Abstract
This study aims to describe the role of open surgical treatment for focal brainstem gliomas (FBSGs) with the assistance of multimodal neuronavigation and intraoperative neurophysiological monitoring (IOM) in children to investigate the efficacy of microsurgical treatment in pediatric FBSGs. Also the prognostic factors related to the overall survival (OS) of FBSGs to describe the patient and tumor characteristics relevant to prognosis/outcome were focused on. Clinical data of 63 pediatric patients below 16 years of age with FBSGs admitted to the Neurosurgical Unit of Beijing Tiantan Hospital from January 2012 to December 2018 were retrospectively analyzed. All patients underwent initial surgical treatment, followed by magnetic resonance diffusion tensor imaging (DTI), neuronavigation and IOM. Gross or near total resection (GTR or NTR) was achieved in 57/63 (90.5%) cases, and subtotal resection (STR) was achieved in 6/63 (9.5%) cases. Postoperative adjuvant therapy was received by 27/63 (42.9%) cases. Postoperative pathological examination revealed that 36/63 (57.1%) cases had grade I gliomas, 22/63 (34.9%) had grade II, and 5/63 (8.0%) had grade III–IV gliomas according to the WHO classification. The mean Karnofsky score preoperatively was 60, and at the time of follow-up was 90. Consecutively, 6 cases demonstrated disease progression, and 5 of these were deceased. The OS in all patients was 81.2% at 5 years. Histological grade (P < .001) and age at diagnosis (P = .023) showed significant association with prolonged OS. Multimodal neuronavigation and IOM allow very precise intracranial surgery, contributing to a maximally safe resection that might decrease the postoperative disability and mortality rate. This study also showed that pediatric FBSGs were mostly low-grade tumors with excellent surgical outcomes. Consequently, it is suggested that microsurgery can be used to treat FBSGs in children in order to provide better prognosis and survival outcomes.
Keywords: focal brainstem gliomas, intraoperative neurophysiological monitoring, magnetic resonance diffusion tensor imaging, microsurgery, neuronavigation
1. Introduction
Brainstem gliomas (BSGs) account for approximately 10% to 20% of all pediatric central nervous system (CNS) tumors, and are characterized by poor prognosis.[1] According to magnetic resonance imaging (MRI) characteristics, BSGs are divided into two groups, either focal or diffuse, regardless of tumor epicenter.[2,3] Compared with highly malignant diffuse tumors, pediatric focal brainstem gliomas (FBSGs) are mostly benign lesions histologically with prolonged duration of disease and more favorable surgical outcomes.[5,6] Histologically low grade malignancy and insensitivity to chemotherapy and radiotherapy allows radical surgical resection to be the primary therapy for FBSGs.[7,8] Although complete surgical resection is remarkably effective, it cannot be conducted with the expense of brainstem function, and it also showed close association with poor prognosis postoperatively.[9,10] The fundamental aim of FBSG surgical treatment is to provide safe resection and protect the brainstem.[11] As the BSGs were surrounded by dense fiber bundles, cranial nerve nuclei and the vital center, operations on these lesions more easily cause dysfunction or mortality due to destruction of these important structures.[12,13] So, these are still considered as one of the most difficult tumors to treat.[14] In general, invasive approaches should not be performed to avoid permanent damage to the nerve tissues.
In recent years, advanced imaging techniques have been widely used to improve safe resection of neurosurgical operations. Among them, neuronavigation provides real-time images of tumor edges through multi-modal image fusion and three-dimensional (3D) reconstruction.[15] Additionally, neuronavigation not only helps surgeons to plan reconstruction steps before surgery and identify important structures that might be hidden by tumors, but also modulates craniotomy to obtain optimal access to the tumors, especially when the tumor is close to the eloquent brain or is deeply seated.[16,17] It is critically important to understand the microsurgical anatomy, and also the function of the location of the brainstem in which the lesion lies. Of note, this might be facilitated by the use of intraoperative neurophysiological monitoring (IOM).[18] IOM is also used to locate the nerve route to indicate stimulation and damage caused to the nerve, and assists in understanding and protecting the brainstem function during resection.[19,20]
Therefore, multimodal neuronavigation systems and IOM-assisted microsurgical resection are regarded as favorable therapeutic strategies for brainstem tumors. The current study aimed to evaluate the effectiveness of microsurgery on pediatric FBSGs as well as to investigate the usefulness and accuracy of neuronavigation system based on preoperative imaging and neurophysiological technology in locating the intracranial structures that aid to facilitate these surgeries.
