Abstract
In China, there is a significant lack of awareness of diabetes and its complications. Screening of diabetic retinopathy has important for early detection, prevention, and treatment. This large, cross-sectional study aimed to characterize the demographic, physical, serological, and ocular characteristics of subjects with diabetes mellitus in Shijiazhuang, China. It also aimed to associate these characteristics with the presence of diabetic retinopathy.
From May 2, 2018 to August 25, 2019, under diabetes care program, the diabetic patients (n = 1008) were subjected to standardized questionnaires to collect demographical characteristics. Also, telescreens and laboratory tests were performed for the enrolled patients. Multivariate logistic regression analysis was used to evaluate factors associated with diabetic retinopathy.
Forty percent of diabetics in its population had some form of diabetic retinopathy. Diabetic retinopathic patients were likely to be elder (P = .0003), men (P = .018), hypertensive (P < .0001), and had high body mass index (P < .0001), metabolic abnormalities, and longer duration of diabetes (P < .0001). Higher intraocular pressure (P = .0008), fasting blood glucose (P < .0001), serum total cholesterol (P < .0001), serum triglyceride (P = .0006), % glycated hemoglobin (HbA1c) (P < .0001), and disc asymmetry including cup–disc ratio (P = .041) reported in patients with diabetic retinopathy. Age (P = .049), male sex (P = .048), hypertension (P = .048), duration of diabetes (P = .012), diabetic neuropathy (P = .048), diabetic nephropathy (P = .048), diabetic foot ulcer (P = .041), foot amputation (P = .042), fasting blood glucose (P = .022), serum total cholesterol (P = .028), serum triglyceride (P = .035), and HbA1c (P = .042) were associated with diabetic retinopathy.
Diabetic retinopathy was the most common ocular fundus disease in diabetic patients. Also, aging, the other comorbidities, and metabolic syndrome are associated with diabetic retinopathy.
Level of Evidence: III.
Keywords: diabetes, diabetic retinopathy, metabolic abnormalities, ocular fundus diseases, telescreening
1. Introduction
China has the largest number of individuals affected by diabetes,[1] which rise a huge burden over the healthcare system.[2] Diabetes may lead to cardiovascular, cerebrovascular, neuropathic, nephropathic, retinopathic, and foot diseases in the Chinese population.[3] Even, there is a need for the management of diabetes prevention and its control in China.[4,5] Vascular autoregulation is affected by diabetes mellitus also damages the microvascular system, especially in the retina and optic nerve.[6] Diabetes is also associated with age-related macular degeneration,[7] glaucoma,[8] and retinopathy.[6] Diabetes and retinopathy are associated with blindness in working-age Chinese individuals.[9] The prevalence of diabetic retinopathy is associated with the duration of type I and type II diabetes in the Chinese people.[10] The prevalence of diabetic retinopathy is different in different regions of China. Different physical and clinical factors influence the risk of diabetic retinopathy.[11] In China, there is a significant lack of awareness of diabetes and its complications, which may affect eyes.[11] Also, the early detection of diabetic retinopathy helps in prevention and treatment. Screening of diabetic retinopathy has important to detect cases where prevalence is significantly high[12] because fundus pathology is asymptomatic.[6] Therefore, annual examinations of the eyes are recommended in diabetic patients but general practitioners of China do not routinely check eyes of their diabetic patients. The telescreening has potential to detect ocular fundus disease by professional ophthalmologists.[13]
This large, cross-sectional study aimed to characterize the demographic, physical, serological, and ocular characteristics of subjects with diabetes mellitus in Shijiazhuang, China. It also aimed to associate these characteristics with the presence of diabetic retinopathy.
2. Materials and methods
2.1. Ethics approval and consent to participate
The protocol of the study (FHS/CL/15/19 dated September 11, 2019) was approved by the human ethics committee of the First Hospital of Shijiazhuang. An informed consent form was obtained from all participants after clarifying the nature of the study and possible outcomes. The study reporting adheres to the strengthening the reporting of observational studies in epidemiology (STROBE) statement: cross-sectional studies,[14] and the V2008 Helsinki Declaration.
