Abstract
Retrospective observational cohort study.
We used observational measures and retrospective chart reviews to compare elderly patients with osteoporosis who underwent multi-level anterior lumbar interbody fusion (ALIF) with either posterolateral fusion (PLF) or percutaneous pedicle screw fixation.
Multi-level ALIF with PLF is used to save the posterior element of the spine and improve fusion rates in elderly patients with osteoporosis. To minimize perioperative invasiveness and improve patients’ postoperative quality of life, we perform minimal percutaneous screw fixation.
Fifty-three elderly patients with osteoporosis who underwent either PLF with open pedicle screw fixation (n = 28) or percutaneous pedicle screw fixation (PPF) (n = 25) for treatment with 2-level ALIF between January 2010 and December 2013 were compared for clinical outcome including operation time, intraoperative and postoperative blood loss, and hospital day and radiological outcome.
Average operation times were significantly shorter and intra- and postoperative blood loss was significantly reduced in the PPF group. There were no significant differences, preoperative and postoperative, in observational measures including visual analog scale, Oswestry disability index, and Rolland-Moris disability. There were no significant differences in the degree of lordosis, changes of motion, or adjacent segmental degeneration. Fusion rates were increased in the PLF group compared to the PPF group 6 months post-surgery, but from 1 year to the last follow-up, the rates were statistically equivalent. There were fewer minor complications in the PPF group, and no major complications at all.
Two-level ALIF with PPF results in shorter operation times, less blood loss and minor complications, and similar fusion rate as 2-level ALIF with PLF. It; therefore, represents an effective method, leading to rapid recovery and less complications in elderly patients with osteoporosis.
Keywords: anterior lumbar interbody fusion, elderly patient, minimal invasive surgery, multilevel, osteoporosis, percutaneous screw fixation, spinal stenosis
1. Introduction
The anterior lumbar interbody fusion (ALIF) procedure is known to stabilize the anterior spinal column, provide additional indirect decompression for the neural foramen and spinal canal resulting from disc height restoration, and maintain normal lordotic curvature of the lumbar spine.[1–4] The ALIF procedure can be preferable to lumbar decompression and arthrodesis with instrumentation which yields a relatively high perioperative complication rate[5–8] in elderly patients. Moreover, instrumentation using minimally invasive techniques, such as percutaneous pedicle screw fixation (PPF), can be favorable for elderly patients. However, there has been a debate regarding the use of ALIF with posterolateral fusion (PLF)[9] versus ALIF with PPF[2] for clinical and radiological results in L5-S1 isthmic spondylolisthesis and foraminal stenosis. Additionally, multi-level ALIF with PLF was used largely to improve the fusion rate in elderly patients with osteoporosis although in elderly patients it is especially important to have minimal bleeding, a short operation time, and minimal invasiveness without fusion failure. Therefore, to minimize perioperative invasiveness and complications and to improve the patient's postoperative quality of life, we have recently been performing minimal percutaneous screw fixation for multilevel operation instead of PLF with open pedicle screw fixation. The surgical strategy for elderly patients with osteoporosis involves a minimally invasive approach of 2 spine levels without posterior decompression. Shim et al[9] suggested that a 1 level ALIF with PPF may be an alternative treatment for elderly patients for whom lengthy operative periods accompanied with significant bleeding should be avoided.
We aimed to compare the clinical and radiological results between ALIF with PLF versus ALIF with PPF for 2 levels in elderly patients with osteoporosis, and to assess the feasibility of the ALIF with PPF procedure.
2. Methods
2.1. Patient population
From January 2010 to December 2013, we treated 64 elderly osteoporosis patients (>65 years of age), who had complained of neurogenic intermittent claudication, using a 2-level ALIF without posterior decompression. From January 2010 to December 2011, we performed ALIF with PLF (group A), and from January 2012 to December 2013, we performed ALIF with PPF (group B). In spinal stenosis cases, ALIF is not indicated for severe stenosis over grade D[10] which was needed for direct decompression was not indicated for ALIF. The selection criteria for this study were neurogenic intermittent claudication due to spinal stenosis with instability, foraminal stenosis, or mixed-type stenosis. This included patients over 65 years old with osteoporosis. Patients were excluded from the study if they had been operated with trauma, had concomitant infection, required additional laminectomy or posterior decompression, or had previously undergone lumbar surgery. Among the total 64 patients, 11 were lost to follow-up for the following reasons: 2 patients due to a change of their phone number, 4 patients due to living in a remote area far from the hospital, and 5 patients who declined further radiological study; A total of 53 patients were selected for this retrospective cohort study after 6 patients from group A and 5 patients from group B were excluded.
