Abstract
Objective:
To examine the efficacy of combined inspiratory and expiratory respiratory muscle training (RMT) with respect to the swallowing function, pulmonary function, functional performance, and dysarthria in patients with stroke.
Design:
Prospective, randomized controlled trial.
Setting:
Tertiary hospital.
Participants:
The trial included 21 subjects (12 men, 9 women) aged 35 to 80 years presenting with 6 months history of unilateral stroke, respiratory muscle weakness (≥70% predicted maximal inspiratory pressure (MIP) and/or ≤70% maximal expiratory pressure (MEP)), dysphagia, or dysarthria. These subjects were randomly assigned to the control (n = 10, rehabilitation) and experimental (n = 11, rehabilitation with RMT) groups.
Intervention:
Inspiratory RMT starting from 30% to 60% of MIP and expiratory RMT starting from 15% to 75% of MEP for 5 days/week for 6 weeks.
Main outcome measures:
MIP, MEP, pulmonary function, peak cough flow, perception of dyspnea, Fatigue Assessment Scale, Modified Rankin Scale, Brunnstrom stage, Barthel index, Functional Oral Intake Scale (FOIS), and parameters of voice analysis.
Results:
Significant differences were observed between both groups in terms of MIP, forced vital capacity (FVC), and forced expiratory volume per second (FEV1) of the percentage predicted. Significant difference was found with respect to the change in fatigue, shimmer percent, amplitude perturbation quotient, and voice turbulence index (VTI) according to the acoustic analysis in the RMT group. The FEV1/FVC ratio was negatively correlated with jitter percent, relative average perturbation, pitch perturbation quotient, and VTI; the maximum mid-expiratory flow (MMEF) and MMEF% were also negatively correlated with VTI. Significant differences among participants of the same group were observed while comparing the Brunnstrom stage before and after training of the affected limbs and the Barthel scale and FOIS scores in both the groups.
Conclusions:
Altogether, 6-week combined inspiratory and expiratory RMT is feasible as adjuvant therapy for stroke patients to improve fatigue level, respiratory muscle strength, lung volume, respiratory flow, and dysarthria.
Clinical trial registration number (Clinical Trial Identifier): NCT03491111.
Keywords: stroke, dysphagia, respiratory muscular training, acoustic analysis, functional performance
1. Introduction
Stroke patients often experience respiratory muscle weakness, swallowing disturbances,[1–3] decreased peak expiratory flow, blunted reflexive cough, impaired voluntary cough,[4] impairment of the cardiorespiratory fitness,[5] and voice dysfunction in dysarthria.[6]
An 8-week inspiratory muscle training (IMT) can increase the inspiratory muscle strength and endurance in chronic stroke patients with > 90% of predicted maximal inspiratory pressure (MIP),[7] while a 6-week IMT can increase the forced expiratory volume in the first second (FEV1), forced vital capacity (FVC), vital capacity, force expiratory flow rate 25% to 75%, and maximal voluntary ventilation in patients with unilateral stroke during the previous 12 months; this finding was also correlated with the exercise capacity, sensation of dyspnea, and quality of life.[8] Expiratory muscle training (EMT) can improve the MIP and peak expiratory flow rate in stroke patients[2] and improve the voice aerodynamics,[9] MEP, and swallowing ability, in acute stroke patients along with reducing vallecular residue and penetration-aspiration.[3]
Messaggi-Sartor et al reported that 3-week IMT of patients with 30% MIP and EMT of patients with 30% MEP could improve the inspiratory and expiratory muscle strength and potentially reduce the occurrence of respiratory complications at 6 months after the onset of acute stroke.[10] Furthermore, Guillen-Sola et al reported that 3-week inspiratory/expiratory muscle training could improve inspiratory and expiratory muscle strength and swallowing function.[11] However, the efficacy of combined IMT and EMT in subacute stroke patients (within 6 months) with respiratory muscle weakness, swallowing disturbance, and dysarthria has not been reported.
Respiration and swallowing require the activation of common anatomical structures. EMT can facilitate the contraction of submental muscles, elevate the hyolaryngeal complex,[12,13] pull the hyoid bone in the anterior-superior direction, and invert the epiglottis towards the pharynx during swallowing.[14–16] Dysarthria (including wet voice) and dysphagia have similar pathogeneses in stroke patients, especially those related to the laryngopharyngeal functions.[17] The acoustic change in phonation following a swallow is a high-risk indicator of fluid aspiration.[18] Moreover, the subglottal pressure initiates and maintains the vocal fold vibration that facilitates voice production.
Five-week EMT followed by 6 sessions of traditional voice therapy increased the subglottal pressure leading to a higher vocal intensity and increased voice dynamic range in professional voice users.[9] Meanwhile, a multi-dimensional voice program (MDVP) is suitable for voice analysis in dysarthria associated with various neurologic diseases of different severity,[6] and the MDVP Model 5105 (KayPENTAX) is reliable and advanced for speech analysis and acquisition.[19]
We hypothesized that the repetitive resistance, pressure, and force generated by threshold RMT could improve the respiratory muscle strength, swallowing function, and voice quality via sensory stimulation and motor activation of the oropharynx and respiratory muscles. RMT can also assist in the upregulation of reflex cough.[2] To our knowledge, this is the first follow-up study that investigated the feasibility and efficacy of a combined IMT and EMT with respect to pulmonary dysfunction, swallowing dysfunction, voice dysfunction due to dysarthria, and activities of daily living of subacute stroke patients.