2.2. Study population
From May 2, 2018 to August 25, 2019, a total of 1022 diabetic patients (diagnoses as per World Health Organization [WHO] criteria: fasting capillary whole blood glucose level ≥6.1 mM/L or plasma glucose ≥7 mM/L and/or 2-hour postprandial blood glucose ≥11.1 mM/L[4]) of Hebei province of China were referred to the project by their treating physicians (general practitioners) for evaluation of diabetes related complications during patients’ diabetes care program. The “diabetes care program” is part of healthcare practice at the First Hospital of Shijiazhuang, Shijiazhuang, Hebei, China. Patients who died for any reason(s) during the study period (n = 2), those data could not be collected for any reason(s) (n = 12), acute renal failure patients, and those who were on dialysis were excluded from the study. The flow diagram of the study is presented in Fig. 1.
Figure 1.
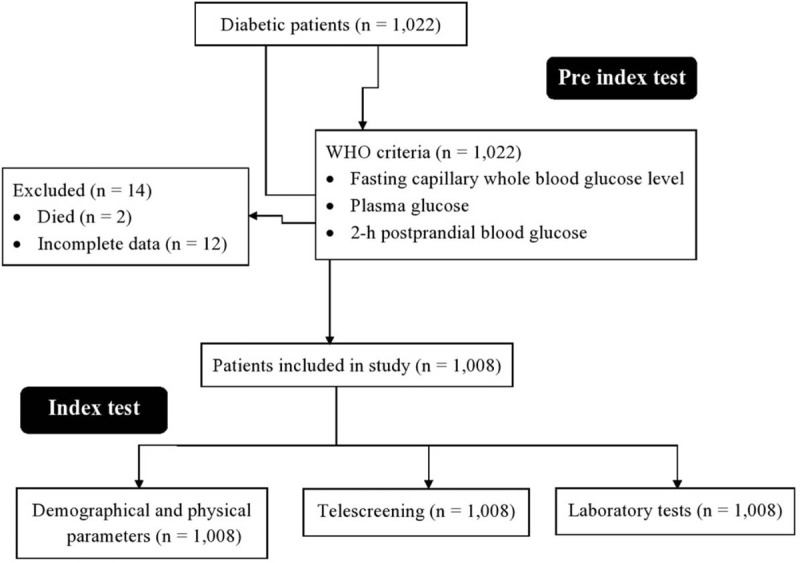
Flow diagram of the study.
2.3. Sample size calculation
Under prospective study, it was assumed that the maximum 15% patients can lost to collect data for analyses and the sample size of 1008 was used for the study.
2.4. Data collection
Demographical, arthrological, habits, and the other health-related information for each participant were obtained by standardized face-to-face questionnaires (validated by the authority) performed by the nursing staff of the institutes during the cross-sectional study. The data regarding telescreening and laboratory test were also collected for the study purposes.
2.5. Intraocular pressure
Each patient was subjected to measure intraocular pressure (IOP) by Tonometry procedure using Icare HOME tonometer (Icare Finland Oy Vantaa, Finland). IOP was measured by physicians.
2.6. Cup-to-disc ratio
It is a ratio of the diameter of the cup portion of the optic disc to the total diameter of the optic disc.[15]
2.7. Telescreening
Two fundus photographs (the macular fovea and the optic center) of each eye of each patient[6,13] had been taken by physicians (minimum 2-years of experience) using 45° CR-DGi NM Fundus Camera (Canon, Ota, Tokyo, Japan). The photographs were digitally stored.
2.8. Laboratory tests
All patients had been instructed to fast overnight (>8 hours) before a collection of blood samples. The blood samples were collected from an antecubital vein by pathologists (minimum 3-years of experience).[6] The collected blood samples were subjected to evaluate fasting plasma glucose levels, total cholesterol, triglyceride, glycated hemoglobin (HbA1c), and serum creatinine by pathologists (minimum 3-years of experience).
