Abstract
The global incidence of pyogenic liver abscess (PLA) is increasing, but related reports of malignant tumor-related PLA are infrequent. Potential malignant tumors of PLA have been reported, but there is no relevant predictive model for this subsection of patients.
To explore the risk factors of malignant tumor-related PLA.
A retrospective analysis about a total of 881 patients who had been diagnosed with PLA from January 2005 to May 2018 was performed. The incidence of malignant tumor-related PLA in the study was 9.99% (88/881) out of all PLA cases. And that of potential malignant tumors with PLA was 4.65% (41/881). There were 62 patients with malignant tumor-related PLA in the observation group, while 146 cases without malignant tumor-related PLA were considered as control group. The data from 52 cases of malignant tumor and nonmalignant tumor-related PLA was verified.
The malignant tumor type was mainly hepatobiliary malignant tumor, which occupies 72.3% (45/62) in all malignant tumor related PLA cases used to the model. Compared with nonmalignant tumor group, the rate of ineffective and mortality was higher in the malignant tumor group [19.4%(12/62) vs 7.5%(11/148), P = .01]. Multivariate analysis suggested that hepatobiliary interventional therapy or surgery, hepatitis B virus infection, multiple abscesses, portal embolism, and bile duct dilatation were independent risk factors for potential malignant tumors within the patients who combined with PLA.
PLA could be considered as an early warning sign of potential malignant tumors. Malignant tumor-related PLA had a poor prognosis. Patients with PLA who have more than one independent risk factor or logit(P) > −1.694 may be considered as the high risk group for potential hepatobiliary or colorectal malignant tumors.
Keywords: colorectal cancer, malignant tumor, primary liver cancer, pyogenic liver abscess
1. Introduction
PLA refers to a bacterial infection of the liver parenchyma that is followed by infiltration of inflammatory cells and formation of abscesses, which is often associated with hepatobiliary diseases, abdominal infections, and even malignant tumors.[1,2] Additionally, the global incidence of PLA continues to rise.[2]
Recently, reports of PLA associated with malignant tumor are increasing. Since PLA can be resulted from interventional treatments of liver cancer such as transarterial chemoembolization (TACE),[3–5] and thermal ablation (radiofrequency ablation, microwave ablation).[6,7] Additionally, studies have shown that PLA can be served as a warning sign of liver cancer,[8–10] cholangiocarcinoma,[10–12] gastrointestinal carcinoma,[11,13–17] lung cancer,[11] or early adenosquamous carcinoma.[18] Early diagnosis and treatment are the key for PLA with potential malignant tumors. A difficult challenge for clinicians is to identify high-risk populations with potential malignant tumors in patients with PLA.
Most of the PLA associated with malignant tumors are case reports so far, while few original study was published. Yet, there is few predictive model for assessing PLA occurrence risk of the patients who recieved transcatheter oily chemoembolization (TACE) therapy.[3] In our previous research, we found that both PLA combined with potential malignant tumors and malignant tumors that had common clinical features and imaging findings. However, the data collected in most studies were not comprehensive enough, and the commonalities between PLA and carcinoma have not been well defined. Therefore, we analyzed the clinical features and imaging features of these patients and developed a predictive model for potential malignant tumors.
2. Materials and methods
2.1. Research population
A total of 881 patients with PLA included 88 (9.99%) cases of malignant tumor-related PLA were hospitalized in the First Affiliated Hospital of Guangxi Medical University from January 1, 2005 to May 31, 2018. And that of the patients with potential malignant tumors with an initial presentation of PLA was 4.65% (41/881) (Table 1). For retrospective research and modeling, first, the medical records of 62 patients (observation group) with malignant tumor-related PLA from 2012 to 2018 were collected, and then the medical records of 146 patients (control group) with non-malignant tumor-related PLA were collected according to the ratio of 1:2 and the time matching principle (year). Clinical data of patients with malignant tumor-related PLA (n = 26) and non-malignant tumor-related PLA (n = 26) was collected as validated data to test the efficacy of the predictive model. Inclusion criteria:
Table 1.
Incidence of cases of malignant tumor-associated liver abscess and potential malignancy with the initial manifestation of liver abscess.

-
1.
Meet the diagnostic criteria for PLA.
-
2.
Meet the diagnostic criteria for hepatocellular carcinoma.
-
3.
Other types of malignant tumors were diagnosed by biopsy.