2. Materials and methods
2.1. Study size and design
In this retrospective study, the medical records of all children diagnosed with FBSGs who underwent treatment at the Neurosurgical Unit of Beijing Tiantan Hospital from January 2012 to December 2018 were reviewed. The inclusion and exclusion criteria were strictly followed. The inclusion criteria were as follows: (1) pediatric patients with less than or equal to 16 years, (2) patients with initial gliomas, (3) pediatric patients diagnosed with FBSG by a neuroimaging doctor and two neurosurgery experts with high professional titles, and (4) patients with typical clinical symptoms and MRI findings of FBSGs, or patients histopathologically diagnosed with BSGs. Patients with the following characteristics were excluded: (1) patients with recurrent gliomas or other diseases in the brain, such as space-occupying lesions and cerebrovascular diseases in other parts, (2) BSGs co-existing with other diseases that might seriously affect the survival time and quality of life, such as severe cardiopulmonary diseases, (3) complicated with serious systemic diseases or mental illnesses, and (4) patients who previously received surgical intervention or adjuvant chemo-/radiation therapy. Clinical data concerning demographic, clinical manifestations, imaging characteristics, histopathology, treatment, clinical outcomes, and follow-up were systematically collected for all patients. Informed consent form was provided by all subjects who participated in this study. This retrospective study was approved by the Institutional Review Board (XTYB201822).
Resection was planned with the goal to maximally resect the tumor by preventing the nerve (and function) of the brainstem from damage. All operations were performed by senior neurosurgeons with >10 years of experience in the field of neurosurgery. The pathological specimens were independently reviewed by neuropathologists in our hospital. All diagnoses were conducted according to the recommendations of the 2016 World Health Organization (WHO) Classification of Tumors of the Central Nervous System.
2.2. Imageological examination
Preoperative comprehensive radiographic examination was performed, which included the following: T1-weighted MRI with and without contrast, T2-weighted MRI, and diffusion tensor imaging (DTI). All tumors were assessed for size, signal characteristics, solid and/or cystic nature, degree of enhancement and location by MRI. The MRI images were interpreted by a board-certified fellowship-trained neuroradiologist. According to the intraoperative estimate and findings of postoperative MRIs, the extent of resection was defined as gross tumor resection (GTR, ≥99% resection), near total resection (NTR, 90%–99% resection), subtotal resection (STR, 70%–90% resection), or partial resection (PR, <70% resection). Exophytic patterns were defined as BSGs that are expanded outwards but do not infiltrate inwards. Endophytic limitation patterns were defined as well-defined localized.
2.3. Neurophysiological monitoring
Intraoperative examination of neurophysiological monitoring (Nicolet, Madison, WI, USA) mainly included the brainstem auditory-evoked potential (BAEP), brainstem somatosensory-evoked potential (SSEP), brainstem motor-evoked potential (MEP), and cranial nerves monitoring. These were used to identify specific nerve structures and evaluate their functions, monitor the systemic changes during the operation and predict the postoperative functional status of cranial nerves. For monitoring cranial nerves, BAEP, SEP and MEP aim at brainstem sensation, motor conduction pathway, and auditory somatosensory conduction pathway, respectively. Therefore, to determine the effect of intraoperative operation on brainstem function, and guide the surgical process and extend the resection, joint monitoring was conducted.
2.4. Surgical methods
-
(1)
Intraoperative neurophysiological monitoring:
The choice of surgical approach was determined based on tumor location and results of the course of corticospinal tract (DTI tractography). With the cooperation of the Anesthesiology Department, the inhaled muscle relaxant drug was ceased after opening of the bone flap, and the amount of intravenous anesthesia was maintained in sufficient amounts to reduce the interference of other anesthetics on electrophysiology studies. Neurophysiological monitoring techniques such as somatosensory evoked potentials (SSEPs), membrane evoked potentials (MEPs), brainstem evoked potentials (BAEPs), and cranial nerves monitoring techniques were introduced to preserve the continuity of the corticospinal tract, accurately locate the anatomical trend of the cranial nerves and provide feedback regarding the functional status of the brainstem.
-
(2)
Intraoperative neuronavigation:
Due to large number of brainstem neurovascular structures and complex shapes, even the localized tumors have likely caused compression, distortion as well as damage to these structures. DTI was used to determine the degree of tumor infiltration before surgery and visualize the anatomical relationship between the tumor and the adjacent white matter bundle. To elucidate the brainstem and tumor structure, radiographic imaging data, including MRI and DTI, were transferred into the neuronavigation system (Stealth Station 7; Medtronic, Minneapolis, MN, USA) for multimodal image fusion and three-dimensional (3D) image reconstruction using Stealth-Merge software (Medtronic). The reconstruction demonstrated the spatial relationship of the bones, blood vessels, and lesions with different colors in a single illustration (Fig. 1). Based on the 3D reconstruction model, the surgeon's field of vision was simulated to select the best surgical approach.
Figure 1.