Preoperative workup included anteroposterior and lateral radiography including dynamic view, computed tomography (CT) imaging, and magnetic resonance imaging (MRI). Medical charts, radiographic features, and outcomes determined through a patient interview in an outpatient clinic were also assessed. This study was approved by the Institutional Review Board of the Leon Wiltse Memorial Hospital.
2.2. Clinical and radiological evaluation
We performed clinical and radiological assessments preoperatively and postoperatively (postoperatively at 3 months, 6 months, 1 year, 2 years, and at last follow-up), in a similar manner to assessments performed in other patients who had undergone fusion surgery in our hospital. For the measurement of clinical outcomes, the postoperative improvement of leg pain was quantified by a visual analog scale (VAS). Additionally, the Oswestry disability index (ODI), the Roland-Morris disability (RMD), and the modified MacNab criteria were used to investigate residual axial symptoms and daily living activities of the 2 groups for increased accuracy of self-reporting. The recovery rate was determined based on the final ODI and RMD using the standard formula.[11]
The 2 groups were compared according to clinical outcome measures of improvement and the radiological outcomes of lordosis, flexibility, fusion rate, and adjacent segment degeneration (ASD) of the lumbar spine. Additionally, the following intergroup variables, including basic demographic data, were examined to ensure that the characteristics in both groups were comparable: operation time, intra and postoperative blood loss, perioperative complications, and hospital length of stay were assessed to compare the perioperative parameters between the 2 types of screw fixation.
The level of lordosis was measured between the superior endplate of the upper body and the inferior endplate of the lower body at the fusion level according to the Cobb angle on the lateral plain radiograph taken with the patient in the neutral position.[2] The range of motion (ROM) was evaluated by the difference between the segments on a flexion and extension radiograph. The ASD was diagnosed using the plain radiographic criteria or CT, whereby a diagnosis was based on the radiographic disc height grade of degenerative changes related to an adjacent degeneration level. The criteria were as follows:
-
(1)
disc degeneration involving a loss of disc height of more than 10%;
-
(2)
a form of listhesis (anterolisthesis or retrolisthesis) greater than 4 mm;
-
(3)
a change in angle greater than 10° between adjacent vertebral bodies on flexion and extension radiographs;
-
(4)
occurrence of symptomatic disc herniation or spinal stenosis as confirmed by CT or MR imaging;
-
(5)
scoliosis; and
- (6)
We classified the fusion criteria into 3 types, stable, probably fused, and pseudoarthrosis (nonunion).[16] In a PLF, the fusion was defined as solid when there was a bony trabecular continuity between the vertebral bodies or a paravertebral bone bridge between the transverse processes and the lateral facets and <4° of mobility between the segments in flexion and extension radiographs or CT scans.[17] We analyzed lumbar CT scans, including reconstructed axial and coronal finecut scans, to evaluate the degree of fusion at 6 months, 1 year, 2 years, and at the last follow-up. Nonunion was defined as follows: the presence of a visible gap; graft collapse; motion >4° in the motion study[11,16,18–20]; small, thin fusion masses; graft resorption; or bilateral pseudarthrosis in PLF.[17] Probably fusion indicates that partial radiolucency was only observed at 1 interface of the graft-endplate contact with no presence of bone resorption. We included this type of probable fusion to stable in our study. We used dual-energy X-ray absorptiometry which targets the patient's bones, including cortical bone and the spongy interior bone, while soft tissue absorption is subtracted out to measure bone mineral density.
2.3. Surgical techniques
Two neurosurgeons, trained extensively in anterior approaches to the spine, performed all ALIF procedures. The same 2 experienced neurosurgeons also performed all posterior procedures (instrumented PLF and PPF, Fig. 1).
Figure 1.

Postoperative plain radiographic anteroposterior (A), and lateral (B) in ALIF with PLF and anteroposterior (C), and lateral (D) in ALIF with PPF. ALIF = anterior lumbar interbody fusion, PPF = pedicle screw fixation.