Exclusion criteria:
-
1.
Amoebic liver abscess, liver tuberculosis.
-
2.
Cases with incomplete data.
-
3.
Malignant tumor diagnosed after PLA had been cured.
A diagnosis of hepatocellular carcinoma depends on a needle biopsy or surgical specimens or was based on the discovery of typical radiological features in at least 2 image examinations, including ultrasound, contrast-enhanced dynamic computer tomography, magnetic resonance imaging and elevated levels of serum alpha-fetoprotein. The Sequential Organ Failure Assessment (SOFA) was performed to assess the severity of a patient's condition use the SOFA scale of International Sepsis, version 3.0. The origins of the PLA were defined as follows: cryptogenic: the doctor cannot find the origin of the infection by assessing the patient's clinical data; biliary: clinical manifestations of biliary calculi and/or cholecystitis or cholangitis, or liver fluke infection, excluding the effects of recent abdominal surgery or interventional therapy; adjacent: abscess in the adjacent abdomen; portal: abdominal infection recorded after appendicitis or diverticulitis; arterial: with a history of recorded bacteremia, no other history of intra-abdominal infections; and traumatic: abscess secondary to a previous abdominal surgery or interventional intervention. Additional terms are defined as follows: intervention or surgery affecting the hepatobiliary system: the intervention or surgery that was performed before the occurrence of PLA, including radiofrequency ablation, TACE, endoscopic retrograde cholangiopancreatography, various types of hepatobiliary surgery, or a gastrointestinal physiologic reconstruction surgery affecting the biliary tract. Ineffectiveness: after active treatment, the patient's symptoms and signs did not improve, and the size of the abscess did not change. Death refers to the ineffective treatment of patients who died from infection or related complications during hospitalization.
2.2. Investigation of risk factors for PLA
The retrospective analyses of the medical records included age, gender, symptoms, signs, diabetes, liver flukes, metastatic abscess, hepatitis B viral infection (HBV Infection), hepatitis C viral infection (HCV Infection), intervention or surgery affecting the hepatobiliary system, other types of complications and complications, abscess origin, and microbiological data. The computed tomography, ultrasound or magnetic resonance imaging data provided information on the maximum abscess size, multiple abscesses (the number of abscess ≥ 2), abscess location, portal embolism, gas formation, and biliary abnormalities, which include biliary dilatation (internal and extrahepatic), gallbladder neoplasms, and thickening of the biliary wall.
2.3. Statistical analysis
Data analysis was performed using SPSS 21.0 (IBM, Chicago, IL). Continuous variables were analyzed by a t test or a Mann-Whitney U test for univariate analysis based on whether the data followed a normal distribution. The categorical variables were analyzed by Chi-square test or Fisher's exact probability method for univariate analysis. The multivariate analysis was performed using a binary logistic regression. Due to the substantial time requirement and difficulty of microbial culture, the multivariate analysis of these data will not be performed. A predictive model of PLA with potential malignant tumors was established based on statistically significant factors in multivariate analysis. Model scores were scored by the SPSS software's scoring guide. The model was validated by external validation data and receiver operating characteristic curves (ROC curves). P < .05 was considered statistically significant.
2.4. Ethical statement
This study was only an observational study. The patient's tissue was not used and the patient's privacy information was not exposed. Therefore, the ethical approval was waived or not necessary.
3. Results
3.1. Clinical features
Chills (35.5% vs 59.6%, P = .001), diabetes (24.2% vs 40.4%, P = .03), and chronic cardiovascular and cerebrovascular disease (3.2% vs 14.4%, P = .02) were significantly lower in the malignant tumor group. While Chronic liver disease (41.9% vs 21.9%, P = .003), hepatitis B viral infection (32.3% vs 11.6%, P < .001), cirrhosis (24.2% vs 6.8%, P < .001), an intervention or surgery affecting hepatobiliary (51.6% vs 11.6%, P < .001), sepsis 3.0 (61.3% vs 41.8%, P = .01), and gastrointestinal bleeding (6.5% vs 0.7%, P = .03) were significantly higher in the malignant tumor group. Sinus or fistula (4.8%) occurred only in the malignant tumor group, so their prevalence differed significantly from that of the nonmalignant group (Table 2). The SOFA score (3.323±0.48 vs 2.048 ± 0.25, P = .003) was significantly higher in the malignant tumor group, indicating that the more severe disease in the malignant tumor group. The incidence of biliary PLA (including biliary and traumatic abscess) was significantly higher in the nonmalignant tumor group, while that of traumatic PLA (including cryptogenic and traumatic abscess) was significantly higher in the malignant tumor group. The first hemoglobin measurement (102.2±2.3 g/L vs 112.1 ± 1.8 g/L, P = .001) in the hospital was significantly lower in the malignant tumor group (Table 3).