All data were digitally recorded and analyzed by ophthalmologists (minimum 3-years of experience) of the institutes. In cases of differences of opinions among ophthalmologists, the expert opinion was taken by external ophthalmologists (minimum 5-years of experience).
2.9. Definitions of fundus pathology and the other factors
2.9.1. Diabetic retinopathy
The presence of intra-retinal neovascularization and/or microvascular abnormalities, any microaneurysms, hard exudates, hemorrhages, macular edema, or cotton-wool spots were considered as diabetic retinopathy.[12]
2.9.2. Glaucoma
IOP >21 mmHg, a cup–disc ratio of >0.6, and disc asymmetry including a cup–disc ratio of >0.2 was considered as glaucoma.[15,16]
2.9.3. Age-related macular degeneration
The large drusen and retinal pigment epithelial changes were considered as age-related macular degeneration.[6]
Patients who smoke at least one cigarette in a day was considered as smokers (self-reported). The consumption of at least one small peg of alcohol in a day was considered as an alcoholic (self-reported). The systolic blood pressure 140 mmHg or more and/or a diastolic blood pressure 90 mmHg or more was considered as hypertension (a result of medical chart review).[6] The time from the first diagnosis of diabetes to enrollment in the program was considered as the duration of diabetes.
2.10. Statistical analysis
SPSS Statistics version 26 (IBM Corporation, Armonk, New York, NY) was used for statistical analysis. Fisher exact test[11] was performed for categorical data and the Mann–Whitney U test[16] was performed for continuous variables. Multivariate logistic regression analysis was used to evaluate factors associated with diabetic retinopathy.[10] All results were considered significant at a 95% level of confidence.