2.3.1. The ALIF with instrumented PLF procedure (Group A)
The patient was placed in a supine position and the dissection of vasculature and soft tissue via a retroperitoneal approach was performed by a neurosurgeon through a paramedian incision. After removing the disc material, a polyetheretherketone (PEEK) cage (Fidji, Zimmer, Warsaw) filled with allograft bone chips was inserted at the disc level as an interbody device. Patients received PEEK integral cage devices ranging from 10 to 20 mm in height, with either a 4°, 8°, or 12° lordotic angle to ensure sufficient distraction and lordosis. Additionally, a 4 mm diameter cancellous screw (Solco Healthcare US, Somerset, NJ) was anchored to the upper endplate of the sacral body at the L5-S1 level to prevent the cage from being pulled out. Following completion of the ALIF, the patient's position was changed to prone. Bilateral paraspinal dissection was performed using the Wiltse approach, and decortications were carried out on the L3 or L4 and L-5 transverse processes, the S-1 alar area, or the lateral portion of the L5-S1 facet. Pedicle screws and rods (GSS, GS Medical Co. Ltd., Cheongju, Korea) were applied. Cancellous bone chips were harvested from the posterior iliac crest and placed on the decorticated lateral side.
2.3.2. The ALIF with PPF procedure (Group B)
The ALIF was performed as described above. After the positional change to prone, the pedicle screws and rods (GSS, GS Medical) were inserted percutaneously under fluoroscopic guidance without PLF.
2.4. Statistical analysis
All parameters were analyzed using Student t tests, Mann–Whitney U tests, and Wilcoxon signed-rank tests. Differences were considered significant when P < .05. All data are expressed as the mean ± standard deviation. The analyses were performed using the IBM SPSS Statistics-Essentials for R software (for Windows, version 3.2.5; IBM Corp., Armonk, NY).
3. Results
Of the 64 patients included in this study, 2 patients were completely lost at 1-year follow-up due to a change of their phone number, 4 patients were lost to follow-up due to living in a remote area from the hospital and 5 patients were lost to follow-up as they declined the X-ray surveillance of the study. The final 53 included 10 males and 43 females, ranging in age from 65 to 83 years (mean, 72.1 ± 6.6 years). The follow-up evaluations were conducted over 39.2 to 86.6 months (mean, 64.6 ± 17.6 months). There were no significant differences in age, duration of preoperative symptoms, and follow-up period between group A and group B. Table 1 provides a summary of the demographic characteristics, including the preoperative diagnoses, for the entire series. The mean operation time was significantly shorter in group B (240.4 ± 22.6 minutes) than in group A (313.6 ± 17 minutes). The mean intraoperative blood loss was also significantly less in group B (281.1 ± 37.5 mL) than in group A (486 ± 79.5 mL). The mean postoperative blood loss over 2 days was also significantly less in group B (108.5 ± 16.0 mL) than in group A (177.2 ± 21.3 mL). The hospital stay was slightly shorter in group B (8.9 ± 0.9 days) than in group A (11.0 ± 0.7 days), although this did not reach the level of statistical significance. There were no major complications such as death, non q-wave myocardial infarction, stroke, pulmonary embolism, or retroperitoneal hematoma reported in either group following the operation. Minor complications such as urinary retention, urinary tract infection, ileus, delirium and confusion, temporary leg dysesthesia, and edema were reported at a frequency of 17.9% in group A and 8% in group B (Table 2). Preoperatively, all patients complained of neurogenic intermittent claudication regardless of whether back pain was present. After surgery, radiating pain, including neurogenic intermittent claudication, was significantly improved in both groups (P < .05, Table 3, Fig. 2.). Postoperatively, the ODI and RMD were also significantly improved in both groups (P < .05, Table 3), and the overall recovery rate was 37.8% and 46.9% according to the ODI, 37.1% and 40.5% according to the RMD in group A (PLF) and group B (PPF), respectively. The recovery rates of all parameters were more improved in group B than in group A, although this difference was not significant (ODI, P = .860, RMD, P = .93).
Table 1.
Demographic summaries of 53 patients in 2 study group.

Table 2.
Comparison of perioperative outcome.

Table 3.
Comparison of clinical results.

Figure 2.

Indirect decompression of preoperative T2 weighted MRI sagittal (A), and axial (B) views, and postoperative T2 weighted MRI sagittal (C), and axial (D) views in a case of spinal stenosis and foraminal stenosis, L3-4-5. MRI = magnetic resonance imaging.