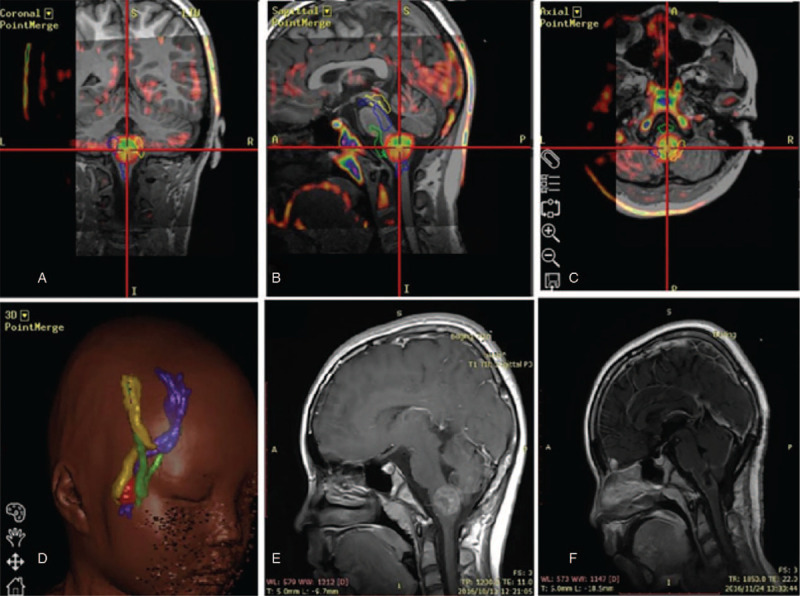
A 10-year-old female pediatric patient underwent tumor resection under the assistance of magnetic resonance diffusion tensor imaging (DTI) multimodal neuronavigation. (A–C) The results revealed that the tumor was located in the medulla and the surrounding cone bundle was compressed and displaced. (D) The green ones represent the pyramidal bundles, and the yellow and blue ones represent the bilateral sensory bundles. (E) Preoperative MRI scans displayed the lesion with limited enhancement. (F) Postoperative MRI scans at 3 months showed a gross-total resection (GTR) of the lesion.
Under the guidance of fiberoptic bundle navigation and intraoperative neurophysiological monitoring, the cranial nerve around the brainstem, which is the important conduction bundle, and the nucleus in the brainstem were avoided, and cut the brainstem along the direction of the fiber bundle to select the nearest area to the tumor on the surface of the brain stem. During surgery, the mechanical traction on the brainstem should be minimized, and the damage to the normal blood supply arteries and drainage veins of the brainstem should be avoided as much as possible.
2.5. Postoperative treatment and follow-up
The patients with residual tumors received additional adjuvant radiotherapy, while those with pathologically confirmed high-grade gliomas postoperatively received routine chemotherapy with temozolomide. All patients underwent long-term follow-up regularly (every 3–6 months) and underwent head MRI scan periodically. The follow-up period ranged from 6 months to 60 months (mean follow-up 38 months). The long-term quality of life was assessed using the Karnofsky performance status (KPS) score. To assess the treatment efficacy, the overall survival (OS) rates were calculated.
2.6. Statistical methods
SPSS 19 statistical software package (IBM Co., Armonk, NY, USA) was used for statistical analysis. Kaplan–Meier and Cox regression models were used for calculating the survival probability. OS was defined as the time from diagnosis till death due to any cause or the last follow-up visit. The variables in the Kaplan–Meier curves were evaluated by log-rank test. P < .05 was regarded as significant for all.
3. Results
3.1. Clinical features
A total of 63 children who met the inclusion criteria were enrolled in this study. There were 32 (50.8%) males and 31 (50.2%) females with a gender ratio of approximately 1:1. The age at diagnosis ranged from 1 mto 16 years (median 8 years), and the disease course ranged from 0.5 to 72 months (mean 12.8 months). The patient demographics, tumor location, and presenting symptoms are presented in Table 1. The following symptoms were reported by patients during admission to the Department of Neurosurgery: limb weakness by 24 (38.1%); hydrocephalus by 23 (36.5%); headache by 18 (28.6%); vomiting by 18 (28.6%); cranial nerve disorder (including dysphagia, double vision) by 16 (25.4%) and ataxia by 9 (14.3%) patients.
Table 1.
Patient characteristics of this group (n = 63).

3.2. Location of the tumor
According to lesion location, 21 (33.3%) patients had lesions in the pons, 12 (19.0%) had lesions in the medulla, 11 (17.5%) had lesions in the midbrain, and 19 (30.2%) had lesions involved in multiple sites. Next, 14 (22.2%) endophytic and 49 (77.8%) exophytic limitations were observed (Table 1). Additionally, 16 (25.4%) patients had cystic changes. Except a case with midbrain tumor and 2 with pons medullary tumor, the remaining showed contrast-enhancement, in which most of them were mainly localized homogeneous enhancements, while a few demonstrated non-uniform or non-typical annular reinforcement. DTI examination revealed that brainstem nerve fiber bundles in all patients were completely continuous or slightly damaged and squeezed by the lesions.
