t test was used for continuous variables according to the distribution of the data and the Chi-squared test was used for categorical variables. The correlation between levels of serum VILIP-1 and infarct volume was evaluated with Pearson correlation coefficient test. We identified risk factors for functional outcomes of stroke by multivariate logistic regression analysis with adjustments for age, initial NIHSS score and other variables in the univariate analyses. A statistical significance was defined when P < .05.
3. Results
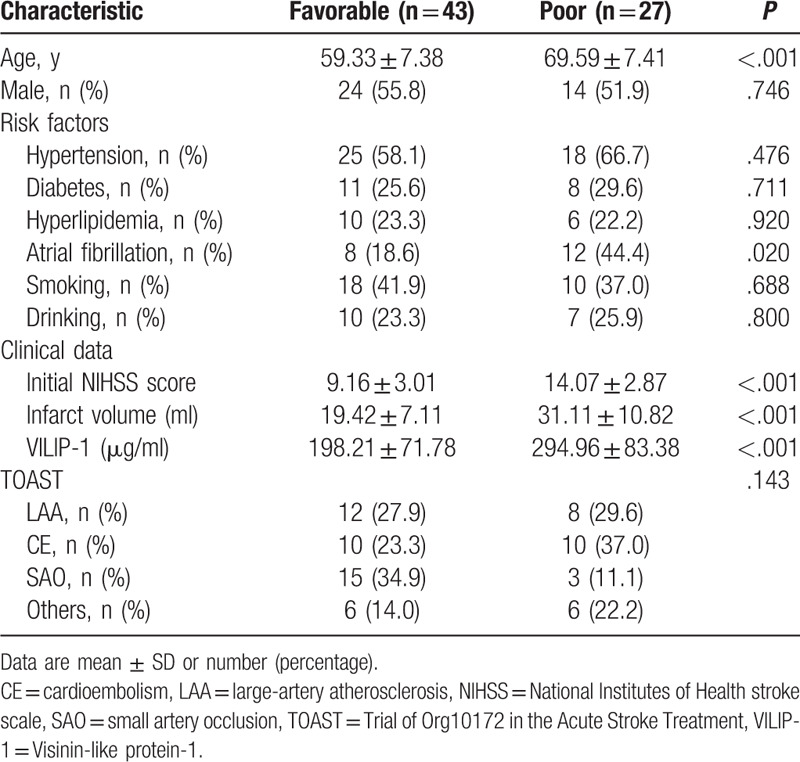
Background characteristics included age, gender, comorbidities (hypertension, diabetes, hyperlipidemia and atrial fibrillation), history of smoking, alcohol intake, initial NIHSS score, infarct volume, levels of serum VILIP-1 and TOAST classification. Table 1 shows the clinical characteristics of patients between the favorable and poor outcome groups. The age, initial NIHSS scores, infarct volume and levels of VILIP-1 were significantly higher in patients with poor outcome than patients with good outcomes (P < .001). The frequencies of atrial fibrillation significantly increased with a poorer outcome (P < .05). Concerning stroke subtypes, there was no significant difference between poor outcome group and favorable outcome group.
Table 1.
Demographic and clinical characteristics in favorable and poor outcome groups.
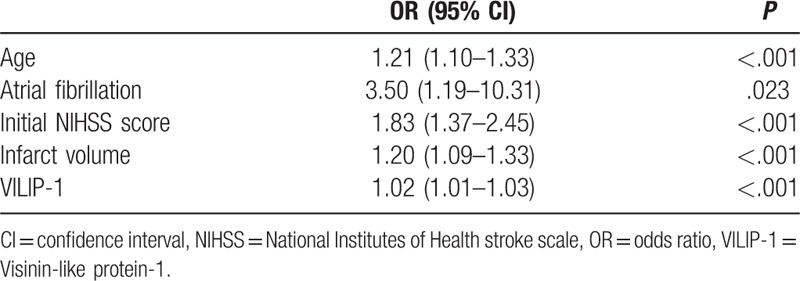
The univariate logistic regression model indicates that age, atrial fibrillation, initial NIHSS score, infarct volume and VILIP-1 were all significantly related to poor prognosis (P < .05 or P < .001; Table 2). Although infarct volume was associated with stroke outcome, we excluded the variable in the multivariate logistic regression model for multi-collinearity. We found infarct volume and serum concentrations of VILIP-1 were highly and significantly correlated in Pearson correlation analysis (r = 0.93, P < .001). Finally, age, atrial fibrillation, initial NIHSS score and VILIP-1 entered into a multivariate logistic regression model.
Table 2.
Univariate logistic regression analysis between favorable and poor outcome groups.