Table 2.
Clinical features of malignant tumor-related and nonmalignant tumor-related PLA.

Table 3.
Laboratory investigation of malignant tumor-related and nonmalignant tumor-related PLA.

The species of microbes in the malignant tumor group was mainly Escherichia coli (26.9% vs 11.7%, P = .02), followed by Klebsiella pneumonia (17.3%) and Enterococcus faecium (13.5%) (Table 4). Primary liver cancer was the most common tumor type in both groups (63.6% vs 68.9%), followed by gastrointestinal malignant tumors (20.5% vs 21.2%). It seemed that the metastatic rate (31.0% vs 9.1%, P = .05) was higher in the potential malignant group, but the difference was not statistically significant (Table 7). This difference may be related to a misdiagnosis by the clinicians, leading to delays in treatment and to metastases.
Table 4.
Microbiological diagnostic procedures and microbiological diagnoses of patients with malignant tumor and nonmalignant tumor with PLA.

Table 7.
Tumor types of potential malignant tumor and nonpotential malignant tumor groups.
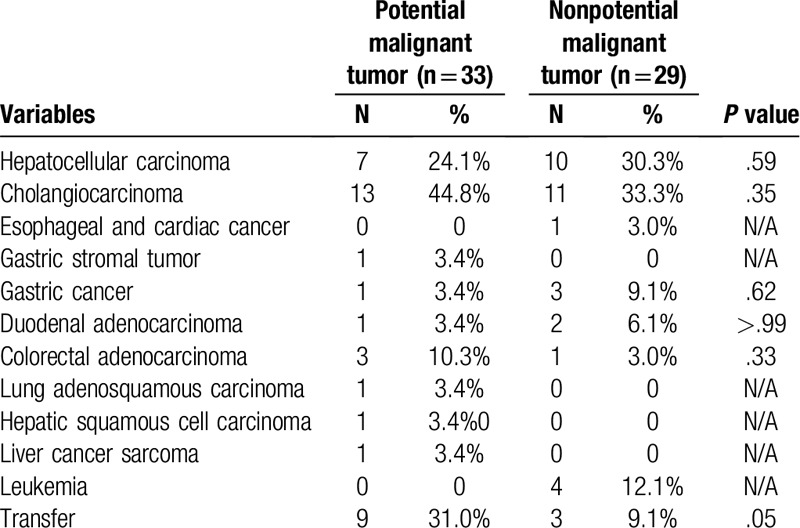
In the potential malignant tumor group, 1 case had been diagnosed with rectal cancer, 1 case with nasopharyngeal carcinoma (T3N2M0), and 1 case with pelvic embryonal tumor. All three patients were diagnosed with cholangiocarcinoma at this time. One of them had multiple intrahepatic and intraabdominal metastasis lesions, and one patient only had intrahepatic metastasis. This finding suggested that the patients with PLA who had a history of malignant tumors may have suffer a potential malignant tumor that was different from previous malignant tumor types.
The diameter of the abscess (6.3 ± 0.4 cm vs 7.2 ± 0.2 cm, P = .04), both lobes (24.2% vs 11.0%, P = .01), multiple abscesses (62.9% vs 36.3%, P < .001), portal embolism (11.3% vs 2.7%, P = .01), biliary tract abnormalities (69.4% vs 52.7%, P = .03), and bile duct dilatation (61.3% vs 34.9%, P < .001)in malignant tumor group showed statistically significant differences in the imaging analyses, comparing to nonmalignant tumor group. (Table 5).
Table 5.
Imaging findings of patients with hepatic abscess in the malignant and nonmalignant tumor groups.

3.2. Prognosis
In the maligant and nonmaligant tumor group, the most prevalent treatment was antibiotics combined with percutaneous puncture drainage (58.1% vs 48.6%), followed by treatment with antibiotics alone (24.2% vs 39.7%), respectively. In addition to these therapeutic methods, treatment of the malignant tumor group also included the combined use of antibiotics and surgical resection 16.1%. The total hospitalization days, and ineffectiveness and mortality (19.4% vs 7.5%, P = .01) are significantly higher in the malignant tumor group comparing to the nonmalignant tumor group (Table 6). This finding suggested that patients suffering malignant tumor-associated PLA showed worse prognosis and higher medical cost.