3.3. Degree of surgical resection and pathological grade
All 63 patients underwent initial surgical resection, and subsequent postoperative MRI re-examination confirmed 57 (90.5%) patients with GTR or NTR, whereas 6 (9.5%) patients with STR. Histopathological examination of tumor specimens revealed 36 (57.1%) cases with grade I pilocytic astrocytoma, 22 (34.9%) with grade II astrocytoma, and 5 (8.0%) with grade III-IV gliomas (WHO classification), including anaplastic astrocytomas and glioblastomas (Table 2).
Table 2.
Postoperative outcomes.

3.4. Survival and prognosis
In this group, 15 (23.8%) patients received postoperative adjuvant radiotherapy alone, 7 (11.1%) had chemotherapy alone, and 5 (7.9%) had combination radiotherapy plus chemotherapy following surgery (Table 2). The preoperative mean KPS score was 60, whereas it was 70 during discharge from the hospital. Postoperatively, 33 (52.4%) patients showed improvement in their neurological deficits, while 24 (38.1%) showed deterioration. During the follow-up period, 6 patients showed progression, and 5 of these died achieving an OS of 92% (Table 2). Pathological examination confirmed that 3 patients died due to grade III FBSGs and 2 from grade IV. In addition, the tumors in all 5 cases involved the pons, and the remaining tumors showed no progression in the remaining 57 (90.5%) patients. Most of them had a complete or significant reduction of neurological deficits within 6 months, and school-aged children could go to school and lead a normal life. More interestingly, the mean KPS score reached 90 during the last follow-up visit.
The Kaplan-Meier survival curves illustrated the overall disease progression in patients with FBSGs (Fig. 2). The OS of the entire cohort with localized lesions was 81.2% at 5 years. In our study, the survival rates were directly related to tumor pathology. For patients with low-grade FBSGs, the mean OS was 23 months and that of high-grade FBSGs was 13 months, showing statistically significant differences (P < .001). Furthermore, the survival rates showed association with age at diagnosis. The mean OS in patients <10 years of age was 18.7 months, while those in patients ≥10 years was 23.9 months (P = .023), indicating a significant increase in the survival time of older children. Nevertheless, the extent of resection, either GTR/NTR or STR, showed no correlation with OS (P = .306, Fig. 3).
Figure 2.
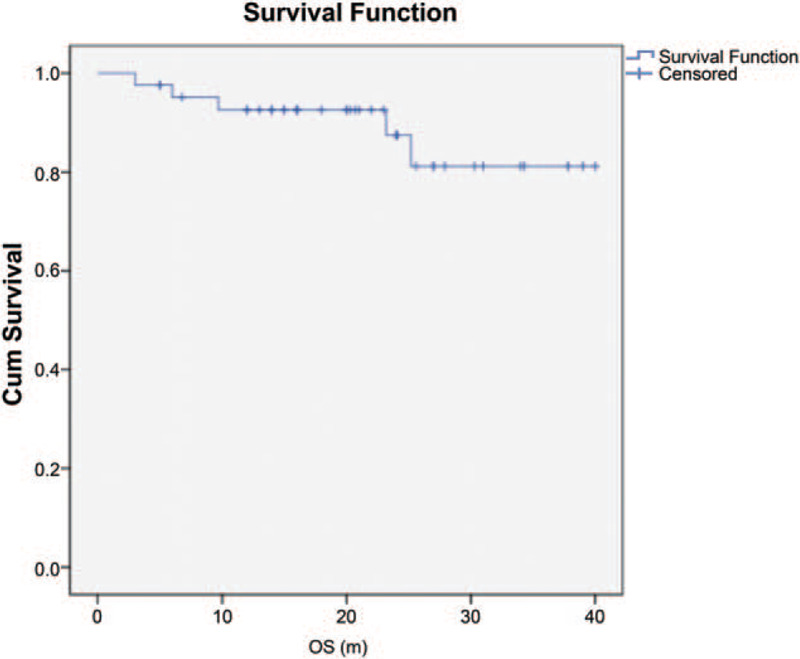
Kaplan-Meier survival curves in all patients with FBSGs. 5 yr OS: 81.2% ± 8.4%. Cum, cumulative; OS, overall survival; m, months.
Figure 3.