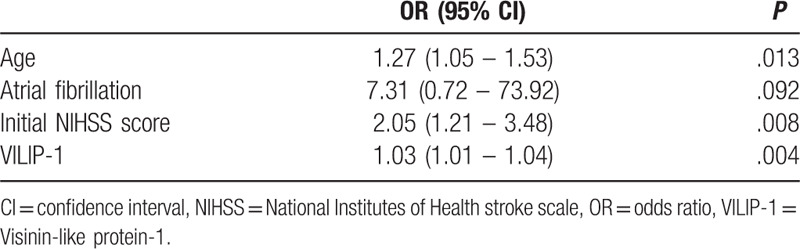
In the multivariate analysis of clinical variables, the levels of VILIP-1 still showed a strong association with poor stroke outcome (OR: 1.03, 95% CI: 1.01–1.04, P = .004; Table 3), while atrial fibrillation had no relevance to stroke outcome (P > .05). And the multivariate logistic regression analysis identified age and initial NIHSS score as independent predictors of short-term outcome of AIS. No multi-collinearity was found among the variables.
Table 3.
Multivariate logistic regression analysis between favorable and poor outcome groups.
4. Discussion
The prediction of functional outcome is important in the management of AIS, because early initiation of the corresponding treatment and intervention to reduce secondary neuronal damage is required for the prevention against morbidity and mortality caused by stroke.[3] Blood biomarker is a promising technique, for the measurement is fast, cheap and convenient.[6] VILIP-1 is one of the biomarkers for stroke. But up to now, few researchers have investigated the relationship between VILIP and functional outcome of AIS. Our result revealed levels of serum VILIP was associated with poor stroke outcome for the first time. The multivariate logistic regression analysis showed age, initial NIHSS scores and levels of VILIP were risk factors of clinical outcome in stroke patients.
VILIP-1 belongs to the family of NCSs. The family consists of 14 members, acting as Ca2+-dependent molecular switches.[10] All of the members have a highly evolutionarily conserved structure: 4 specific EF-hand motifs for Ca2+ and proteins binding.[11,12] NCS proteins are primarily located in central nervous system (CNS), such as retinal photoreceptors, neurons or neuroendocrine cells.[13] So is VILIP-1. Although there are small amounts of VILIP-1 in peripheral organs,[14] like stomach, skin, heart, and lung, which may be involved in cell migration, VILIP-1 is widely expressed in cortical pyramidal cells, interneurons, septal neurons, subthalamic neurons and hippocampal neurons, essential for the maintenance of neurological function.[15] Basically, VILIP-1 works as a calcium sensor, in the regulation of neuronal activity, growth, differentiation and synaptic plasticity as well as learning and memory.[10,16] An increase in the intracellular Ca2+ concentration results in the displacement of VILIP-1 to subcellular membrane compartments,[17,18] including axons, dendrites and trans-Golgi membranes in the hippocampus, then activates diverse signaling cascades, such as cAMP and cGMP signaling pathways, through bindings to different proteins in the living neurons.[19,20] In addition, VILIP-1 could also interact with proteins in the apo form.[21–24] For example, it forms a functional protein complex with Glu6R receptors of the kainite subtype in the absence of calcium ions, which plays an important role in the regulation of synaptic transmission and neuronal excitability.[21]
Calcium homeostasis plays a critical role in neuronal physiology.[25] The disruption of calcium homeostasis was one of the notable features in the pathogenesis of ischemic stroke.[6] Calcium overload, a persistent increased cytosolic Ca2+ concentrations in neurons, may result in the involvement of NCS proteins mediating a variety of necrotic and apoptotic pathways in the CNS.[26] VILIP-1 participates in various pathological processes leading to neuronal loss under the condition of destabilization of calcium homeostasis in the development of the disease.[26,27] Thus, it is generally recognized that VILIP-1 is a biomarker of neuronal injury. Since 2006 Laterza et al discovered the increased expression of VILIP-1 in plasma of stroke patients, a great number of studies have confirmed the diagnosis value of VILIP-1 as a stroke biomarker.[28] In the present study, we found VILIP-1 as an independent predictor of short-term outcome of AIS. Furthermore, our data show a high and significant association between VILIP-1 concentrations and infarct volume (r = 0.93, P < .001). These results are very similar to the classic stroke biomarker S100B. S100B was also highly correlated with infarct volumes (r2 = 0.81, P < .001), indicating both of the biomarkers reflect the extent of substantial brain damage.[29] Interestingly, the release patterns of VILIP-1 and S100B are alike, for the peak levels of both proteins are delayed for several days after stroke onset.[29] That may be attributed to later extensively responses in the pathophysiological cascade of ischemic stroke for both biomarkers. Additionally, several case-control studies showed that blood levels of S100B were associated with poor stroke outcome.[30,31] In contrast, Park et al found neither of VILIP-1 and S100B had a relevance to the long-term outcomes of stroke.[4] In the study, the lack of an association between blood protein levels and stroke outcome could be explained by the sampling of blood markers within 24 hours after stroke onset, when the release of proteins from damaged brain to peripheral blood has not reached the peak yet.