3. Results
3.1. Prevalence of ocular fundus diseases
Maximum numbers of patients reported with diabetic retinopathy 409 (40%), followed by glaucoma 309 (31%), and age-related macular degeneration 219 (22%). A small fraction of patients also reported the other pathogenesis 71 (7%). The detailed prevalence of ocular fundus diseases is reported in Table 1.
Table 1.
Results of telescreens.
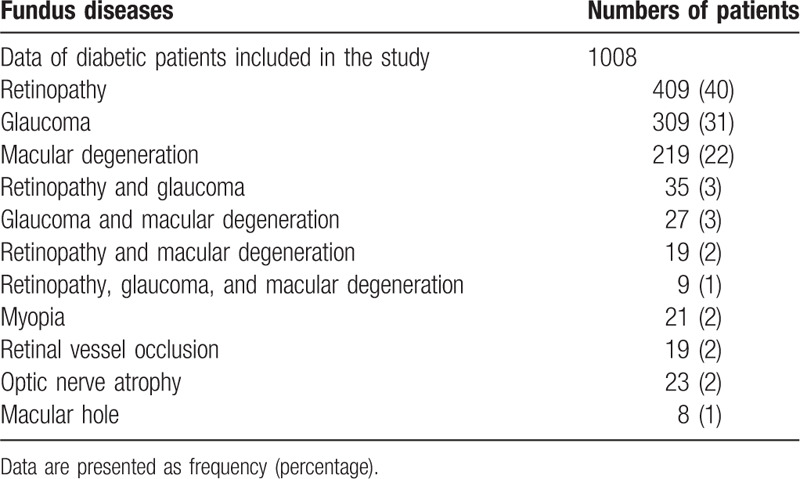
3.2. Demographical and physical parameters
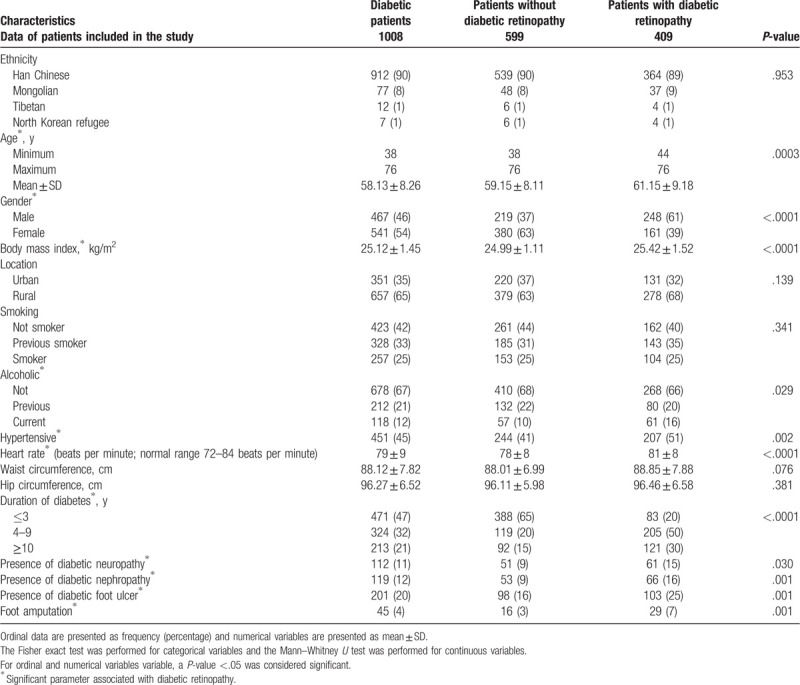
Patients had history of diabetes from 1-month to 15-years. Diabetic retinopathy reported in elder patients (P = .0003). More male patients reported diabetic retinopathy (P < .0001), patients with diabetic retinopathy had high body mass index (BMI, P < .0001), hypertension (P = .002), higher heart rate (P < .0001), and longer duration of diabetes (P < .0001). Also, higher population reported with neuropathy (P = .03), nephropathy (P = .001), foot ulcer (P = .001), and foot amputation (P = .001) for those were suffering from diabetic retinopathy. The other demographical parameters of diabetic patients are reported in Table 2.
Table 2.
Demographical and physical parameters.
3.3. Results of laboratory tests
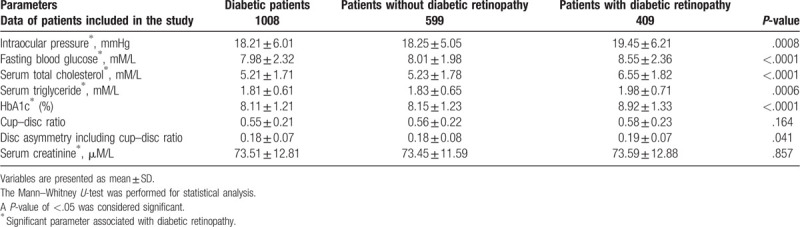
Higher IOP (P = .0008), fasting blood glucose (P < .0001), serum total cholesterol (P < .0001), serum triglyceride (P = .0006), % HbA1c (P < .0001), and disc asymmetry including cup–disc ratio (P = .041) reported in patients with diabetic retinopathy. The detailed values of laboratory tests are reported in Table 3.
Table 3.
Results of laboratory tests.