2. Methods
2.1. Participants and setting
This prospective, single-blinded, randomized controlled study was conducted in a tertiary hospital from April 2016 to October 2018 with 47 unilateral stroke patients aged 35 to 80 years with respiratory muscle weakness, swallowing disturbance, or dysarthria for 6 months. The patients were screened by attending physicians and randomly divided into the control (conventional rehabilitation) and experimental (rehabilitation with RMT) groups by a research assistant using a random number generator algorithm. Signed informed consent from the patients or a family member was obtained, and the Institutional Review Board approved the study.
Sixteen subjects declined to participate, not meeting the inclusion criteria regarding inspiratory and expiratory muscle weakness (≥70% predicted MIP and/or ≤ predicted MEP).[20,21] In addition, patients with increased intracranial pressure, uncontrolled hypertension, decompensated heart failure, unstable angina, recent myocardial infarction, complicated arrhythmias, pneumothorax, bullae/blebs in the preceding 3 months, severe cognitive function or infection, recurrent stroke, brain stem stroke, and aphasia were excluded.
Each patient underwent physical and neurological examination, and assessment of clinical characteristics, height, weight, body mass index, duration of stroke, Modified Rankin scale (MRS), Brunnstrom stage, hand grip of unaffected upper limb, Barthel activity of daily living index, spirometry, peak cough flow, MIP, MEP, resting heart rate, perception of dyspnea using modified Borg scale,[22] resting oxyhemoglobin saturation, fatigue assessment scale (FAS),[23] functional oral intake scale (FOIS),[24] and voice quality.[18] These parameters were recorded before and after the 6-week RMT. The technician was blinded to the group allocation.
2.2. Intervention
Patients were trained using the Dofin Breathing Trainer (DT 11 or DT 14 GaleMed Corporation), a hand-held threshold trainer with a spring-loaded valve and a colored ball that indicates whether breathing strength exceeds the set target pressure. Ten training levels were set for IMT and EMT. The DT11 has a pressure range of 5 to 39 cmH2O during inspiration and 4 to 33 cmH2O during expiration, while DT14 has a pressure range of 5 to 79 cmH2O during inspiration and 4 to 82 cmH2O during expiration.
For IMT, the subjects were instructed to tightly seal their lips around the breathing trainer with a nose clip in a sitting position, and inhale deep and forceful breathes that were sufficient for opening the valve with a whistling sound (due to the movement of the colored ball inside the trainer). Then, they were instructed to exhale slowly and gently through the mouthpiece. The inspiratory training pressure ranged from 30% to 60% of each individual's MIP for 6 sets of 5 repetitions. For EMT, the subjects were instructed to blow fast and forcefully which could open the valve following maximal inhalation. Expiration training pressure commenced from 15% to 75% of threshold load of an individual's MEP for 5 sets of 5 repetitions, 1 to 2 times per day, 5 days a week for 6 weeks[2,25,26]; 1 to 2 minutes of rest was allowed between each set.
The training resistance was adjusted according to tolerance. We requested the patients to stop if they experienced discomfort and, in case of desaturation, the threshold load was decreased. The patients were called once a week for checking their compliance with the program and were encouraged to continue with it. A training diary was provided for them to keep a record.
In addition to RMT, both the groups underwent the regular rehabilitation, which included postural training, breathing control, improving cough technique, checking chest wall mobility, fatigue management, orofacial exercises, thermal-tactile stimulation, Mendelsohn maneuvering, effort swallowing, or supra-glottic maneuver among others.
2.3. Main outcome measurement
The primary outcome variables were: change in MIP (cmH2O) and MEP (cmH2O). For MIP, negative pressure is favorable and for MEP, positive pressure is favorable. The secondary outcome variables were the pulmonary functional parameters including FVC (liter), FVC (% prediction), FEV1 (liter), FEV1 (% of prediction), FEV1/FVC (%), maximum mid-expiratory flow (MMEF) (liter/s), MMEF%, peak cough flow (liter/s), resting heart rate, resting respiratory rate, FOIS [7-point scale, from 1 (nothing by mouth) to 7 (total oral diet with no restrictions)],[24] Modified Borg scale (0.5 to 10),[22] FAS (10-item, 5 levels (1: never to 5: always), score: 10 to 50),[23] non-affected hand grip strength, Barthel index (0 to 100),[27] MRS (5: severe disability to 0: no symptoms),[28] and the variables of acoustic analysis.