Table 6.
Treatment and outcome of patients in the malignant tumor and nonmalignant tumor groups.

Table 8.
Multivariate analysis of patients with malignant tumor-associated liver abscesses.

3.3. The independent risk factors of malignant tumors occurrence in the cases with PLA
A multivariate analysis was then performed. Chills (OR 0.375, CI 95% 0.162–0.868, P = .02), high hemoglobin (OR 0.965, CI 95% 0.945–0.984, P < .001), abscess diameter (OR 0.803, CI 95% 0.695–0.928, P = .003), and cholelithiasis or cholecystitis (OR 0.120, CI 95% 0.040–0.356, P < .001) suggested a lower risk of the occurrence of a potential malignant tumor. Intervention or surgery affecting hepatobiliary function (OR 10.342, CI 95% 3.684- 29.031, P < .001), hepatitis B viral infection (OR 4.237, CI 95% 1.558–11.519, P = .005), multiple abscesses (OR 3.655, CI 95% 1.584–8.435, P = .002), portal embolism (OR 16.099, CI 95% 2.938–88.207, P = .001), and bile duct dilatation (OR 2.966, CI 95% 1.200–7.332, P = .02) were independent risk factors for patients with PLA suffering potential malignant tumors.
The predictive model that was constructed by binary logistic regression using SPSS software predicts the validated data (malignant tumor group and nonmalignant tumor group in 26 cases) and constructs the receiver operating characteristic curve of the predictive model. The area under the curve (AUC) 0f was calculated to be 0.932 [(95% confidence interval, 0.865–0.999), P < .001, Fig. 1]. In addition, the corresponding optimal risk was 0.155 (sensitivity = 0.962, specificity = 0.808). By the equation logit (p) = 3.260 + (−.982) ×Chills + 2.336 × Intervention or surgery affecting hepatobiliary + (−.036) × First Hemoglobin + 1.444 ×HBV infection +(−.219) ×Side diameter (cm) + 1.296 × Multiple abscesses (≥ 2) + 2.779 × Portal embolism + (−2.123) × Gallstone or cholecystitis + 1.087 × Bile duct dilatation and using the value  the equation was derived to obtain logit (P) = -ln5.442 = −1.694. It was concluded that a logit (P) > −1.694, suggested that patients with PLA had a higher possibility of potential malignant tumors.
the equation was derived to obtain logit (P) = -ln5.442 = −1.694. It was concluded that a logit (P) > −1.694, suggested that patients with PLA had a higher possibility of potential malignant tumors.
Figure 1.

Receptor characteristic curve of predictive model of malignant tumor-related PLA. The predictive model constructed by binary logistic regression using SPSS software predicts the validated data (malignant and non-malignant in 26 cases, respectively) and constructs the receiver operating characteristic curve of the predictive model. The area under the curve (AUC) was calculated to be 0.932 ([95% confidence interval, 0.865–0.999], P = .000, Fig.1).
4. Discussion
An infection secondary to biliary tract obstruction is now the most easily identifiable cause of PLA. However, studies from Western countries have shown that due to developments in the treatment of hepatobiliary and pancreatic tumors, cases that were reported of PLA have changed from relatively benign obstructions to malignant obstructions.[1,19]
Malignant tumor-related PLA were uncommon. Recent studies have mainly focused on potential malignant tumors with PLA as an initial clinical manifestation. There have still been few studies on patients with PLA with a history of previous malignant tumors, and these studies were mainly from Asian countries.[3,11] Hepatobiliary malignant tumors,[8–11,20–22] colorectal tumors,[11,16,21] and other gastrointestinal tumors were the top three malignant tumor types that had PLA as an initial manifestation, as reported by previous studies.[21] Malignant tumor-related PLA have been reported to be mainly caused by TACE and RFA treatment of hepatocellular carcinoma.[3–7,23] Previous studies have shown that potential malignant tumor-related PLA had common clinical features with nonpotential malignant tumor-related PLA. Our study showed that the overall incidence of malignant tumor-related PLA was 9.88%, and that of potential malignancies rate of PLA was 4.65%, which was higher than the overall incidence that was reported in previous reports (2.15%–3.5%).[8,10] The type of potential malignant tumor was mainly hepatobiliary malignant tumor (72.3%), of which hepatobiliary cell carcinoma accounted for the highest proportion (44.8%), followed by gastrointestinal malignant tumor (20.5%). It is worth noting that in the group of potential malignant tumors, there were 3 patients with previous malignant tumors, including colorectal cancer surgery, nasopharyngeal carcinoma (T3N2M0), and pelvic embryonic tumor. The pathological type of malignant tumor diagnosed in these 3 patients were hepatobiliary cell carcinoma. One suffered multiple intrahepatic and intraabdominal metastases and another one suffered only intrahepatic metastasis. This finding suggested that even in patients with PLA with a history of malignancies, there may be a possibility of potential malignant tumors that were different from previous malignant tumors types, which warns of the existence of potential malignant tumors.