3.1. Radiological outcomes
3.1.1. Lordosis
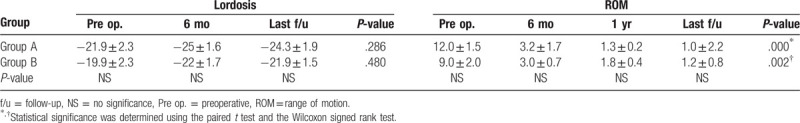
In both groups, the lordosis was improved until 6 months and there was a tendency for the lordotic angle to be maintained or decrease at the last follow-up (Table 4). However, there was no significant difference between the 2 groups at the last postoperative follow-up. There were 5 patients, 3 in group A and 2 in group B, whose lordosis was more decreased at the last follow-up than preoperatively. Three of these 5 patients had concomitant symptomatic ASD and the other 2 patients had symptoms related to pseudoarthrosis. There was a similar degree of lordosis in both groups, and there was no statistically significant intergroup difference in the data associated with lordosis.
Table 4.
Comparison of radiological results I.
3.2. ROM
The ROM was significantly decreased in both groups at the last postoperative follow-up and, this was especially apparent from the 6-month follow-up onward. A decreased ROM was more rapid in the PLF group than in the PPF group (74% and 66% at 6-month, and 89% and 80% at 1 year follow-up, respectively), although there was no statistical significance (Table 4). Although the decreased ROM was more rapid in group A, the decrease in the ROM at the last follow-up was similar in both groups. At the last follow-up, a total of 5 patients had >4° of mobility between the segments on flexion and extension radiograph (2 in group A and 3 in group B). Two of the 5 patients were correlated with a failure of complete fusion and the remaining 3 patients demonstrated minor motion (4.5°, 5.4°, and 6.1°) without any complications.
3.3. Fusion rate
The fusion rate was 85.8% and 76% in the PLF and the PPF group, respectively, 6 months after surgery. At the last follow-up, the fusion rate increased to 96.5% and 96% in the PLF and PPF groups, respectively. The fusion rate was higher in group A at 6 months follow-up, although this was not significant (P = .297); however, the fusion rate was similar between the 2 groups at the 1-year and 2-year follow-up (Fig. 3). In group A, the rate of dorsolateral bone bridge formation at 6 months after surgery was 100%. However, among these, 4 cases had >4° of mobility between the segments on flexion and extension radiograph, and a visible gap in the disc space. Therefore, we classified these 4 cases as “nonunion.” The remaining patients in group A (24 of 28) had bony trabecular continuities between the vertebral bodies, as well as paravertebral bone bridges between the transverse processes and lateral facets at 6 months postoperatively. In group B, 6 cases of 25 had >4° of mobility between the segments on flexion and extension radiograph, and a visible gap in the disc space at 6 months postoperatively. At 1 year follow-up, the fusion rate was similar between the 2 groups (96.5% and 96% for group A and group B, respectively) with no significant statistical difference. However, in group A, 1 patient who complained of back pain had non-union and screw loosening at the L4-5 level and radiolucency was observed in an interface with additional bone resorption. In group B, there was 1 case of L4 screw migration caused by nonunion at the L4-5 level, so we performed an extension to L3 using an interbody cage (Fig. 4).
Figure 3.

Plain radiographic anteroposterior (A), and lateral (B) in ALIF with PLF and anteroposterior (C), and lateral (D) in ALIF with PPF at a 2 yr follow-up. ALIF = anterior lumbar interbody fusion, PPF = pedicle screw fixation.
Figure 4.

Pseudoarthrosis with screw loosening at L4 was found in sixty-eight female patient 1 yr after surgery. Plain radiographic anteroposterior (A), and lateral (B), and computed tomography anteroposterior (C), and lateral (D).