However, the limitation in our study still exists. First, this study was a short-term research trial with small sample size. Second, we did not perform serial measurements to research the temporal pattern of the release of VILIP-1. Lastly, we did not study multiple blood markers together. In the future, a large-scale prospective cohort with a refined scheme is required to determine whether VILIP-1 could be applied to routine clinical practice for prediction of risk of the clinical outcome in AIS patients.
In conclusion, we found that levels of blood VILIP-1 are associated with poor clinical outcome in patients with AIS.
Author contributions
Data curation: Dengjun Liu, Xiaoli Dong.
Investigation: Rui Yang, Hao Guo, Tao Wang, Guodong Xu.
Supervision: Dengjun Liu, Xiaoli Dong.
Writing – original draft: Dengjun Liu, Xiaoli Dong.
Writing – review & editing: Dengjun Liu, Xiaoli Dong.
Footnotes
Abbreviations: AIS = acute ischemic stroke, CNS = central nervous system, CSF = cerebrospinal fluid, DWI = diffusion weighted imaging, mRS = modified Rankin Scale, NCSs = neuronal calcium sensors, NIHSS = National Institutes of Health Stroke Scale, TOAST = Trial of Org 10172 in Acute Stroke Treatment, VILIP-1 = visinin-like protein-1.
How to cite this article: Liu D, Dong X, Yang R, Guo H, Wang T, Xu G. Visinin-like protein-1 level is associated with short-term functional outcome of acute ischemic stroke: A prospective cohort study. Medicine. 2020;99:9(e19252).
XD and RY contributed equally to this work.
HG, TW, and GX contributed equally to this work.
The authors have no funding and conflicts of interests to disclose.
All data including in this study can be obtained by proper requests from three authors.
References
- [1].Thrift AG, Thayabaranathan T, Howard G, et al. Global stroke statistics. Int J Stroke 2017;12:13–32. [DOI] [PubMed] [Google Scholar]
- [2].Pan F, Hernandez L, Ward A. Cost-effectiveness of stroke treatments and secondary preventions. Expert Opin Pharmacother 2012;13:1751–60. [DOI] [PubMed] [Google Scholar]
- [3].Boyd LA, Hayward KS, Ward NS, et al. Biomarkers of stroke recovery: consensus-based core recommendations from the Stroke Recovery and Rehabilitation Roundtable. Int J Stroke 2017;12:480–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Park S-Y, Kim J, Kim O-J, et al. Predictive value of circulating interleukin-6 and heart-type fatty acid binding protein for three months clinical outcome in acute cerebral infarction: multiple blood markers profiling study. Crit Care 2013;17:R45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Pettigrew LC, Bradley-Whitman MA, Lovell MA. Visinin-like protein-1 as a stroke biomarker. Ann Neurol 2015;78:S32–132. [Google Scholar]
- [6].Groblewska M, Muszynski P, Wojtulewska-Supron A, et al. The role of visinin-like protein-1 in the pathophysiology of Alzheimer's disease. J Alzheimers Dis 2015;47:17–32. [DOI] [PubMed] [Google Scholar]
- [7].Laterza OF, Modur VR, Crimmins DL, et al. Identification of novel brain biomarkers. Clin Chem 2006;52:1713–21. [DOI] [PubMed] [Google Scholar]
- [8].Stejskal D, Sporova L, Svestak M, et al. Determination of serum visinin like protein-1 and its potential for the diagnosis of brain injury due to the stroke - a pilot study. Biomed Pap-Olomouc 2011;155:263–8. [DOI] [PubMed] [Google Scholar]
- [9].LI Y, WU X-Q, Fan Q, et al. A study on the correlation of cognitive dysfunction after stroke with the levels of vilip-1 and hs-crp in serum. Acta Medica Mediterranea 2018;34:1895–9. [Google Scholar]
- [10].Burgoyne RD. Neuronal calcium sensor proteins: generating diversity in neuronal Ca2+ signalling. Nat Rev Neurosci 2007;8:182–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Burgoyne RD, O’Callaghan DW, Hasdemir B, et al. Neuronal Ca2(+)-sensor proteins: multitalented regulators of neuronal function. Trends Neurosci 2004;27:203–9. [DOI] [PubMed] [Google Scholar]
- [12].Braunewell K-H, Szanto AJK. Visinin-like proteins (VSNLs): interaction partners and emerging functions in signal transduction of a subfamily of neuronal Ca2+-sensor proteins. Cell Tissue Res 2009;335:301–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ames JB, Ishima R, Tanaka T, et al. Molecular mechanics of calcium-myristoyl switches. Nature 1997;389:198–202. [DOI] [PubMed] [Google Scholar]