Pulmonary function test: Pulmonary function was assessed using a spirometer (Vitalograph, Serial Spirotrac, Buckingham, VA) as per the American Thoracic Society standards.[29]MIP and MEP: MIP was measured after maximal expiration near residual volume. MEP was measured after maximal inspiration near total lung capacity while patients were sitting and wearing a nose-clip in an upright position. All pressure measurements were maintained for at least 1 second. The highest recorded value was used for calculations only when two technically satisfactory measurements were obtained.[30,31]
Voice quality analysis: Voice quality was assessed with the Computerized Speech Lab (CSL), Model 4500 (Multi-Dimensional Voice). The participant was asked to phonate the vowel ‘a’ at their most comfortable speaking pitch and loudness for at least 3 seconds while sitting at a 30 cm distance from the microphone. The lowest pitch and highest pitch with increasing and decreasing loudness were measured.[6] The parameters of voice analysis included jitter percent (Jitt), relative average perturbation (RAP), and pitch perturbation quotient (PPQ) for frequency perturbation. Amplitude was determined based on the shimmer in decibels (ShdB), shimmer percent (Shim), amplitude perturbation quotient (APQ), and peak-to-peak amplitude variation, while the noise-related parameters included noise-to-harmonic ratio and voice turbulence index (VTI).[6]
2.4. Sample size calculation
Based on the study by Sutbeyaz et al,[8] the mean differences of MIP between experimental group and control group before and after IMT training were fixed at 7.87 cmH2O and 2.90 cmH2O, respectively, with standard deviation of 6.6 cmH2O and 1.9 cmH2O. After calculation, we realized that the study required at least 17 subjects in each group. While setting these conditions at a two-sided significance level at 0.05 with a statistical power of 0.80, the number of subjects in each group should be 24 under the estimation that the dropout rate was about 30%. Number of participants in the RMT group to that in the control group was set at 1:1 ratio.
2.5. Data analysis
Values were expressed as the mean ± standard deviation for continuous variables and number (%) for categorical variables. Linear regression analysis was used to adjust for sex, BMI, and the Brunnstrom stage of the distal part of the affected upper limb. Clinical characteristics were compared using the Mann–Whitney U test for continuous variables and the Fisher exact test for categorical variables. The Wilcoxon signed-rank test was used to examine the change in clinical data from baseline in both the groups, and the Mann-Whitney U test was applied for comparisons between the groups. The Spearman rank correlation coefficient was calculated to analyze the correlations between cardiopulmonary function parameters and clinical characteristics. All collected data were analyzed using the SPSS Statistics version 22.0 software (IBM, Armonk, NY). P value < .05 was considered statistically significant.
3. Results
A total of 47 patients were determined to be eligible initially. After exclusion of 16 patients, 31 were randomly allocated to the RMT (15 patients) and control (16 patients) groups. During training, 10 patients (32.2%) dropped out of the study, 5 from the RMT group (reasons being: they lived far away from the study venue, insisted to stay at home or in the nursing home, and had impaired vision in one eye and upper gastrointestinal bleeding) and 5 from the control group (reasons being: 4 patients did not undergo follow-up at the outpatient department and 1 patient had another disease). Finally, 21 patients completed the study (RMT group, n = 10; control group, n = 11) (Fig. 1). The Intention-To-Treat and Per Protocol analysis for all the data is shown in Tables 1 and 2.
Figure 1.

Design and flow of participants through the study.
Table 1.
Characteristics of patients in the training and control groups.
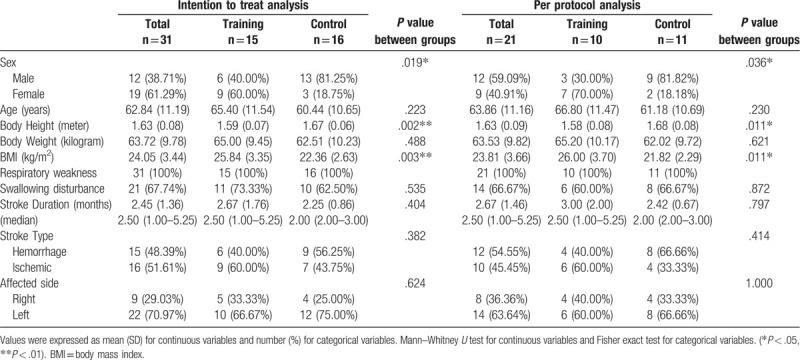
Table 2.
Functional and pulmonary baselines of patients in the training and control groups.