3.4. Adjacent segment disease
Radiological ASD was found in 7 patients (25%) of group A and 6 patients (24%) of group B at the last follow-up (Table 5). However, symptomatic ASD was also found in 3 patients (10.7%) in the PLF group and 4 patients (16%) in the PPF group at the last follow-up. Therefore, ASD was similar between the 2 groups at the last follow-up with no significant difference. One patient in group A with symptomatic ASD was re-operated on at 69 months after the first surgery. The radiographs shown in Figure 5 and Table 5 are examples of ASD in both groups. There was no statistically significant intergroup difference in the rate of ASD development (P = .56). The ASD was located primarily at the cranial segment of the fused level in both groups. In group A, ASD was found in 2 patients (66.7%) at the cranial segment, in 1 patient (33.3%) at the caudal segment, and in group B, ASD was found in 2 patients (100%) at the cranial segment excepting the L4-5-S1 level. Parameters related to radiological ASD were duplicated in 7 of the 20 patients. The most common type of ASD in both groups was spinal stenosis and the remainder of the patients had retrolisthesis or compression fracture. Reoperations for ASD were performed in 1 patient from group A (1.9%). Posterior lumbar interbody fusion with extension to L3-4 was performed for spinal stenosis.
Table 5.
Comparison of radiological results II (adjacent segment disease).

Figure 5.

Fifty-four male patients complained of neurogenic intermittent claudication in both legs, 4 yr after ALIF with PLF at L4–5-S1. Adjacent segment disease of spinal stenosis is shown in T2 weighted MRI sagittal (A), and axial (B), postoperative plain radiographic anteroposterior (C), and lateral (D). ALIF = anterior lumbar interbody fusion, PPF = pedicle screw fixation.
4. Discussion
The use of surgery to treat lumbar spinal stenosis has increased during the past decade as has the complexity of surgical procedures.[21] Among the various surgical treatment options for spinal stenosis with instability or foraminal stenosis, and lumbar degenerative disease, surgeons worldwide have performed simple decompression, PLF with or without instrumentation, 270° fusion procedures (posterior lumbar interbody fusion [PLIF] or transforaminal lumbar interbody fusion), and until recently, circumferential fusion procedures.[22–25] Regarding fusion, although there is an ongoing debate as to whether the anterior approach is better than the posterior approach, Jiang et al[26] reported in a systematic review that clinical outcomes and failed fusion rates were similar in both techniques. However, posterior fusion of 2 levels for older patients with osteoporosis could bring about worse results than ALIF which is successful in saving posterior elements by indirect decompression of the neural element. ALIF with less bleeding, rapid recovery, and wider cage is also more advantageous than the posterior approach for older patients with osteoporosis, especially in cases of long level over 2 levels. We had performed 2 level ALIF and PLF for elderly patients with osteoporosis to improve the fusion rate. However, this technique led to increased bleeding and longer operation times for PLF and also increased perioperative morbidity. As a result of this, we performed 2 level ALIF and PPF for elderly osteoporosis patients to decrease perioperative morbidity. To the best of our knowledge, this is the first study to demonstrate the effectiveness of 2-level ALIF and PPF in elderly osteoporosis patients.
Clinical results including VAS, ODI, and RMD were more improved in group B compared to group A, although there was no significant statistical difference. Rapid recovery, including a short operation time, less blood loss, and a shorter hospital day are important advantages demonstrated by group B. Elderly patients have generally been considered to be at high risk of postoperative complications after conventional lumbar arthrodesis due to their age and age-related comorbidities.[5,6,27] Deyo et al[7] reported an increased risk of in-hospital complications in elderly patients who underwent fusion compared with elderly patients who underwent laminectomy alone. With respect to lumbar interbody fusion, Okuda et al[8] reported postoperative complications in 16% of elderly patients after PLIF with pedicle screw placement. There were no major complications reported in our study; in terms of minor complications, we recorded 17.9% in group A and 8% in group B. We believe that the reduced bleeding and short operation time following ALIF is one of the main reasons for the lower complication rate. This was especially true in group B where PPF was performed PPF, which showed more reduced bleeding and a shorter operation time. Though there were fewer complications this did not reach the level of statistical significance.
To obtain greater lordosis in our cases, positioning was performed with both legs elevated to a backward keeping prone position and maximum rod compression in consideration of the osteoporosis. The Lordosis was slightly improved in both groups following the operation. Similarly, a last follow-up the lordosis of the majority of patients was slightly decreased than the postoperatively improved lordosis due to ambulation before the complete fusion.
