Abstract
Background:
Some studies assessed the effect of aerobic exercise on diabetic obese patients with hepatic disease, while very limited studies compared high-intensity interval (HII) versus moderate-intensity continuous (MIC) on diabetic obese patients with non-alcoholic fatty liver disease (NAFLD).
Objectives:
This study was designed to assess the effects of HII versus MIC on intrahepatic triglycerides (IHTG) and visceral lipids in diabetic obese patients with NAFLD.
Design:
Randomized controlled trial.
Methods:
Forty-seven diabetic obese individuals with NAFLD were enrolled in this study. The individuals were randomly divided into 16 in HII group, 15 in MIC group, and 16 in the controls. HII group received HII exercise, MIC group received 8-week MIC exercise while the control group did not receive any exercise intervention. IHTG and visceral lipids were assessed pre- and post-intervention.
Results:
Baseline and clinical characteristics showed nonsignificant difference among the 3 groups (P > .05). Both HII and MIC groups showed a significant reduction in hepatic fat and visceral lipids (P < .05), while the controls showed nonsignificant difference (P > .05) after completing the study intervention. Postintervention analysis showed nonsignificant changes between the HII and MIC groups (P > .05).
Conclusions:
Exercise training wither HII or MIC aerobic exercise reduces IHGT and visceral lipids in diabetic obese patients with NAFLD. No differences were observed between the effects of both exercise programs on diabetic obese patients with NAFLD.
Keywords: diabetes, hepatics, high-intensity interval exercise, moderate-intensity continuous exercise, obesity
1. Introduction
Recently, the obesity worldwide epidemic caused a potential increase in the widespread of type 2 diabetes mellitus (T2DM). Diabetes, obesity, and fatty liver disease in combination with lower physical activity are potential causes to increase mortality and morbidity rates.[1,2] Nearly, third of the world population experienced obesity with dominant medical complications such as impairments of glucose and fat metabolism, insulin sensitivity.[3] The manifestations of nonalcoholic fatty liver disease (NAFLD) and another metabolic disorders may lead to high rate of mortality in hepatic and cardiac patients.[4,5]
Many complications of obesity were documented, particularly visceral adipose tissue and intrahepatic triglycerides (IHTG) that were increased and affected the cardiovascular and metabolic outcomes.[6] Accumulation of IHTG is commonly one of the major characteristics of obesity that lead to impairments of cardiovascular function, metabolism, and insulin sensitivity.[7]
Reduction of IHTG is commonly associated with increase of metabolism and restore normal blood glucose in T2DM.[8] Previous studies approved the positive influences of exercise training and dietary control on IHTG and also concluded that no definitive medical prescription can outlive reduce hepatic fats.[9]
NAFLD is a common complication of obesity, associated with serum hypertriglyceridemia and impairments of liver lipoprotein metabolism.[10] Prior studies provided that accumulation of IHTG and visceral lipid plays an important role in the pathogenesis of fatty liver disease and have been recognized as the main biological indicators to NAFLD.[11,12] Lifestyle modifications such as exercise training and dietary control reduce IHTG and improve the metabolic function in patients with nonalcoholic fatty liver disease.[13,14]
Several documents provided that exercise and physical activity training are well-advised as an important protocol in management of NAFLD.[15,16] These documents are based on the combination among obesity, T2DM, and NAFLD, while poor documents explain the role of exercise training in the treatment of NAFLD. Previous study assessed the correlation between the level of physical activity and the changes of hepatic histology and concluded the non-significant correlation between them in patients with NAFLD, while that study observed the high measure of maximal oxygen uptake (VO2peak) in mild NAFLD, confirming the vital function of exercise training in the management of NAFLD.[17] Also, another research approved that 3 months of exercise and dietary control reduced steatosis significantly in NAFLD.[18] The effects of physical and exercise training in NAFLD have been assessed using NAFLD manifestations such as imaging of steatosis and liver functions test or using restricted hepatic histology.[19,20]
Oh et al concluded that exercise training solely improved hepatic function by decrease of visceral adipose fat level and body weight. That study explained that exercise training decreased liver fat and improved the hepatic function.[21] Other studies provided the positive effects of exercise training on NAFLD with high liver fat content[22] and ventilatory marker dysfunctions.[23]
Previous studies showed that high-intensity interval exercise (HII) improves hepatic function and glucose metabolism [24–26] and other studies provided that moderate-intensity exercise (MIC) reduces hepatic fat and increases glucose oxidation [27,28] while there are limited studies that assess the effects of HII versus MIC on diabetic obese patients with NAFLD. Hence, this study was designed to assess the effects of HII versus MIC on hepatic fat content and visceral lipids in diabetic obese patients with NAFLD.