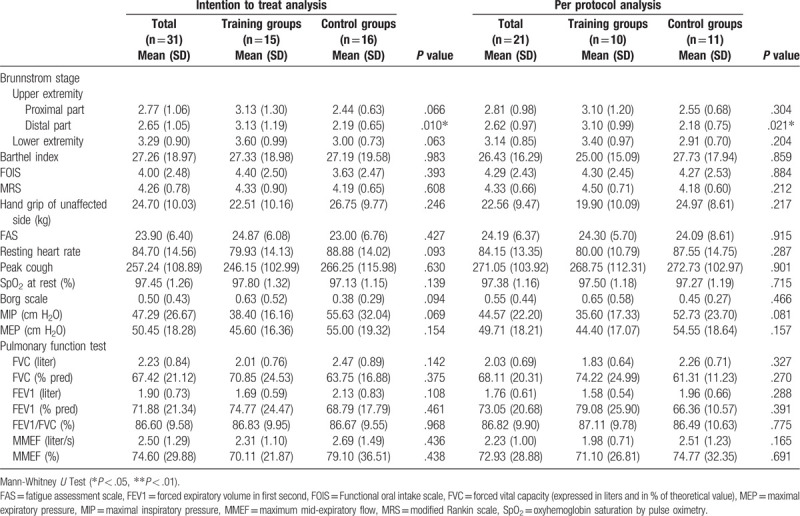
No statistically significant difference between the groups was noted in the clinical characteristics, cardiopulmonary function, and acoustic analysis parameters (Tables 1–3), except sex (P = .036), height (training vs control group: 1.58 ± 0.08 vs 1.68 ± 0.08 cm, P = .011), body mass index (BMI) (26.0 ± 3.7 vs 21.82 ± 2.29, P = .011 kg/m2) (Table 1), and Brunnstrom stage of the distal part of affected upper extremity (3.10 ± 0.99 vs 2.18 ± 0.75, P = .021) (Table 2).
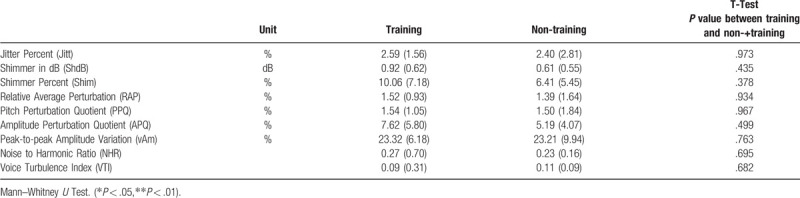
Table 3.
Data of Multi–Dimensional Voice report in the training and control groups.
Significant correlations were found between MIP and MEP (r = 0.632, P < .01); peak cough and MEP (r = 0.504, P < .05), FVC (r = 0.781, P < .01), and FEV1 (r = 0.739, P < .01); Borg scale and MEP (r = −0.505, P < .05); age and FVC (r = −0.536, P < .05), FEV1 (r = −0.590, P < .01), and MMEF (r = −0.584, P < .01); post-stroke duration and FVC (% predicted) (r = 0.594, P < .01), FEV1 (% predicted) (r = 0.458, P < .05), and FEV1/FVC (% predicted) (r = −0.456, P < .05) (Table 4).
Table 4.
Relationships between cardiopulmonary function and clinical characteristics.

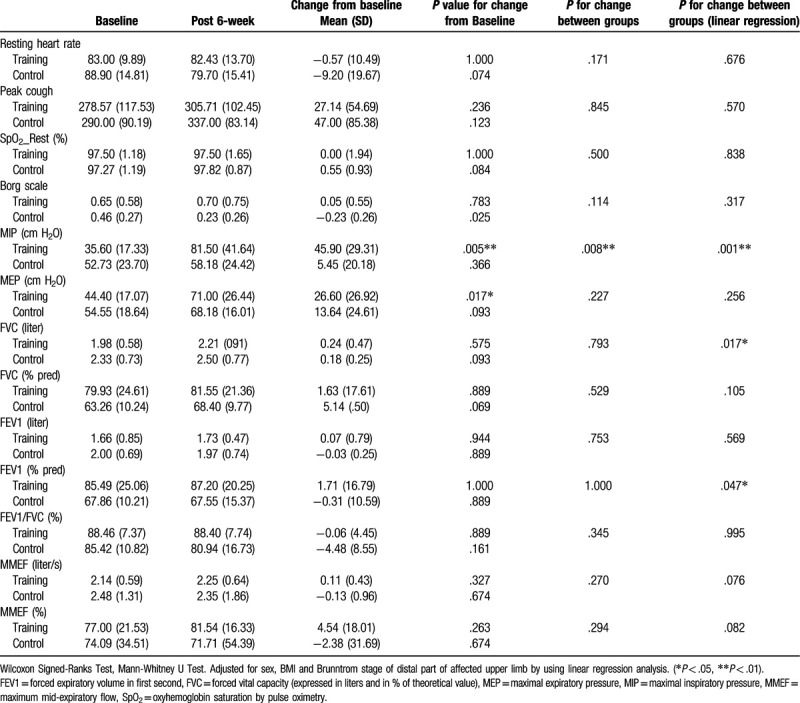
Significant differences within each group were noted for the change from baseline of the Brunnstrom stage of the affected upper and lower limbs, Barthel scale, and FOIS. However, no significant difference between the groups was observed (Table 5). Significant change from the baseline was seen in fatigue (P = .007) (Table 5), MIP (P = .008) only in the RMT group, and significant between-group differences were seen for MIP (P = .001), FVC (P = .017), and FEV1 (% predicted) (P = .047) according to the linear regression analysis adjusted for the differences already present between the groups in terms of sex, BMI, and Brunnstrom stage of the distal part of affected limb (Table 6).
Table 5.
Clinical data before and after the 6-week study in the training and control groups.