In general, the recent fusion rate of ALIF is reported as ranging from 88.6% to 97.3%.[3,28–30] A previous study reported that, at the last follow-up, the radiographs of all patients operated to 1-level ALIF with PPF showed solid fusion.[2] In our study, the fusion rate of a 2 level ALIF was higher in the PLF group than in the PPF group 6 months after surgery (85.8% and 76%, respectively), although this difference was not significant. However, the fusion rate at the 1-year and 2-year follow-up was nearly identical in each group (96.5% and 96% in the PLF group and the PPF group, respectively). Bone chips and demineralized bone matrix was used as the fusion material for the ALIF in both groups, and additional autografts of iliac bone were employed in the PLF group (group A). Fusion occurs earlier with PLF but eventually the fusion rate in both groups equalized after a year.
The decreased disc height in patients with ASD overlapped with the spinal stenosis of ASD or had no specific symptoms. We used strict radiologic criteria for ASD including a disc height loss of 10%. Some authors evaluate a disc height loss of 50% for ASD.[31] Therefore, the radiologic criteria for ASD might be different across different studies; it is true that measurement or selection bias always exists. The rate of symptomatic adjacent segment disease is also higher in patients with transpedicular instrumentation (12.2%–18.5%) compared to patients fused with other forms of instrumentation or with no instrumentation (5.2%–5.6%). We also believe that it is more important to confirm symptomatic ASD although radiologic factors may also indicate risk. The present study demonstrated that 10.7% (3 patients) in group A and 16% (4 patients) in group B showed symptomatic ASD for an average of 64 months with no statistical difference. One patient in group A (1.9%) with ASD of spinal stenosis at L3-4 was re-operated on at 69 months after the first operation. Reoperation surgery rates of ASD ranged from 4.5% to 23.1%.[32,33] There were no differences in the ASD and reoperation rates caused by ASD between the 2 groups, and the reoperation rate caused by ASD for ALIF is likely to be lower than for posterior fusion. In our study, for the overall clinical and radiologic results including complications, the PPF group had more benefit than the PLF group although the fusion rate was slower than with the PLF group.
5. Limitations
This study has several limitations. First, the 4- to 6-year follow-up period of ASD with fusion is relatively short. Second, the study has a small sample size: 53 patients altogether, with 9 patients having incomplete follow-up data and 2 having missing phone numbers (6 in group A and 5 in group B). The patients with missing information may have affected the comparability of the 2 groups. However, the number of incomplete follow-up and follow-up loss between both groups is similar, and we could surely evaluate the clinical results in 9 patients who lived far from the hospital and did not want to undergo follow-up radiological study. Furthermore, clinical results obtained using questionnaires were excellent and similar to those of the included study group, although 9 patients with incomplete follow-up data were not included in this study. Future research involving more patients, thereby allowing for enhanced comparability between the groups, and a longer follow-up period to monitor ASD are needed to confirm the findings of this study.
6. Conclusions
Two-level ALIF with PPF resulted in less blood loss, fewer minor complications, shorter operation time, and a rapid recovery. Although 2-level ALIF with PLF results in a fusion that is achieved earlier, ALIF with PPF is the more advantageous method in elderly patients with osteoporosis for the treatment of multiple spinal stenosis with instability, foraminal stenosis, and mixed type stenosis
Acknowledgment
I would like to express my special thanks of gratitude to Han Na, Lee, one of academic team who helped me to collect and organize data. I would also like to thank epidemiologist Yoon Sik, Kang who helped me compiling statistics and came to know about new things about statistics.
Author contributions
Conceptualization: Choon Keun Park.
Data curation: Dong Geun Lee.
Formal analysis: Dong Geun Lee, Dong Chan Lee.
Investigation: Dong Geun Lee.
Methodology: Dong Geun Lee, Choon Keun Park.
Project administration: Dong Geun Lee.
Resources: Dong Geun Lee, Choon Keun Park, Dong Chan Lee.
Software: Dong Geun Lee.
Supervision: Choon Keun Park, Dong Chan Lee.
Validation: Dong Geun Lee.
Visualization: Dong Geun Lee.
Writing – original draft: Dong Geun Lee.
Writing – review & editing: Dong Geun Lee.
Footnotes
Abbreviations: ALIF = anterior lumbar interbody fusion, ASD = adjacent segment degeneration, BMD = bone mineral density, CT = computed tomography, MRI = magnetic resonance imaging, ODI = Oswestry disability index, PEEK = polyetheretherketone, PLF = posterolateral fusion, PLIF = posterior lumbar interbody fusion, PPF = percutaneous pedicle screw fixation, RMD = Roland-Morris disability, ROM = range of motion, VAS = visual analog scale.