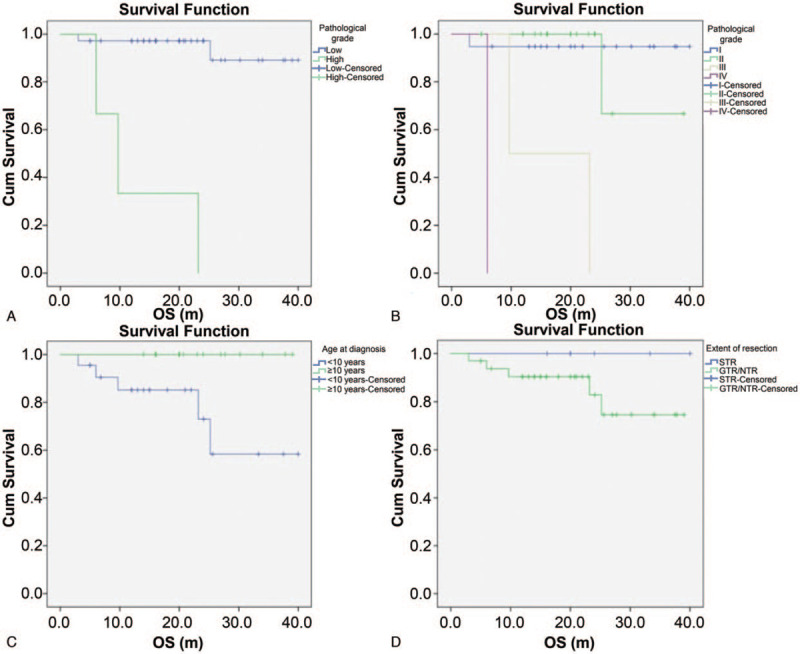
Kaplan-Meier survival curves in all patients with FBSGs according to the pathological grade (P < .001) (A, B), the age at diagnosis (P = .023) (C) and the degree of surgical removal (P = .306) (D). Cum, cumulative; OS, overall survival; m, months.
4. Discussion
Although FBSGs are low malignant tumors with relatively good prognosis, the important physiological function and complex anatomical structure of brainstem have significantly limited the treatment effectiveness of surgical resection.[21,22] With the development of imaging technology and the progression of surgical techniques, the results of surgical resection of low-grade BSGs have been greatly improved, and the postoperative quality of life of patients has been significantly improved. However, research on surgical treatment of FBSGs in children is still lacking. Our preliminary study showed that children with FBSGs achieved good results after receiving microsurgical treatment. The integration of multimodal neuronavigation system with IOM technology added a great value for microsurgical treatment of pediatric patients with FBSGs, providing abundant information with regard to preoperative planning, intraoperative guidance and postoperative complications prediction.
In the current study, all children with FBSGs received microsurgical resection under the assistance of multimodal neuronavigation system and IOM. Encouragingly, the results achieved a GTR or NTR (≥90% tumor removal) in 57 patients (90.5%), with a higher percentage than that reported previously by Lesniak MS and Sandri A for standard microsurgical removal of FBSGs without intraoperative imaging guidance, and showed significant improvement in postoperative neurological outcomes.[23,24] This demonstrated excellent prognosis in children with FBSGs, and a real-time imaging guidance for tumor resection was provided by neuronavigation and IOM, significantly improving the surgical outcomes of resection of FBSGs when compared with traditional microsurgery.
The following are the key points in microsurgical treatment for pediatric FBSGs in analyzing the diagnosis and treatment process:
4.1. Indications and contraindications for surgery
The extent of FBSGs is limited, and the tumor pushes the important nerve fibers and nuclei to one side, thus making the surgical area relatively safe. As microsurgical treatment of low-grade and localized tumors achieves satisfactory results, aggressive surgery for patients with FBSGs was recommended. However, not all patients with focal lesions are suitable for undergoing microsurgical resection. Preoperatively, patients based on the severity of symptoms, rate of disease progression, general condition and MRI examination results were deemed to be suitable for surgical treatment. The indications for surgery were as follows: patients (1) presenting significant clinical symptoms; (2) presenting mass effect by occupying, pressing or pushing adjacent nerves and tissues; (3) showing tumor progression on imaging; (4) with hydrocephalus; and (5) in some cases, although the tumor is large, the progression remains slow or the neurologic deficits is not obvious, and the high intracranial pressure caused by hydrocephalus is regarded as the main performance. Such a condition indicates that the tumor has low malignancy, without any distinct invasion and damage. After removing the tumor by surgery, the morphology and function of severely compressed and deformed brainstem might return to a normal state. Therefore, these patients primarily undergo surgical resection. Conversely, the pediatric patients with multiple cranial nerve injury before surgery, especially those with severe posterior cranial nerve function injury or severe failure of brainstem function, were recommended for contraindications of surgery.