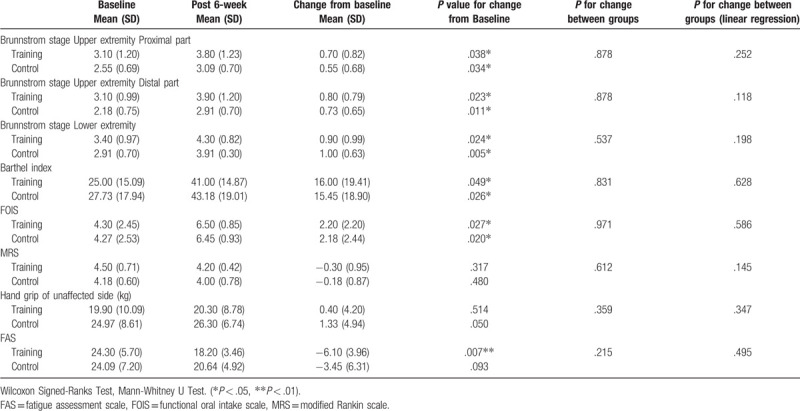
Table 6.
Data changes in cardiopulmonary function before and after the 6-week study in the training and control groups.
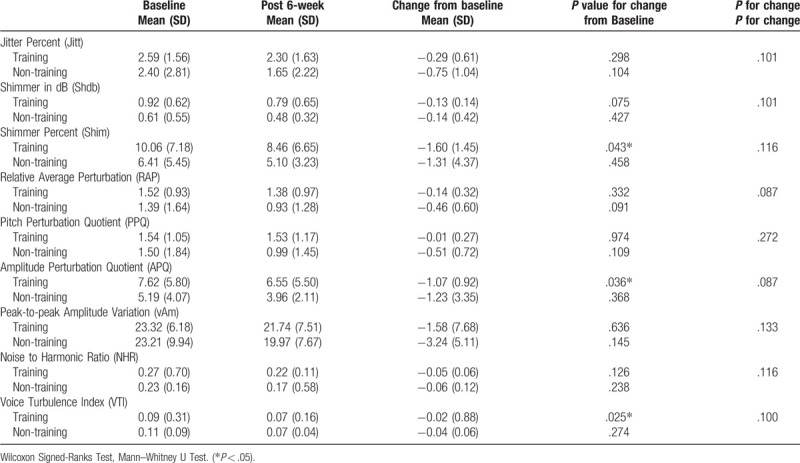
Regarding voice analysis, there were significant changes among participants of the RMT group in the Shim (P = .043), APQ (P = .036), and VTI (P = .025) values (Table 7). Significant negative correlations were found between FEV1/FVC and Jitt (r = −0.574, P < .05), RAP (r = −0.574, P < .05), PPQ (r = −0.538, P < .05), and VTI (r = −0.835, P < .01). MMEF (r = −0.659, P < .05) and MMEF% (r = −0.692, P < .05) were negatively correlated with VTI (Table 8).
Table 7.
Data of Multi–Dimensional Voice report before and after the 6-week study in the training and non-training groups.
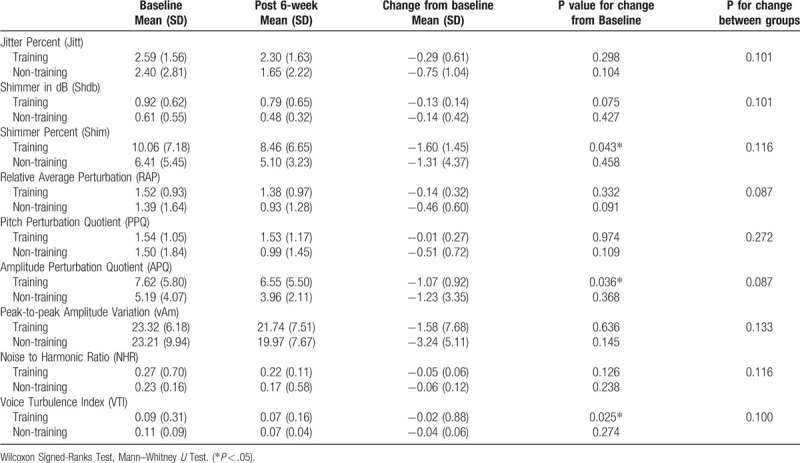
Table 8.
Data of Multi–Dimensional Voice report before and after the 6-week study in the training and non-training groups.
4. Discussion
Both RMT and control groups showed significant changes from the baseline in Brunnstrom stage of the affected limb, Barthel index, and FOIS; the stroke duration positively correlated with FVC and FEV1 (% prediction) and negatively correlated with FEV1/FVC%. These findings can be partially explained by neurologic recovery with time and the effectiveness of regular rehabilitation after stroke onset.