PLA are part of a chronic inflammatory process that destroys the liver parenchyma, and they may be involved in the development of liver malignant tumors. However, studies have shown that it took a long time for inflammation to develop into a liver malignant tumors.[24] Our study suggested that there may be potential malignant tumors in patients with PLA. PLA is an early warning sign for malignant tumors in people who are at a high risk of malignant tumors, but it is not a trigger for malignant tumors. This hypothesis is consistent with previous studies.[9–12,16,17,21,25]
The pathogenesis of PLA in patients with potential malignant tumors has not yet been fully illuminated. The mechanism of primary hepatocellular carcinoma with PLA is mainly caused by spontaneous malignant tumors necrosis, since a dropping tumor thrombosis and biliary obstruction results in a malignant tumors thrombus with a bacterial infection.[26] This is consistent with our findings. In malignant tumor group, the proportion of patients with chills was significantly lower than that of the nonmalignant tumor group (59.6% vs 35.5%, P = .001), and the proportion of bile duct dilatation was significantly higher than that of the nonmalignant tumor group (61.3% vs 34.9%, P < .001). Chills (OR 0.375) suggested that the patients with the PLA had a lower risk of suffering from potential malignant tumors, which may be related to malignant tumors. The fever in patients with PLA was mainly due to the absorption of heat by the malignant tumors, which was caused by spontaneous necrosis of the malignant tumors and was consistent with previous studies.[26]
To the best of our knowledge, some malignant tumors, especially large malignant tumors, develop spontaneous necrosis when the blood supply was insufficient. Malignant tumor necrosis and biliary obstruction also exist in the cases of liver cancer that are treated with TACE and thermal ablation. The pathogenesis of PLA in patients with liver cancer who are undergoing interventional therapy is similar to that of potential liver cancer. In the case of potential colorectal cancer (CRCs), bacteria enters into the portal system due to a deficiency in the malignant tumor mucosa or to potential vascular exposure; subsequently, blood diffuses into the liver, and this is considered to be the key process.[11,27]
Other types of potential gastrointestinal malignant tumors also have mucosal deficiencies. In our study, some gastrointestinal malignant tumors had sinus formation on the liver surface, and the bacteria that spread through the sinus or mucosal defects in the malignant tumor mucosa enter the portal system. Then, the blood moves the bacterial infection to the liver. To the best of our knowledge, asymptomatic colorectal cancer combined with PLA rarely occur tumor metastasis, but all cases of pancreatic maligant tumor and stomach stromal tumor combined with PLA that were enrolled suffer liver metastasize.
Studies had shown that hospitalized patients suffering from hepatocellular carcinoma with PLA had a hospital mortality rate of 40.9%.[28] In the study by Yeh et al, patients suffering from hepatocellular carcinoma with PLA had a poor prognosis and an average survival of 3.5 months..[26] Lin et al showed that patients who were diagnosed with HCC that initially manifested as PLA had a worse prognosis than patients with HCC without PLA.[8] They believed that if the doctors were not alerted to the PLC, the treatment of the PLA may lead to delays in diagnoses, and drainage may cause the malignant tumors to spread. In this study, compared with the nonmalignant tumor group, the SOFA score, the proportion of serious complications (gastrointestinal bleeding, sepsis 3.0, gastrointestinal bleeding), total time of hospital stay, and cost were significantly higher in the malignant tumor group. The rate of ineffectiveness and mortality (19.4% vs 7.5%, P = .02) was significantly higher in the malignant tumor group than in the nonmalignant tumor group, and sinus or leakage only occurred in the malignant tumor group. These results suggested that patients suffering malignant tumor-associated PLA showed worse prognosis and higher medical cost. The potential malignant tumor group had a higher metastatic rate than the nonpotential malignant tumor group (31.0% vs 9.1%, P = .05). In one case, the malignant tumor cells metastasized to the drain from the sinus, which indicates that the initial manifestation of PLA can lead to the diagnosis and delayed treatment of malignant tumors. If the clinicians ignore the possibility of malignant tumors, drainage will lead to the spread of malignant tumors.