4.2. Key points in surgery
All patients with FBSGs were treated with craniotomy microsurgery. The primary principle of this surgical procedure is to maximize safe resection by decreasing the postoperative neurologic deficits and improving the survival rate.[11] The choice of surgical approach is based on the principle that the weakest part of the brain stem or exophytic tumor can be reached to the base of brain stem under direct vision. The brain stem was cut along the direction of the fiber bundles by avoiding important nerve vessels. Combined with DTI fusion navigation, the intratumoral decompression is performed in the position of the tumor without the conduction bundle and the nucleus, and then the treatment within the minimum damage range is performed for the interface between the lesion and the conduction beam, and the tumor is removed according to the natural channel of the tumor. In case of exophytic tumors, it is not necessary to open the brainstem. The tumor inside the brainstem is further removed by excising the extra-brain tumor. For limited endophytic tumors, most of the superficial part of the tumor is selected to open the brainstem vertically. For tumors located in the medulla oblongata, care should be taken to avoid the obex and the changes in the respiratory and circulatory systems during the operation. Consecutively, the extent of lesion resection and its correlation with the surrounding fiber bundles and cranial nerves were dynamically elucidated by neuronavigation to achieve maximum resection of the lesion and protection of the functional areas.[25,26]
However, the potential disadvantage of intraoperative brain shift, which is mainly caused by cerebrospinal fluid (CSF) drainage and tumor tissue removal, severely reduced the accuracy of neuronavigation system in identifying the important brainstem structures, leading to poor surgical outcomes.[20,27] In this study, intraoperative electrical stimulation was employed under neurophysiological monitoring to map the limitations by identifying the displaced structures, correcting the misjudgments, and guiding the surgery in real-time.[18] In addition, to minimize the loss of CSF or cystic fluid before reaching the target site, the occurrence of drift and the impact of surgical accuracy were reduced.[28,29]
For monitoring cranial nerves, the SEP, MEP and BAEP mainly targeted the brainstem sensory pathway, motor conduction pathway and pons auditory somatosensory pathway, respectively. Therefore, combined monitoring was adopted to determine the influence of intraoperative operation on brainstem function. If changes occur in neurophysiological monitoring during tumor resection, then the surgery should be discontinued. After monitoring returns to normal, then the tumor should be removed from other parts of the lesion.
4.3. Factors affecting prognosis
In this study, the factors affecting the prognosis of FBSG in pediatric patients included the pathological grade of the tumor, and decreased survival time with increasing WHO grade, which were basically consistent with the results of domestic and foreign studies.[9,24] In addition, the age of the children showed correlation with survival. This study showed that patients younger than 10 years had worse prognosis, which might be due to young age of the patients that worsen their tolerance to diseases and treatment measures.
Several recent studies have analyzed the effect of contrast-enhanced MRI on the prognosis of BSGs, but revealed mixed results. In the studies conducted by Moghrabi and Albright et al,[4] no correlation was observed between contrast-enhanced lesions and OS. However, Lesniak et al believed that MRI enhanced the signs of brainstem tumors in children, suggesting a good prognosis.[23] This study showed that the presence or absence and the mode of enhancement in pediatric FBSGs demonstrated significant effects on prognosis. Our study deduced that the tumor with overall enhancement was mostly pilocytic astrocytomas, while those with central enhancement or cavity wall enhancement were mostly astrocytomas or oligo-astrocytomas. The pathological grade of these tumors remained low, and usually no brainstem tissue was detected inside the enhanced lesion, and clinical prognosis was relatively well. In contrast, the cases with multi-center enhancement or sparse enhancement have high pathological grades, the brainstem conduction bundles or nerve nucleus could putatively occur in the tumor lesions, and the possibility of new neurological deficits caused by surgery remained relatively higher.[30,31]
Previous study has suggested that the extent of surgical resection is the most important factor affecting the prognosis.[28] The present study showed no statistically significant differences in the survival rate between patients with varied extent of resections, attributing to a positive result for intraoperative navigation and neurophysiological monitoring in predicting the respectability of the tumor. In addition, the tumor grade in these patients remained low and associated with slow progression. The short follow-up time might lead to errors, which require further improvement in the future studies.
4.4. Treatment of postoperative complications
The common complications after brainstem tumor surgery included brainstem edema, respiratory dysfunction, and posterior cranial nerve dysfunction (expressed by cough reflex, drinking cough, and dysphagia). The focus of the treatment with regard to these complications involves stability of respiratory and circulatory systems and timely tracheotomy if necessary, which is determined to be crucial for good clinical outcomes in patients with FBSGs.
The major drawbacks in our study include limited number of patients and their clinical data. Due to rarity of these tumors in children, the results might be biased by the sample size. Nevertheless, this is a preliminary study, and patients with FBSGs showed significant benefits after surgical removal. This further encourages conduction of more studies in the future to validate the effect of surgery, as well as the effectiveness and accuracy of neuronavigation and IOM in patients with FBSGs.
5. Conclusions
This single-center retrospective study demonstrated that the histopathological grade of pediatric FBSGs remained generally low, and satisfactory results can be achieved by surgical treatment. The combination of multimodal neuronavigation systems with IOM techniques demonstrated obvious advantages during surgical resection of brainstem functional area, increasing the tumor resection rate and decreasing the postoperative complications while protecting the functional fiber bundles. Also it is suggested that microsurgery can be actively pursued for pediatric FBSGs with clear indications.