3.4. Association of parameters with diabetic retinopathy
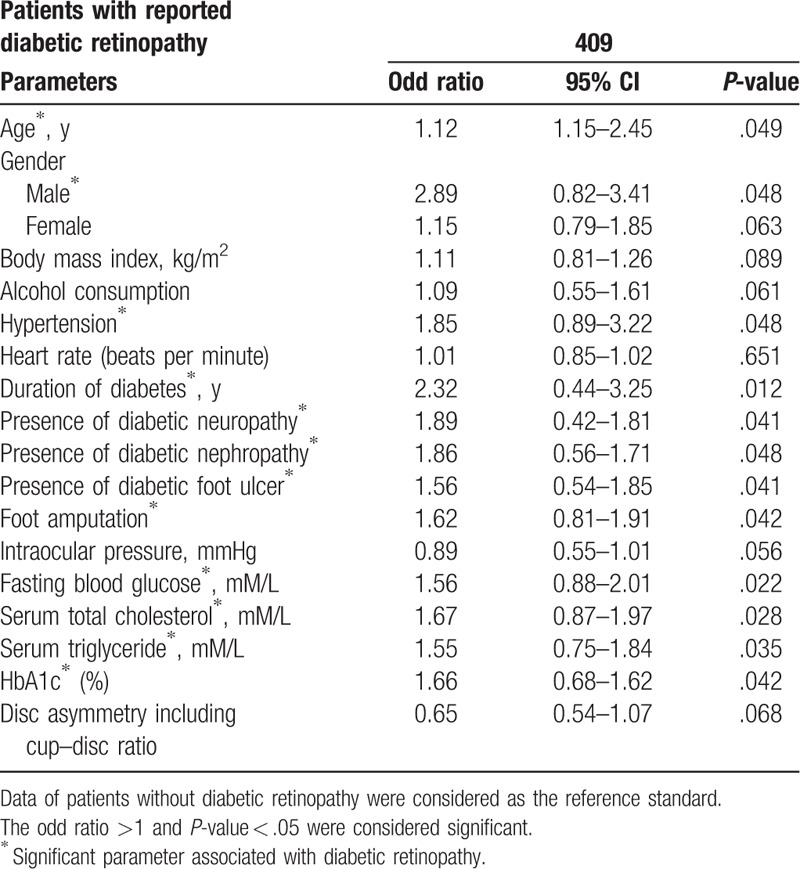
Significant demographical parameters, clinical conditions, and the laboratory tests were selected in the multivariate analyses for prediction of association with retinopathy. Age (P = .049), male sex (P = .048), hypertension (P = .048), duration of diabetes (P = .012), presence of diabetic neuropathy (P = .048), presence of diabetic nephropathy (P = .048), presence of diabetic foot ulcer (P = .041), foot amputation (P = .042), fasting blood glucose (P = .022), serum total cholesterol (P = .028), serum triglyceride (P = .035), and HbA1c (P = .042) were associated with diabetic retinopathy (Table 4).
Table 4.
Association of parameters with diabetic retinopathy.
4. Discussion
The study performed with 1008 participants of the Shijiazhuang, China with confirmed diabetes. Also, the evaluation reported 409 participants with diabetic retinopathy as the most frequent ocular fundus disease. Diabetic patients with retinopathy had elevated blood chemistry, hypertension, additionally nephropathy, neuropathy, and/or a foot ulcer, and a long duration of diabetes. The results of the study were consistent with a study on the Chinese community,[3,10,11,17] the study of the Fengyutan community of China,[6] and the study of the Fengyutan sub-district.[18] Low vision and blindness are severe public health problems in China.[9] Monitoring of blood parameters and early screening of ocular fundus diseases are required in a long duration of diabetes to prevent damage to eyes.
The study reported age, male sex, hypertension, duration of diabetes, diabetic neuropathy, diabetic nephropathy, presence of diabetic foot ulcer, and foot amputation as independent risk factors for diabetic retinopathy. The results of the study were consistent with multi-hospital-based cross-sectional studies on the Chinese community,[3,10] cross-sectional study of the Fengyutan community of China,[6] and study of the Fengyutan sub-district.[18] Ageing and the other comorbidities have a significant association with prevalence of diabetic retinopathy. For treatment, management, and prevention of retinopathy special attention is required to patients with the other comorbidities.
The study reported that fasting blood glucose, serum total cholesterol, serum triglyceride, and HbA1c were independent risk factors for diabetic retinopathy. The results of the study were consistent with a population-based study of the Fengyutan sub-district.[18] Metabolic abnormalities have a significant role in the prevalence of diabetic retinopathy because it may worsen the chronic complications of diabetes.
The study reported that IOP, cup–disc ratio, and disc asymmetry including the cup–disc ratio were not associated with diabetic retinopathy. The results of the study were consistent with the study on the Malay population.[8] Unlike, comorbidities and metabolic syndrome the small amount of increase in IOP would not lead to diabetic retinopathy.