2. Materials and methods
2.1. Subjects
This randomized controlled trial included 47 diabetic obese patients with NAFLD, their age was 40 to 60 years. All patients were diagnosed with NAFLD, type II DM, and obesity (body mass index [BMI] ≥30 kg/m2). The diagnostic criteria of NAFLD based on the diagnostic guidelines for NAFLD in the Asia-Pacific region.[29] All study participants were non-smokers. The 47 patients were randomly classified into 3 groups. Group I included 16 patients, received medical treatment with a program of HII exercise 3 times/wk for 8 weeks (HII group), group II included fifteen patients, received moderate-intensity continuous (MIC) exercise 3 times/wk for 8 weeks (MIC group), and group III included 16 patients, received only medical treatment without exercise program (control). Any patient had a severe life limiting illness (cancer, renal failure), uncontrolled heart disease, neuromuscular limitations, orthopedic problems, and endocrine disorders that could affect physical exercise was excluded from the study. This study was ethically accepted by the research ethical committee, Faculty of Physical Therapy, Cairo University (P.T.REC/012/002146) with clinical trial registration number (NCT03774511) in accordance with the guidelines of the Helsinki Declaration. All patients have signed consent form before starting the study program.
2.2. Randomization
From 53 patients, 48 have been enrolled in the study program. Three subjects did not meet the inclusion criteria of the study and 2 subjects refused to participate in the study without informative reason. Allocation was carried out before commencing the study program by blinded physiotherapist using secured envelopes, which included a piece of red sheet indicated HII group, a piece of green sheet indicated MIC group, and a piece of white sheet indicated control group. The flowchart of the study is presented in Figure 1.
Figure 1.
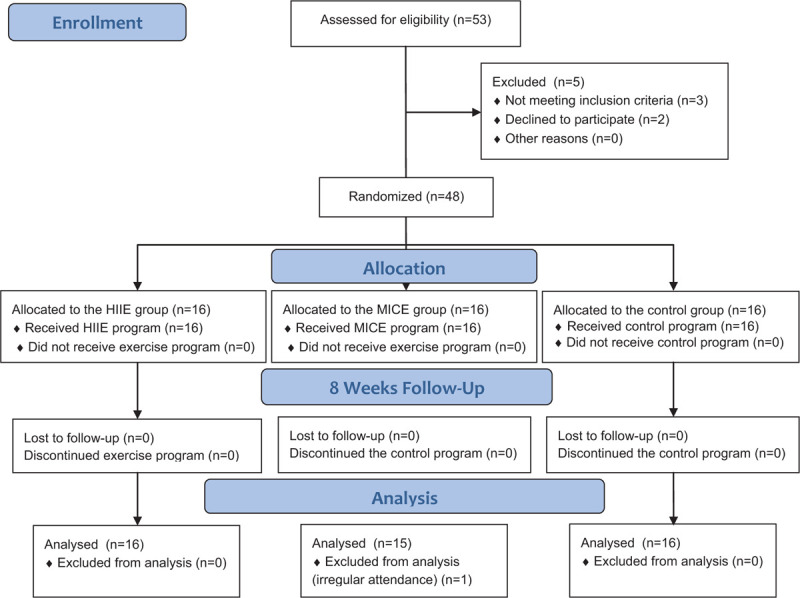
Flow diagram of the study.
3. Procedures
3.1. Assessment
All patients were evaluated for the IHTG, visceral lipids, and insulin resistance before commencing the study program and at the end of 8 weeks of the program by the same examiner who was blinded concerning the group to which each patient appointed.
All patients were informed about the nature, procedure, and benefits of the study. The test of IHTG, visceral lipids, lipid profile, glycated hemoglobin (HbA1c), and alanine-transaminase (ALT) were recorded at the initial assessment and at the end of the program. Assessment of hepatic fat was performed using magnetic resonance imaging.[30] The venous blood sample was taken at the morning after fasting at least 10 hours for biochemical analysis.
3.2. Intervention
During the study program, all patients of the study were instructed to adhere to physicians’ advice including medications, dietary control, and home activity such as walking and stretching exercise. The 2 exercise programs were conducted at the outpatient physiotherapy clinic, Cairo university hospitals and were handled by trained physiotherapists.
HII group, each patient in this group conducted a program of high-intensity aerobic exercise for 8 weeks, 3 times per week, each exercise session lasting for nearly 40 minutes morning. Each patient was instructed to not eat for 2 hours before the exercise session to avoid exercise induced airway obstruction.