Chronic cholangitis and hepatolithiasis were closely related to intrahepatic cholangiocarcinoma.[29] The proportion of patients with cholelithiasis or cholecystitis in the nonmalignant tumor group was higher than that in the malignant tumor group, which was inconsistent with previous studies.[9] The multivariate analysis suggested that patients with PLA had a lower risk of potential malignant tumors. Most patients in the malignant tumor group had a previous history of gallstone removal surgery, and the determination of cholelithiasis or cholecystitis in this study was done with imaging tools. In the case of a diagnosis of cholelithiasis or cholecystitis, the sensitivity of the imaging examination was limited, which meant that some patients with chronic recessive biliary tract infections and tiny biliary stones were not identified through the imaging methods, so the predictive capabilities of the imaging examination on cholelithiasis or cholecystitis and the determination of whether PLA function as a protective factor for potential malignant tumors required further research. The higher first hemoglobin (OR 0.965) and the larger abscess diameter (OR 0.803) suggested that PLA carried a lower risk of potential malignant tumors. The malignant tumor-related PLA group was characterized by a more serious condition and multiple abscesses that resulted in a higher consumption of hemoglobin. In addition, we found that an intervention or surgery affecting hepatobiliary function, hepatitis B viral infection, multiple abscesses (number of abscesses ≥ 2), portal embolism, and bile duct dilatation were independent risk factors for PLA in patients with potential malignant tumors. There were always multiple liver abscesses in the malignant tumor cases, which was consistent with previous studies.[3]
Hepatocellular carcinoma (HCC) and cholangiocarcinoma were the two most common PLCs, accounting for 90% and 8% of all primary liver cancers in this study, respectively.[30] The main risk factors for HCC were cirrhosis, chronic hepatitis B, chronic hepatitis C, alcohol consumption, obesity and diabetes.[31] The main risk factors for ICC were similar to those for HCC[32] and included intrahepatic bile duct stones.[29] Biliary dilatation was a manifestation of poor bile drainage in hepatobiliary malignant tumors and was a risk factor for malignant tumor-related PLA, and this finding was consistent with previous studies.[3] In this study, hepatitis C viral infection was not an independent risk factor, but this may be due to a lack of cases. Additional cases should be researched further.
Few studies have previously reported the characteristic imaging findings of PLA. This study collected comprehensive clinical data to study PLA with potential malignant tumors and PLA based on previously identified malignant tumors. A diagnostic prediction model was developed further. The area under the curve (AUC) for the diagnostic prediction model was 0.932. The diagnostic model had an excellent diagnostic performance and extensive application for patients with the risk factors. This model is suitable for determining the risk of a potential malignant tumors in patients with new-onset PLA.
The present study had several limitations. First, this was a retrospective analysis with a small sample size for the malignant tumor group. Second, patients with colorectal adenomas were not included in the study. Patients with high-risk cryptogenic PLA did not undergo colonoscopy, which may lead to a misdiagnosis of most patients with colorectal malignant tumors. Third, the number of cases of hepatitis C viral infection is too low, and the diagnostic efficacy of elevating the number of HCV patients in the model was poor. Fourth, this predictive model for malignant tumor-related PLA has not been validated in independent populations outside our hospital, and it needs to be further studied in independent populations in other regions.
In conclusion, first, A PLA is an early warning sign of potential malignant tumors. Second, Malignant tumor-related PLA carries a poor prognosis. Third, Patients in PLA with a previous malignant tumor exist a potential hepatobiliary malignant tumors. Patients with PLA who have more than one independent risk factor or logit (P) > -1.694 may be considered as a high risk group for potential hepatobiliary or colorectal malignant tumors. Liver biopsy is recommended in patients with PLA, and colonoscopy is recommended in patients with high-risk PLA, especially in areas of primary liver cancer and colorectal cancer.