Author contributions
Sun Tao: Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Software, Visualization, Roles/Writing - original draft, Writing - review & editing. Xu Yan: Data curation, Formal analysis, Investigation, Methodology, Software, Visualization, Roles/Writing - original draft, Writing - review & editing. Pan Changcun: Formal analysis, Validation. Liu Yuhan: Data curation, Methodology. Tian Yongji: Investigation, Methodology, Resources. Li Chunde: Investigation, Methodology, Software. Di Fei: Conceptualization, Data curation, Funding acquisition, Methodology, Project, Supervision, Writing - review & editing. Zhang Liwei: Conceptualization, Data curation, Methodology, Project, Supervision, Writing - review & editing.
Footnotes
Abbreviations: BAEP = brainstem auditory-evoked potential, BSGs = brainstem gliomas, CI = confidence interval, DIPGs = diffuse intrinsic pontine gliomas, DTI = diffusion tensor imaging, GTR = gross total resection, IOM = intraoperative neurophysiological monitoring, KPS = Karnofsky Performance Scale, MEP = motor-evoked potential, MRI = magnetic resonance imaging, NTR = near total resection, OS = overall survival, PR = partial resection, SSEP = Somatosensory-evoked potential, STR = subtotal resection, WHO = World Health Organization.
How to cite this article: Sun T, Xu Y, Pan C, Liu Y, Tian Y, Li C, Di F, Zhang L. Surgical treatment and prognosis of focal brainstem gliomas in children: A 7 year single center experience. Medicine. 2020;99:36(e22029).
TS and YX contributed equally to this work.
We certify that our manuscript is a unique submission and is not being considered for publication by any other source in any medium.
This work was supported by The Special Fund of the Pediatric Medical Coordinated Development Center of Beijing Hospitals Authority (No. XTYB201822); Beijing-Tianjin-Hebei Collaborative innovation community construction project (No.18247788D). The sponsor had no role in the design or conduct of this research.
The institutional review committee of Beijing Tiantan Hospital approved the research program (XTYB201822). This study adhered to good clinical practice and ethical principles described in the Declaration of Helsinki, and was approved by the IRB of the authors’ institution. Written informed consent was obtained from all participants or their legally authorized representative.
All authors declare that they have no any conflict of interests.
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
- [1].Fisher PG, Breiter SN, Carson BS, et al. A clinicopathologic reappraisal of brain stem tumor classification. Identification of pilocystic astrocytoma and fibrillary astrocytoma as distinct entities. Cancer 2000;89:1569–76.. [DOI] [PubMed] [Google Scholar]
- [2].Barkovich AJ, Krischer J, Kun LE, et al. Brain stem gliomas: a classification system based on magnetic resonance imaging. Pediatr Neurosurg 1990;16:73–83.. [DOI] [PubMed] [Google Scholar]
- [3].Sun B, Wang CC, Wang J. MRI characteristics of midbrain tumours. Neuroradiology 1999;41:158–62.. [DOI] [PubMed] [Google Scholar]
- [4].Moghrabi A, Kerby T, Tien RD, et al. Prognostic value of contrast-enhanced magnetic resonance imaging in brainstem gliomas. Pediatr Neurosurg 1995;23:293–8.. [DOI] [PubMed] [Google Scholar]
- [5].Albright AL, Packer RJ, Zimmerman R, et al. Magnetic resonance scans should replace biopsies for the diagnosis of diffuse brain stem gliomas: a report from the Children's Cancer Group. Neurosurgery 1993;33:1026–9.. discussion 1029-1030. [DOI] [PubMed] [Google Scholar]
- [6].Donaldson SS, Laningham F, Fisher PG. Advances toward an understanding of brainstem gliomas. J Clin Oncol 2006;24:1266–72.. [DOI] [PubMed] [Google Scholar]
- [7].Freeman CR, Farmer JP. Pediatric brain stem gliomas: a review. International journal of radiation oncology, biology, physics 1998;40:265–71.. [DOI] [PubMed] [Google Scholar]
- [8].Hargrave D, Bartels U, Bouffet E. Diffuse brainstem glioma in children: critical review of clinical trials. Lancet Oncol 2006;7:241–8.. [DOI] [PubMed] [Google Scholar]
- [9].Bandopadhayay P, Bergthold G, London WB, et al. Long-term outcome of 4,040 children diagnosed with pediatric low-grade gliomas: an analysis of the Surveillance Epidemiology and End Results (SEER) database. Pediatr Blood Cancer 2014;61:1173–9.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Reyes-Botero G, Mokhtari K, Martin-Duverneuil N, et al. Adult brainstem gliomas. Oncologist 2012;17:388–97.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sinha S, Kale SS, Chandra SP, et al. Brainstem gliomas: surgical indications and technical considerations in a series of 58 cases. Br J Neurosurg 2014;28:220–5.. [DOI] [PubMed] [Google Scholar]