There are several limitations of the study, for example, the cross-sectional study and lack of random sampling. The study reported glaucoma, age-related macular degeneration, and the other pathogenesis for ocular fundus diseases but did not evaluate the risk factors for the same. The intra- and inter-rater agreements are required to perform between ophthalmologists for image analysis of fundus photographs[19] but the study did not perform agreements. The current study reported only 21 (2%) patients with myopia but a recent population-based survey estimated the prevalence of myopia to be nearly 88% in young people in Eastern China.[20] The patients enrolled in the current study were older than a population-based survey. The current study reported 309 (31%) patients with glaucoma but a resent meta-analysis found that the prevalence of glaucoma in China to be just over 2.5% of the population.[21] Serial measurements of IOP, optic nerve, and visual fields, especially early in the disease. As a result, the current study overestimated glaucoma in the population. The current study did not consider the age of the patient when diagnosing the age-related macular degeneration. Relatively young patients can have these findings as a result of an alternative etiology, such as pattern dystrophy or myopic maculopathy. Also, the study did not differentiate between wet and dry the age-related macular degeneration. The study did not differentiate between proliferative and non-proliferative retinopathy.
5. Conclusions
Diabetic retinopathy was the most common ocular fundus disease in diabetic patients. Also, age, male sex, hypertension, duration of diabetes, diabetic neuropathy, diabetic nephropathy, diabetic foot ulcer, foot amputation, fasting blood glucose, serum total cholesterol, serum triglyceride, and HbA1c are independent risk factors for diabetic retinopathy. The study will help physicians to understand the prevalence and association of diabetic retinopathy in the Chinese population.
Acknowledgments
The authors are thankful for the medical and non-medical staff of the First Hospital of Shijiazhuang, Shijiazhuang, Hebei Province, China.
Author contributions
Conceptualization: Delong Zhang.
Data curation: Delong Zhang, Qian Ren.
Formal analysis: Qian Ren, Xian Su.
Investigation: Xian Su.
Methodology: Qian Ren.
Project administration: Li Yin.
Resources: Delong Zhang, Qian Ren.
Software: Li Yin, Delong Zhang.
Supervision: Li Yin, Xian Su.
Validation: Li Yin, Xian Su, Zhaohui Sun.
Visualization: Zhaohui Sun.
Writing – original draft: Zhaohui Sun.
Writing – review & editing: Zhaohui Sun.
Zhaohui Sun orcid: 0000-0002-7260-7835.
Footnotes
Abbreviations: BMI = body mass index, HbA1c = glycated hemoglobin, IOP = intraocular pressure, STROBE = the study reporting adheres to the strengthening the reporting of observational studies in epidemiology.
How to cite this article: Yin L, Zhang D, Ren Q, Su X, Sun Z. Prevalence and risk factors of diabetic retinopathy in diabetic patients: A community based cross-sectional study. Medicine. 2020;99:9(e19236).
The datasets used and analyzed during the current study available from the corresponding author on reasonable request.
The research did not receive any specific financial motivation from the government of PR China, non-government, or non-profitable sectors to perform research.
The authors have no conflicts of interest to disclose.