Acknowledgments
We thank Professor Jiean Huang for his administrative support of Guangxi Medical University Second Affiliated Hospital. We thank Changliang Wu, MD for collecting data. We thank Dr. Mengbin Qin for analyzing the data. We thank Fuqing Cai, MD for the helpful advice and discussion.
Author contributions
Data curation: Changliang Wu.
Formal analysis: Fuqing Cai.
Methodology: Mengbin Qin.
Resources: Jiean Huang.
Writing – original draft: Weizheng Li.
Weizheng Li orcid: 0000-0003-4193-4080.
Footnotes
Abbreviations: AUC = area under curve, CI = confidence interval., CRCs = colorectal cancer, GGT = gamma glutamyl transferase, HBV Infection = hepatitis B viral infection, HCC = Hepatocellular carcinoma, HCV Infection = hepatitis C viral infection, ICC = intrahepatic cholangiocarcinoma, Intervention or surgery∗ = Intervention or surgery affecting hepatobiliary function, K. pneumoniae = Klebsiella pneumoniae, MODS = multiple organ dysfunction syndrome, PLA = pyogenic liver abscess, R/L/B+ Caudate lobe∗ = Right/Left/Both+ Caudate lobe, SOFA = sequential organ failure assessment, TACE = transarterial chemoembolization, TOCE = transcatheter oily chemoembolization.
How to cite this article: Li W, Wu C, Qin M, Cai F, Huang J. The aura of malignant tumor: Clinical analysis of malignant tumor-related pyogenic liver abscess. Medicine. 2020;99:9(e19282).
The authors have no funding and conflicts of interest to disclose.
References
- [1].Branum GD, Tyson GS, Branum MA, et al. Hepatic abscess. Changes in etiology, diagnosis, and management. Ann Surg 1990;212:655–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Tsai FC, Huang YT, Chang LY, et al. Pyogenic liver abscess as endemic disease, Taiwan. Emerg Infect Dis 2008;14:1592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Shin JU, Kim KM, Shin SW, et al. A prediction model for liver abscess developing after transarterial chemoembolization in patients with hepatocellular carcinoma. Dig Liver Dis 2014;46:813–7. [DOI] [PubMed] [Google Scholar]
- [4].Son DJ, Hong JY, Kim KH, et al. Liver abscess caused by Clostridium haemolyticum infection after transarterial chemoembolization for hepatocellular carcinoma: a case report. Medicine 2018;97:e0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hama Y, Kusano S. Liver abscess formation after hepatic chemoembolization for metastatic pancreatic neuroendocrine tumor. Minim Invasive Ther Allied Technol 2005;14:6–7. [DOI] [PubMed] [Google Scholar]
- [6].Su XF, Li N, Chen XF, et al. Incidence and risk factors for liver abscess after thermal ablation of liver neoplasm. Hepat Mon 2016;16:e34588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Yoshikawa T, Ohana M, Fukuda A. High fever after radiofrequency ablation of hepatocellular carcinoma. Gastroenterology 2018;155:e3–4. [DOI] [PubMed] [Google Scholar]
- [8].Lin YT, Liu CJ, Chen TJ, et al. Pyogenic liver abscess as the initial manifestation of underlying hepatocellular carcinoma. Am J Med 2011;124:1158–64. [DOI] [PubMed] [Google Scholar]
- [9].Huang WK, Lin YC, Chiou MJ, et al. Pyogenic liver abscess as a warning sign for primary liver cancer: a nationwide population-based study. Asian Pac J Cancer Prev 2013;14:4727–31. [DOI] [PubMed] [Google Scholar]
- [10].Chong VH, Lim KS. Pyogenic liver abscess as the first manifestation of hepatobiliary malignancy. Hepatobiliary Pancreat Dis Int 2009;8:547–50. [PubMed] [Google Scholar]
- [11].Kao WY, Hwang CY, Chang YT, et al. Cancer risk in patients with pyogenic liver abscess: a nationwide cohort study. Aliment Pharmacol Ther 2012;36:467–76. [DOI] [PubMed] [Google Scholar]
- [12].Chai CY, Ishizaki Y, Fukumura Y, et al. Pyogenic liver abscess complicating early bile duct carcinoma in the middle bile duct: a rare presentation. Intern Med (Tokyo, Japan) 2009;48:325–7. [DOI] [PubMed] [Google Scholar]
- [13].Kurtz LE, Greenberg RE. Pyogenic liver abscess associated with a gastrointestinal stromal tumor of the stomach. Am J Gastroenterol 2010;105:232–3. [DOI] [PubMed] [Google Scholar]