Significant changes in MIP, MEP, and fatigue level from baseline were observed only in the RMT group. However, the linear regression analysis, adjusted for between-group differences in sex, BMI, and Brunnstrom stage of the affected limb, demonstrated significant between-group differences in the change from baseline in mean MIP, FVC, and FEV1 (% predicted). Furthermore, a significant mean change from baseline of MEP was found only in the RMT group. The mean MEP positively correlated with MIP and peak cough flow, which in turn positively correlated with FVC and FEV1; MEP also negatively correlated with the Borg scale. These findings indicate that the 6-week combined RMT could improve the respiratory muscle strength patients. The effect of RMT on MIP was apparently greater than that observed on MEP.
Clinically, the discoordination between inhaling and exhaling should be resolved at the beginning of RMT and the active inspiratory volume needs to be enough for forceful expiration or cough flow. This explains why a significant between-group difference was seen only in MIP and not in MEP or peak cough as a 6-week program may be too short to achieve a significant effect on expiratory muscle force. This finding was consistent with results of a systemic review, which showed that RMT shows greater improvement in MIP, but has no effect on MEP in patients with various neurologic diseases.[32] Further, 5-week EMT for ischemic stroke patients increases the average expiratory muscle strength by approximately 30 cmH2O and improves the urge and strength of reflex cough, but is not effective for voluntary cough or swallow function. Therefore, the efficacy of EMT was attributed to the upregulation of reflex cough.[2] Moreover, a 4-week RMT by using threshold resistance device in acute stroke patients significantly improved the mean MIP by 14 cmH2O, MEP by 15 cmH2O, and the peak expiratory flow rate (74 L/min) of all three groups, regardless of the allocation of expiratory, inspiratory, or sham training; but no between-group differences was noted.[33] Similarly, our study showed no significant between-group difference in MEP and peak cough flow. Furthermore, our study also revealed no difference between both groups in terms of MRS, hand grip strength, and FOIS, which may be attributed to the heterogeneity in neurological lesion characteristics and existence of multiple comorbidities including congestive heart failure, atrial fibrillation, hypertension, and diabetes mellitus. Most of our participants’ brain lesions were located in the middle cerebral artery territory. Moreover, quite a few participants had borderline cardiomegaly or congestive heart.
The physical activity level in stroke patients is usually limited by fatigue and dyspnea. Some patients were too fatigued to attend the program at the time of eligibility screening. However, our RMT group patients showed a significant change from baseline of FAS in contrast to that in the control group.
For stroke patients, the perception of dyspnea is low and blunted, which is due to their dissociation between respiratory effort and dyspnea.[34] This can explain the similar Borg scale scores of both groups.
Regarding voice signals, Shim and ShdB are associated with hoarse and breathy voices; APQ and PPQ indicate the inability of the cords to support a periodic vibration. Hoarse and breathy voices usually have increased APQ, PPQ, or RAP.[19] Moreover, the subglottal pressure initiates and maintains the vocal fold vibration and voice production. Wingate et al reported that 5-week EMT followed by 6 sessions of traditional voice therapy could increase subglottal pressure, which increased the vocal intensity and voice dynamic range.[9] After the 6-week RMT, our stroke patients showed significant changes in Shim, APQ, and VTI from baseline in the voice analysis thus indicating that RMT is beneficial for the improvement of voice quality in stroke patients showing dysarthria. Further, considering that FEV1/FVC% was negatively correlated with Jitt, RAP, PPQ, and VTI, FEV1/FVC% may be correlated to voice quality, although no significant between-group difference after RMT was obtained for this parameter.
No adverse event was reported throughout the program, except in one subject with transient facial muscle soreness, which subsided within 2 to 3 days. Similar to previous studies,[10,11,33] the results proved that RMT could be feasible as adjunct therapy in stroke patients with respiratory muscle weakness, dysphagia, and dysarthria. However, the 6-week combined RMT was considered not long enough to demonstrate efficacy for expiratory muscle strength, swallowing, functional activity, and dysarthria and designing an intervention strategy based on the intensity, frequency, and duration of training program remains a challenge.
Study limitations: This study is limited by the small number of patients recruited. It took us two to three years to recruit the participants and those with apraxia, aphasia, and loose teeth, and those who could not hold a breath or perform a spirometry test were excluded. This study is also limited by the marked degree of drop-out rate (33.3% in RMT and 31.3% in control group). Moreover, the long-term effects and maintenance of RMT were not evaluated.
5. Conclusions:
Altogether, RMT significantly improved the respiratory muscle strength, FVC, FEV1, and fatigue in stroke patients with respiratory muscle weakness. In addition, the improvement in post-stroke dysphagia and dysarthria was also enhanced through RMT. The 6-week combined inspiratory and expiratory RMT is thus feasible as adjuvant therapy in stroke patients.
Acknowledgments
The authors would like to thank Andrew Wei-Hsiang Tiong for his assistance with this research.
Author contributions
Conceptualization: Mei-Yun Liaw, Chau-Peng Leong, Ching-Yi Liao, Cheng-Hsien Lu, Meng-Chih Lin.
Data curation: Mei-Yun Liaw, Chia-Hao Hsu, Chau-Peng Leong, Ching-Yi Liao, Lin-Yi Wang, Cheng-Hsien Lu, Meng-Chih Lin.