References
- [1].Ma RC. Epidemiology of diabetes and diabetic complications in China. Diabetologia 2018;61:1249–60. [DOI] [PubMed] [Google Scholar]
- [2].Xu Y, Wang L, He J, et al. Prevalence and control of diabetes in Chinese adults. JAMA 2013;310:948–59. [DOI] [PubMed] [Google Scholar]
- [3].Liu Z, Fu C, Wang W, et al. Prevalence of chronic complications of type 2 diabetes mellitus in outpatients - a cross-sectional hospital based survey in urban China. Health Qual Life Outcomes 2010;8:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Yang L, Shao J, Bian Y, et al. Prevalence of type 2 diabetes mellitus among inland residents in China (2000-2014): a meta-analysis. J Diabetes Investig 2016;7:845–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Yang W, Lu J, Weng J, et al. Prevalence of diabetes among men and women in China. N Engl J Med 2010;362:1090–101. [DOI] [PubMed] [Google Scholar]
- [6].Liu L, Geng J, Wu J, et al. Prevalence of ocular fundus pathology with type 2 diabetes in a Chinese urban community as assessed by telescreening. BMJ Open 2014;3:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Choi JK, Lym YL, Moon JW, et al. Diabetes mellitus and early age-related macular degeneration. Arch Ophthalmol 2011;129:196–9. [DOI] [PubMed] [Google Scholar]
- [8].Tan GS, Wong TY, Fong CW, et al. Singapore Malay Eye Study. Diabetes, metabolic abnormalities, and glaucoma. Arch Ophthalmol 2009;127:1354–61. [DOI] [PubMed] [Google Scholar]
- [9].Tang Y, Wang X, Wang J, et al. Prevalence and causes of visual impairment in a Chinese adult population: the Taizhou eye study. Ophthalmology 2015;122:1480–8. [DOI] [PubMed] [Google Scholar]
- [10].Zhang G, Chen H, Chen W, et al. Prevalence and risk factors for diabetic retinopathy in China: a multi-hospital-based cross-sectional study. Br J Ophthalmol 2017;101:1591–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sapkota R, Chen Z, Zheng D, et al. The profile of sight-threatening diabetic retinopathy in patients attending a specialist eye clinic in Hangzhou. China BMJ Open Ophthalmol 2019;4:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Walton OB, 4th, Garoon RB, Weng CY, et al. Evaluation of automated teleretinal screening program for diabetic retinopathy. JAMA Ophthalmol 2016;134:204–9. [DOI] [PubMed] [Google Scholar]
- [13].Villena JE, Yoshiyama CA, Sanchez JE, et al. Prevalence of diabetic retinopathy in Peruvian patients with type 2 diabetes: results of a hospital-based retinal telescreening program. Rev Panam Salud Publica 2011;30:408–14. [PubMed] [Google Scholar]
- [14].von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg 2014;12:1495–9. [DOI] [PubMed] [Google Scholar]
- [15].Willekens K, Bataillie S, Sarens I, et al. Funduscopic versus HRT III confocal scanner vertical cup-disc ratio assessment in normal tension and primary open angle glaucoma (The Leuven Eye Study). Ophthalmic Res 2017;57:100–6. [DOI] [PubMed] [Google Scholar]
- [16].Zheng Y, Wong TY, Cheung CY, et al. Influence of diabetes and diabetic retinopathy on the performance of Heidelberg retina tomography II for diagnosis of glaucoma. Invest Ophthalmol Vis Sci 2010;51:5519–24. [DOI] [PubMed] [Google Scholar]
- [17].Pang C, Jia L, Hou X, et al. The significance of screening for microvascular diseases in Chinese community-based subjects with various metabolic abnormalities. PLoS One 2014;9:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Liu L, Yue S, Wu J, et al. Prevalence and risk factors of retinopathy in patients with or without metabolic syndrome: a population-based study in Shenyang. BMJ Open 2015;5:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Thapa R, Bajimaya S, Bouman R, et al. Intra- and inter-rater agreement between ophthalmologist and mid-level ophthalmic personnel to diagnose retinal diseases based on fundus photographs at a primary eye center in Nepal: the Bhaktapur Retina Study. BMC Ophthalmol 2016;16:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chen M, Wu A, Zhang L, et al. The increasing prevalence of myopia and high myopia among high school students in Fenghua city, eastern China: a 15-year population-based survey. BMC Ophthalmol 2018;18:1–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Song P, Wang J, Bucan K, et al. National and subnational prevalence and burden of glaucoma in China: a systematic analysis. J Glob Health 2017;7:114–31. [DOI] [PMC free article] [PubMed] [Google Scholar]