- [14].Gierke P, Zhao CJ, Brackmann M, et al. Expression analysis of members of the neuronal calcium sensor protein family: combining bioinformatics and Western blot analysis. Biochem Biophys Res Commun 2004;323:38–43. [DOI] [PubMed] [Google Scholar]
- [15].Bernstein HG, Baumann B, Danos P, et al. Regional and cellular distribution of neural visinin-like protein immunoreactivities (VILIP-1 and VILIP-3) in human brain. J Neurocytol 1999;28:655–62. [DOI] [PubMed] [Google Scholar]
- [16].Monfort P, Munoz MD, Kosenko E, et al. Long-term potentiation in hippocampus involves sequential activation of soluble guanylate cyclase, cGMP-dependent protein kinase, and cGMP-degrading phosphodiesterase. J Neurosci 2002;22:10116–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Spilker C, Gundelfinger ED, Braunewell KH. Evidence for different functional properties of the neuronal calcium sensor proteins VILIP-1 and VILIP-3: from subcellular localization to cellular function. BBA-Proteins Proteomics 2002;1600:118–27. [DOI] [PubMed] [Google Scholar]
- [18].Braunewell KH. The visinin-like proteins VILIP-1 and VILIP-3 in Alzheimer's disease-old wine in new bottles. Front Molec Neurosci 2012;5:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Braunewell KH, Brackmann M, Schaupp M, et al. Intracellular neuronal calcium sensor (NCS) protein VILIP-1 modulates cGMP signalling pathways in transfected neural cells and cerebellar granule neurones. J Neurochem 2001;78:1277–86. [DOI] [PubMed] [Google Scholar]
- [20].Brackmann M, Schuchmann S, Anand R, et al. Neuronal Ca2+ sensor protein VILIP-1 affects cGMP signalling of guanylyl cyclase B by regulating clathrin-dependent receptor recycling in hippocampal neurons. J Cell Sci 2005;118:2495–505. [DOI] [PubMed] [Google Scholar]
- [21].Coussen F, Perrais D, Jaskolski F, et al. Coassembly of two GluR6 Kainate receptor splice variants within a functional protein complex. Neuron 2005;47:555–66. [DOI] [PubMed] [Google Scholar]
- [22].Zhao CJ, Noack C, Brackmann M, et al. Neuronal Ca2+ sensor VILIP-1 leads to the upregulation of functional alpha 4 beta 2 nicotinic acetylcholine receptors in hippocampal neurons. Mol Cell Neurosci 2009;40:280–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lin L, Jeanclos EM, Treuil M, et al. The calcium sensor protein visinin-like protein-1 modulates the surface expression and agonist sensitivity of the alpha 4 beta 2 nicotinic acetylcholine receptor. J Biol Chem 2002;277:41872–8. [DOI] [PubMed] [Google Scholar]
- [24].Chaumont S, Compan V, Toulme E, et al. Regulation of P2X2 receptors by the neuronal calcium sensor VILIP1. Sci Signal 2008;1:ra8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bezprozvanny I. Calcium signaling and neurodegenerative diseases. Trends Mol Med 2009;15:89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Blandini F, Braunewell KH, Manahan-Vaughan D, et al. Neurodegeneration and energy metabolism: from chemistry to clinics. Cell Death Differ 2004;11:479–84. [DOI] [PubMed] [Google Scholar]
- [27].Braunewell KH, Riederer P, Spilker C, et al. Abnormal localization of two neuronal calcium sensor proteins, visinin-like proteins (VILIPs)-1 and -3, in neocortical brain areas of Alzheimer disease patients. Dement Geriatr Cogn Disord 2001;12:110–6. [DOI] [PubMed] [Google Scholar]
- [28].Tarawneh R, Ford AL, Ohlendorf M, et al. Visinin-like protein-1 (VILIP-1): a novel plasma biomarker for the detection of acute cortical ischemic stroke. Stroke 2011;42:E203–1203. [Google Scholar]
- [29].Wunderlich MT, Ebert AD, Kratz T, et al. Early neurobehavioral outcome after stroke is related to release of neurobiochemical markers of brain damage. Stroke 1999;30:1190–5. [DOI] [PubMed] [Google Scholar]
- [30].Missler U, Wiesmann M, Friedrich C, et al. S-100 protein and neuron-specific enolase concentrations in blood as indicators of infarction volume and prognosis in acute ischemic stroke. Stroke 1997;28:1956–60. [DOI] [PubMed] [Google Scholar]
- [31].Abraha HD, Butterworth RJ, Bath PMW, et al. Serum S-100 protein, relationship to clinical outcome in acute stroke. Ann Clin Biochem 1997;34:366–70. [DOI] [PubMed] [Google Scholar]