Formal analysis: Mei-Yun Liaw, Chia-Hao Hsu, Meng-Chih Lin.
Funding acquisition: Mei-Yun Liaw.
Investigation: Mei-Yun Liaw.
Methodology: Mei-Yun Liaw, Chau-Peng Leong, Ching-Yi Liao, Cheng-Hsien Lu, Meng-Chih Lin.
Resources: Chia-Hao Hsu, Lin-Yi Wang.
Supervision: Lin-Yi Wang.
Writing – original draft: Mei-Yun Liaw, Meng-Chih Lin.
Writing – review & editing: Mei-Yun Liaw.
Footnotes
Abbreviations: APQ = amplitude perturbation quotient, ERMT = expiratory respiratory muscle training, FAS = fatigue assessment scale, FEV1 = forced expiratory volume in first second, FOIS = functional oral intake scale, FVC = forced vital capacity, IRMT = inspiratory respiratory muscle training, Jitt = jitter percent, MEP = maximal expiratory pressure, MIP = maximal inspiratory pressure, MMEF = maximum mid-expiratory flow, MRS = Modified Rankin scale, PPQ = pitch perturbation quotient, RAP = relative average perturbation, RMT = respiratory muscle training, ShdB = shimmer in dB, Shim = shimmer percent, VTI = voice turbulence index.
How to cite this article: Liaw MY, Hsu CH, Leong CP, Liao CY, Wang LY, Lu CH, Lin MC. Respiratory muscle training in stroke patients with respiratory muscle weakness, dysphagia, and dysarthria - a prospective randomized trial. Medicine. 2020;99:10(e19337).
The study was approved by the Institutional Review Board of the Chang Gung Memorial Hospital, Kaohsiung Medical Board (IRB number: 105-1989C).
This research was funded by Chang Gung Memorial Hospital, Taiwan (grant number: CMRPG8E0911; 2016-5-1 to 2018-4-30).
The authors report no conflicts of interest.
The devices used are as follows: Model 4500 (Multi–Dimensional Voice) for Dimensional Voice Program, Model 5105 (KayPENTAX), Computerized Speech Lab (CSL).
Dofin Breathing Trainer (a threshold trainer), (DT 11 GaleMed Corporation), (DT 14 GaleMed Corporation). Product number: PO09000038.
Pulmonary function tests: spirometer (Vitalograph, Serial Spirotrac, Buckingham, USA).
References
- [1].Martino R, Foley N, Bhogal S, et al. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke 2005;36:2756–63. [DOI] [PubMed] [Google Scholar]
- [2].Hegland KW, Davenport PW, Brandimore AE, et al. Rehabilitation of swallowing and cough functions following stroke: an expiratory muscle strength training trial. Arch Phys Med Rehabil 2016;97:1345–51. [DOI] [PubMed] [Google Scholar]
- [3].Moon JH, Jung JH, Won YS, et al. Effects of expiratory muscle strength training on swallowing function in acute stroke patients with dysphagia. J Phys Ther Sci 2017;29:609–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ward K, Seymour J, Steier J, et al. Acute ischaemic hemispheric stroke is associated with impairment of reflex in addition to voluntary cough. Eur Respir J 2010;36:1383–90. [DOI] [PubMed] [Google Scholar]
- [5].Kelly JO, Kilbreath SL, Davis GM, et al. Cardiorespiratory fitness and walking ability in subacute stroke patients. Arch Phys Med Rehabil 2003;84:1780–5. [DOI] [PubMed] [Google Scholar]
- [6].Kent RD, Vorperian HK, Kent JF, et al. Voice dysfunction in dysarthria: application of the multi-dimensional voice program. J Commun Disord 2003;36:281–306. [DOI] [PubMed] [Google Scholar]
- [7].Britto RR, Rezende NR, Marinho KC, et al. Inspiratory muscular training in chronic stroke survivors: a randomized controlled trial. Arch Phys Med Rehabil 2011;92:184–90. [DOI] [PubMed] [Google Scholar]
- [8].Sutbeyaz ST, Koseoglu F, Inan L, et al. Respiratory muscle training improves cardiopulmonary function and exercise tolerance in subjects with subacute stroke: a randomized controlled trial. Clin Rehabil 2010;24:240–50. [DOI] [PubMed] [Google Scholar]
- [9].Wingate JM, Brown WS, Shrivastav R, et al. Treatment outcomes for professional voice users. J Voice 2007;21:433–49. [DOI] [PubMed] [Google Scholar]
- [10].Messaggi-Sartor M, Guillen-Sola A, Depolo M, et al. Inspiratory and expiratory muscle training in subacute stroke: a randomized clinical trial. Neurology 2015;85:564–72. [DOI] [PubMed] [Google Scholar]
- [11].Guillen-Sola A, Messagi Sartor M, Bofill Soler N, et al. Respiratory muscle strength training and neuromuscular electrical stimulation in subacute dysphagic stroke patients: a randomized controlled trial. Clin Rehabil 2017;31:761–71. [DOI] [PubMed] [Google Scholar]