How to cite this article: Lee DG, Park CK, Lee DC. Clinical and radiological comparison of 2 level anterior lumbar interbody fusion with posterolateral fusion and percutaneous pedicle screw in elderly patients with osteoporosis. Medicine. 2020;99:10(e19205).
All authors certify that they have no affiliation with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
The authors have no conflicts of interest to disclose.
References
- [1].Heary RF, Bono CM. Circumferential fusion for spondylolisthesis in the lumbar spine. Neurosurg Focus 2002;13:E3. [PubMed] [Google Scholar]
- [2].Kim JS, Choi WG, Lee SH. Minimally invasive anterior lumbar interbody fusion followed by percutaneous pedicle screw fixation for isthmic spondylolisthesis: minimum 5-year follow-up. Spine J 2010;10:404–9. [DOI] [PubMed] [Google Scholar]
- [3].Lee SH, Choi WG, Lim SR, et al. Minimally invasive anterior lumbar interbody fusion followed by percutaneous pedicle screw fixation for isthmic spondylolisthesis. Spine J 2004;4:644–9. [DOI] [PubMed] [Google Scholar]
- [4].Wang M, Dalal S, Bagaria VB, et al. Changes in the lumbar foramen following anterior interbody fusion with tapered or cylindrical cages. Spine J 2007;7:563–9. [DOI] [PubMed] [Google Scholar]
- [5].Benz RJ, Ibrahim ZG, Afshar P, et al. Predicting complications in elderly patients undergoing lumbar decompression. Clin Orthop Relat Res 2001;116–21. [DOI] [PubMed] [Google Scholar]
- [6].Cassinelli EH, Eubanks J, Vogt M, et al. Risk factors for the development of perioperative complications in elderly patients undergoing lumbar decompression and arthrodesis for spinal stenosis: an analysis of 166 patients. Spine 2007;32:230–5. [DOI] [PubMed] [Google Scholar]
- [7].Deyo RA, Ciol MA, Cherkin DC, et al. Lumbar spinal fusion. A cohort study of complications, reoperations, and resource use in the Medicare population. Spine 1993;18:1463–70. [PubMed] [Google Scholar]
- [8].Okuda S, Oda T, Miyauchi A, et al. Surgical outcomes of posterior lumbar interbody fusion in elderly patients. J Bone Joint Surg Am Vol 2006;88:2714–20. [DOI] [PubMed] [Google Scholar]
- [9].Shim JH, Kim WS, Kim JH, et al. Comparison of instrumented posterolateral fusion versus percutaneous pedicle screw fixation combined with anterior lumbar interbody fusion in elderly patients with L5-S1 isthmic spondylolisthesis and foraminal stenosis. J Neurosurg Spine 2011;15:311–9. [DOI] [PubMed] [Google Scholar]
- [10].Schizas C, Theumann N, Burn A, et al. Qualitative grading of severity of lumbar spinal stenosis based on the morphology of the dural sac on magnetic resonance images. Spine 2010;35:1919–24. [DOI] [PubMed] [Google Scholar]
- [11].Min JH, Jang JS, Lee SH. Comparison of anterior- and posterior-approach instrumented lumbar interbody fusion for spondylolisthesis. J Neurosurg Spine 2007;7:21–6. [DOI] [PubMed] [Google Scholar]
- [12].Park P, Garton HJ, Gala VC, et al. Adjacent segment disease after lumbar or lumbosacral fusion: review of the literature. Spine 2004;29:1938–44. [DOI] [PubMed] [Google Scholar]
- [13].Ishihara H, Osada R, Kanamori M, et al. Minimum 10-year follow-up study of anterior lumbar interbody fusion for isthmic spondylolisthesis. J Spinal Disord 2001;14:91–9. [DOI] [PubMed] [Google Scholar]
- [14].Kumar MN, Jacquot F, Hall H. Long-term follow-up of functional outcomes and radiographic changes at adjacent levels following lumbar spine fusion for degenerative disc disease. Eur Spine J 2001;10:309–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Oda I, Cunningham BW, Buckley RA, et al. Does spinal kyphotic deformity influence the biomechanical characteristics of the adjacent motion segments? An in vivo animal model. Spine 1999;24:2139–46. [DOI] [PubMed] [Google Scholar]