- [12].Leblond P, Vinchon M, Bernier-Chastagner V, et al. Diffuse intrinsic brain stem glioma in children: current treatment and future directions. Archives de Pediatrie 2010;17:159–65.. [DOI] [PubMed] [Google Scholar]
- [13].Mehta VS, Chandra PS, Singh PK, et al. Surgical considerations for ’intrinsic’ brainstem gliomas: proposal of a modification in classification. Neurol India 2009;57:274–81.. [DOI] [PubMed] [Google Scholar]
- [14].Green AL, Kieran MW. Pediatric brainstem gliomas: new understanding leads to potential new treatments for two very different tumors. Curr Oncol Rep 2015;17:436. [DOI] [PubMed] [Google Scholar]
- [15].Dolati P, Gokoglu A, Eichberg D, et al. Multimodal navigated skull base tumor resection using image-based vascular and cranial nerve segmentation: a prospective pilot study. Surg Neurol Int 2015;6:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Enchev Y. Neuronavigation: geneology, reality, and prospects. Neurosurg Focus 2009;27:E11. [DOI] [PubMed] [Google Scholar]
- [17].Ganslandt O, Behari S, Gralla J, et al. Neuronavigation: concept, techniques and applications. Neurol India 2002;50:244–55.. [PubMed] [Google Scholar]
- [18].Sala F, Coppola A, Tramontano V, et al. Intraoperative neurophysiological monitoring for the resection of brain tumors in pediatric patients. J Neurosurg Sci 2015;59:373–82.. [PubMed] [Google Scholar]
- [19].Kim JH, Phi JH, Lee JY, et al. Surgical outcomes of thalamic tumors in children: the importance of diffusion tensor imaging, neuro-navigation and intraoperative neurophysiological monitoring. Brain Tumor Res Treat 2018;6:60–7.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wang X, Li L, Wang Y, et al. Clinical application of multimodal neuronavigation system in neuroendoscope-assisted skull base chordoma resection. J Craniofac Surg 2017;28:e554–7.. [DOI] [PubMed] [Google Scholar]
- [21].Jallo GI, Biser-Rohrbaugh A, Freed D. Brainstem gliomas. Child's Nerv Syst 2004;20:143–53.. [DOI] [PubMed] [Google Scholar]
- [22].Roujeau T, Machado G, Garnett MR, et al. Stereotactic biopsy of diffuse pontine lesions in children. J Neurosurg 2007;107:1–4.. [DOI] [PubMed] [Google Scholar]
- [23].Lesniak MS, Klem JM, Weingart J, et al. Surgical outcome following resection of contrast-enhanced pediatric brainstem gliomas. Pediatr Neurosurg 2003;39:314–22.. [DOI] [PubMed] [Google Scholar]
- [24].Sandri A, Sardi N, Genitori L, et al. Diffuse and focal brain stem tumors in childhood: prognostic factors and surgical outcome. Experience in a single institution. Childs Nerv Syst 2006;22:1127–35.. [DOI] [PubMed] [Google Scholar]
- [25].Choudhri AF, Whitehead MT, Klimo P, Jr, et al. Diffusion tensor imaging to guide surgical planning in intramedullary spinal cord tumors in children. Neuroradiology 2014;56:169–74.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Trinh VT, Fahim DK, Shah K, et al. Subcortical injury is an independent predictor of worsening neurological deficits following awake craniotomy procedures. Neurosurgery 2013;72:160–9.. [DOI] [PubMed] [Google Scholar]
- [27].Rygh OM, Nagelhus Hernes TA, Lindseth F, et al. Intraoperative navigated 3-dimensional ultrasound angiography in tumor surgery. Surgical neurology 2006;66:581–92.. discussion 592. [DOI] [PubMed] [Google Scholar]
- [28].Helton KJ, Phillips NS, Khan RB, et al. Diffusion tensor imaging of tract involvement in children with pontine tumors. AJNR Am J Neuroradiol 2006;27:786–93.. [PMC free article] [PubMed] [Google Scholar]
- [29].Lui YW, Law M, Chacko-Mathew J, et al. Brainstem corticospinal tract diffusion tensor imaging in patients with primary posterior fossa neoplasms stratified by tumor type: a study of association with motor weakness and outcome. Neurosurgery 2007;61:1199–207.. discussion 1207-1198. [DOI] [PubMed] [Google Scholar]
- [30].Epstein F, Wisoff J. Intra-axial tumors of the cervicomedullary junction. J Neurosurg 1987;67:483–7.. [DOI] [PubMed] [Google Scholar]
- [31].Pierre-Kahn A, Hirsch JF, Vinchon M, et al. Surgical management of brain-stem tumors in children: results and statistical analysis of 75 cases. J Neurosurg 1993;79:845–52.. [DOI] [PubMed] [Google Scholar]