- [12].Wheeler KM, Chiara T, Sapienza CM. Surface electromyographic activity of the submental muscles during swallow and expiratory pressure threshold training tasks. Dysphagia 2007;22:108–16. [DOI] [PubMed] [Google Scholar]
- [13].Troche MS, Huebner I, Rosenbek JC, et al. Respiratory-swallowing coordination and swallowing safety in patients with Parkinson's disease. Dysphagia 2011;26:218–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ertekin C, Aydogdu I. Neurophysiology of swallowing. Clin Neurophysiol 2003;114:2226–44. [DOI] [PubMed] [Google Scholar]
- [15].Wheeler-Hegland KM, Rosenbek JC, Sapienza CM. Submental sEMG and hyoid movement during Mendelsohn maneuver, effortful swallow, and expiratory muscle strength training. J Speech Lang Hear Res 2008;51:1072–87. [DOI] [PubMed] [Google Scholar]
- [16].Pearson WG, Jr, Langmore SE, Yu LB, et al. Structural analysis of muscles elevating the hyolaryngeal complex. Dysphagia 2012;27:445–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ko KR, Park HJ, Hyun JK, et al. Effect of laryngopharyngeal neuromuscular electrical stimulation on dysphonia accompanied by dysphagia in post-stroke and traumatic brain injury patients: a pilot study. Ann Rehabil Med 2016;40:600–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ryu JS, Park SR, Choi KH. Prediction of laryngeal aspiration using voice analysis. Am J Phys Med Rehabil 2004;83:753–7. [DOI] [PubMed] [Google Scholar]
- [19].Software instruction manual. Multi-Dimentional Voice Program (MDVP) Model 5105 Lincoln Park, NJ 07035-1488 USA: KayPENTAX; 2008. [Google Scholar]
- [20].Dall’Ago P, Chiappa GR, Guths H, et al. Inspiratory muscle training in patients with heart failure and inspiratory muscle weakness: a randomized trial. J Am Coll Cardiol 2006;47:757–63. [DOI] [PubMed] [Google Scholar]
- [21].Chiappa GR, Roseguini BT, Vieira PJ, et al. Inspiratory muscle training improves blood flow to resting and exercising limbs in patients with chronic heart failure. J Am Coll Cardiol 2008;51:1663–71. [DOI] [PubMed] [Google Scholar]
- [22].Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 1982;14:377–81. [PubMed] [Google Scholar]
- [23].Smith OR, van den Broek KC, Renkens M, et al. Comparison of fatigue levels in patients with stroke and patients with end-stage heart failure: application of the fatigue assessment scale. J Am Geriatr Soc 2008;56:1915–9. [DOI] [PubMed] [Google Scholar]
- [24].Crary MA, Mann GD, Groher ME. Initial psychometric assessment of a functional oral intake scale for dysphagia in stroke patients. Arch Phys Med Rehabil 2005;86:1516–20. [DOI] [PubMed] [Google Scholar]
- [25].Troche MS, Okun MS, Rosenbek JC, et al. Aspiration and swallowing in Parkinson disease and rehabilitation with EMST: a randomized trial. Neurology 2010;75:1912–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Park JS, Oh DH, Chang MY, et al. Effects of expiratory muscle strength training on oropharyngeal dysphagia in subacute stroke patients: a randomised controlled trial. J Oral Rehabil 2016;43:364–72. [DOI] [PubMed] [Google Scholar]
- [27].Collin C, Wade DT, Davies S, et al. The Barthel ADL Index: a reliability study. Int Disabil Stud 1988;10:61–3. [DOI] [PubMed] [Google Scholar]
- [28].van Swieten JC, Koudstaal PJ, Visser MC, et al. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988;19:604–7. [DOI] [PubMed] [Google Scholar]
- [29].Standardization of Spirometry, 1994 Update. American Thoracic Society. Am J Respir Crit Care Med 1995;152:1107–36. [DOI] [PubMed] [Google Scholar]
- [30].American Thoracic Society/European Respiratory S. ATS/ERS Statement on respiratory muscle testing. Am J Respir Crit Care Med 2002;166:518–624. [DOI] [PubMed] [Google Scholar]
- [31].Black LF, Hyatt RE. Maximal respiratory pressures: normal values and relationship to age and sex. Am Rev Respir Dis 1969;99:696–702. [DOI] [PubMed] [Google Scholar]
- [32].Pollock RD, Rafferty GF, Moxham J, et al. Respiratory muscle strength and training in stroke and neurology: a systematic review. Int J Stroke 2013;8:124–30. [DOI] [PubMed] [Google Scholar]
- [33].Kulnik ST, Birring SS, Moxham J, et al. Does respiratory muscle training improve cough flow in acute stroke? Pilot randomized controlled trial. Stroke 2015;46:447–53. [DOI] [PubMed] [Google Scholar]
- [34].Lanini B, Gigliotti F, Coli C, et al. Dissociation between respiratory effort and dyspnoea in a subset of patients with stroke. Clin Sci (Lond) 2002;103:467–73. [DOI] [PubMed] [Google Scholar]