- [16].Rzewuska M, Ferreira M, McLachlan AJ, et al. The efficacy of conservative treatment of osteoporotic compression fractures on acute pain relief: a systematic review with meta-analysis. Eur Spine J 2015;24:702–14. [DOI] [PubMed] [Google Scholar]
- [17].Lenke LG, Bridwell KH, Bullis D, et al. Results of in situ fusion for isthmic spondylolisthesis. J Spinal Disord 1992;5:433–42. [DOI] [PubMed] [Google Scholar]
- [18].Kim JS, Lee KY, Lee SH, et al. Which lumbar interbody fusion technique is better in terms of level for the treatment of unstable isthmic spondylolisthesis? J Neurosurg Spine 2010;12:171–7. [DOI] [PubMed] [Google Scholar]
- [19].Madan SS, Boeree NR. Comparison of instrumented anterior interbody fusion with instrumented circumferential lumbar fusion. Eur Spine J 2003;12:567–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Suk SI, Lee CK, Kim WJ, et al. Adding posterior lumbar interbody fusion to pedicle screw fixation and posterolateral fusion after decompression in spondylolytic spondylolisthesis. Spine 1997;22:210–9. [DOI] [PubMed] [Google Scholar]
- [21].Deyo RA, Mirza SK, Martin BI, et al. Trends, major medical complications, and charges associated with surgery for lumbar spinal stenosis in older adults. JAMA 2010;303:1259–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Mobbs RJ, Phan K, Malham G, et al. Lumbar interbody fusion: techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF,. J Spine Surg 2015;1:2–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Humphreys SC, Hodges SD, Patwardhan AG, et al. Comparison of posterior and transforaminal approaches to lumbar interbody fusion. Spine 2001;26:567–71. [DOI] [PubMed] [Google Scholar]
- [24].Lee N, Kim KN, Yi S, et al. Comparison of outcomes of anterior, posterior, and transforaminal lumbar interbody fusion surgery at a single lumbar level with degenerative spinal disease. World Neurosurg 2017;101:216–26. [DOI] [PubMed] [Google Scholar]
- [25].Schroeder GD, Kepler CK, Millhouse PW, et al. L5/S1 fusion rates in degenerative spine surgery: a systematic review comparing ALIF, TLIF, and axial interbody arthrodesis. Clin Spine Surg 2016;29:150–5. [DOI] [PubMed] [Google Scholar]
- [26].Jiang SD, Chen JW, Jiang LS. Which procedure is better for lumbar interbody fusion: anterior lumbar interbody fusion or transforaminal lumbar interbody fusion? Arch Orthop Trauma Surg 2012;132:1259–66. [DOI] [PubMed] [Google Scholar]
- [27].Carreon LY, Puno RM, Dimar JR. Perioperative complications of posterior lumbar decompression and arthrodesis in older adults. J Bone Joint Surg Am Vol 2003;85-a:2089–92. [DOI] [PubMed] [Google Scholar]
- [28].Rao PJ, Phan K, Giang G, et al. Subsidence following anterior lumbar interbody fusion (ALIF): a prospective study. J Spine Surg 2017;3:168–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kim NH, Lee JW. Anterior interbody fusion versus posterolateral fusion with transpedicular fixation for isthmic spondylolisthesis in adults. A comparison of clinical results. Spine 1999;24:812–6. [DOI] [PubMed] [Google Scholar]
- [30].Phan K, Thayaparan GK, Mobbs RJ. Anterior lumbar interbody fusion versus transforaminal lumbar interbody fusion--systematic review and meta-analysis. Br J Neurosurg 2015;29:705–11. [DOI] [PubMed] [Google Scholar]
- [31].Moreau PE, Ferrero E, Riouallon G, et al. Radiologic adjacent segment degeneration 2 years after lumbar fusion for degenerative spondylolisthesis. Orthop Traumatol Surg Res 2016;102:759–63. [DOI] [PubMed] [Google Scholar]
- [32].Drysch A, Ajiboye RM, Sharma A, et al. Effectiveness of reoperations for adjacent segment disease following lumbar spinal fusion. Orthopedics 2018;41:e161–7. [DOI] [PubMed] [Google Scholar]
- [33].Zhong ZM, Deviren V, Tay B, et al. Adjacent segment disease after instrumented fusion for adult lumbar spondylolisthesis: Incidence and risk factors. Clin Neurol Neurosurg 2017;156:29–34. [DOI] [PubMed] [Google Scholar]