- [14].Weng SW, Liu JW, Chen WJ, et al. Recurrent Klebsiella pneumoniae liver abscess in a diabetic patient followed by Streptococcus bovis endocarditis--occult colon tumor plays an important role. Jpn J Infect Dis 2005;58:70–2. [PubMed] [Google Scholar]
- [15].Qian LM, Ge JG, Huang JM. Rupture of liver abscess following hepatogastric fistula caused by perforation of remnant gastric carcinoma: a case report. Eur Rev Med Pharmacol Sci 2016;20:4535–9. [PubMed] [Google Scholar]
- [16].Huang WK, Chang JW, See LC, et al. Higher rate of colorectal cancer among patients with pyogenic liver abscess with Klebsiella pneumoniae than those without: an 11-year follow-up study. Colorectal Dis 2012;14:e794–801. [DOI] [PubMed] [Google Scholar]
- [17].Jang DK, Jeong SH, Lee SH, et al. Computed tomographic colonography is valuable for post-treatment evaluation and screening of hidden colorectal cancer in patients with cryptogenic pyogenic liver abscess. Digestion 2014;89:175–83. [DOI] [PubMed] [Google Scholar]
- [18].Yeung JT, Fan WC, Cheng RL. Adenosquamous carcinoma presenting as liver abscess. Singapore Med J 2012;53:e110–3. [PubMed] [Google Scholar]
- [19].Huang CJ, Pitt HA, Lipsett PA, et al. Pyogenic hepatic abscess. Changing trends over 42 years. Ann Surg 1996;223:600–7. discussion 607-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lai KC, Cheng KS, Jeng LB, et al. Factors associated with treatment failure of percutaneous catheter drainage for pyogenic liver abscess in patients with hepatobiliary-pancreatic cancer. Am J Surg 2013;205:52–7. [DOI] [PubMed] [Google Scholar]
- [21].Lai HC, Lin CC, Cheng KS, et al. Increased incidence of gastrointestinal cancers among patients with pyogenic liver abscess: a population-based cohort study. Gastroenterology 2014;146:129–37.e121. [DOI] [PubMed] [Google Scholar]
- [22].Chu CS, Lin CC, Peng CY, et al. Does pyogenic liver abscess increase the risk of delayed-onset primary liver cancer? Evidence from a nationwide cohort study. Medicine 2017;96:e7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Song SY, Chung JW, Han JK, et al. Liver abscess after transcatheter oily chemoembolization for hepatic tumors: incidence, predisposing factors and clinical outcome. J Vasc Interv Radiol 2001;12:313–20. [DOI] [PubMed] [Google Scholar]
- [24].Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420:860–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Behrns KE. Hepatobiliary cancer and pyogenic liver abscess: when poking the skunk is not enough: invited commentary on Chen, et al. Am J Surg 2013; 205: 52-7. Am J Surg 2013;205:479–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Yeh TS, Jan YY, Jeng LB, et al. Hepatocellular carcinoma presenting as pyogenic liver abscess: characteristics, diagnosis, and management. Clin Infect Dis 1998;26:1224–6. [DOI] [PubMed] [Google Scholar]
- [27].Lim WC, Lim CC. Silent colorectal carcinoma and pyogenic liver abscess. J Gastroenterol Hepatol 2004;19:945–6. [DOI] [PubMed] [Google Scholar]
- [28].Chok KS, Cheung TT, Chan AC, et al. Liver resection for de novo hepatocellular carcinoma complicated by pyogenic liver abscess: a clinical challenge. World J Surg 2016;40:412–8. [DOI] [PubMed] [Google Scholar]
- [29].Kim HJ, Kim JS, Joo MK, et al. Hepatolithiasis and intrahepatic cholangiocarcinoma: a review. World J Gastroenterol 2015;21:13418–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cong WM, Dong H, Tan L, et al. Surgicopathological classification of hepatic space-occupying lesions: a single-center experience with literature review. World J Gastroenterol 2011;17:2372–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol 2013;47: Suppl: S2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Palmer WC, Patel T. Are common factors involved in the pathogenesis of primary liver cancers? A meta-analysis of risk factors for intrahepatic cholangiocarcinoma. J Hepatol 2012;57:69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]