The HII exercise program was performed on a cycle Ergometer (MonarkRC6 Novo, Langley) with firmly grasping the rails to maintain balance. The exercise session was started with a 5-minute warm-up involving cycling exercise without resistance of the Ergometer followed by 3 sets of 4-minutes cycling sessions at 80% to 85% of the VO2max with 2-minutes interval at 50% of the VO2max between sets. The session was finished with 5 minutes of cool down exercise.
MIC group, each patient in the exercise group was recruited to a MIC aerobic exercise program 3 times weekly for 8 weeks, the duration of the exercise was nearly 40 to 50 minutes. All patients were informed to prevent eating 2-hour before exercise program to nullify exercise-related respiratory dysfunction. The MIC exercise program consisted of 5-minute warming-up followed by cycling Ergometer with continuous intensity at 60% to 70% of the maximum heart rate (max HR) and ended the exercise program with 5-minute cooling-down.
3.3. Statistical analysis
Normality of data was checked using Shapiro–Wilk test. Analysis of variance was used to evaluate the difference between HII, MIC, and control groups and paired t test was performed to measure changes within each group. SPSS version 22.0 (SPSS Inc., Chicago, IL) was used for statistical analysis. P < .05 was considered to be statistically significant for all measurements.
For sample size estimation, an initial power analysis was applied (2-tailed test with statistical power of 0.80, α error = 0.05, and effect size = 0.5). Estimates of mean difference and standard deviation for the IHTG value from the previous study assessed 19 patients who received aerobic exercise.[31] According to that study measures, 13 patients were required in each group. Forty-eight patients were included in the study to account for the dropout rate of 20%.
4. Results
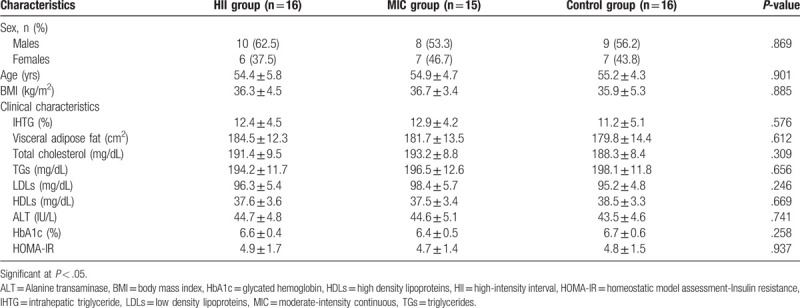
From 48 individuals who enrolled in the study program, 1 patient did not attend regularly the study program in the MIC group and not included in the data analysis. As demonstrated in Table 1, baseline and clinical features of 47 patients (16 in HII group, 15 in the MIC group, and 16 in the control group) showed nonsignificant differences in all measures among the 3 groups before commencing the study program (P > .05).
Table 1.
Baseline characteristics.
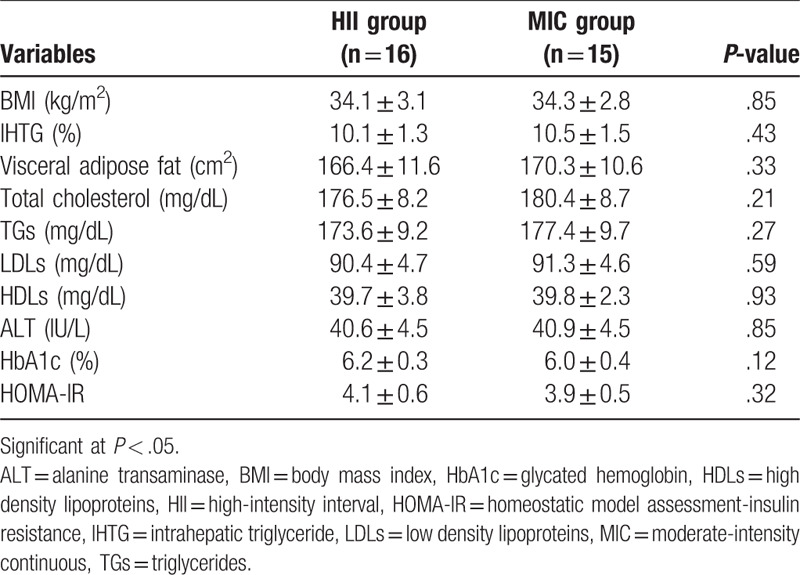
After completing study intervention, the 2 study programs (HII and MIC groups) showed significant decrease in BMI, IHTG, visceral lipids, ALT, HbA1c, and lipid profile (P < .05). While the control group exhibited nonsignificant changes in (P > .05) as shown in Table 2. At the end of the study intervention, comparison between HII and MIC groups showed nonsignificant changes (P > .05) between the 2 groups (Table 3).
Table 2.
Pre- and post-treatment mean values of all measures for HII, MIC, and control groups.
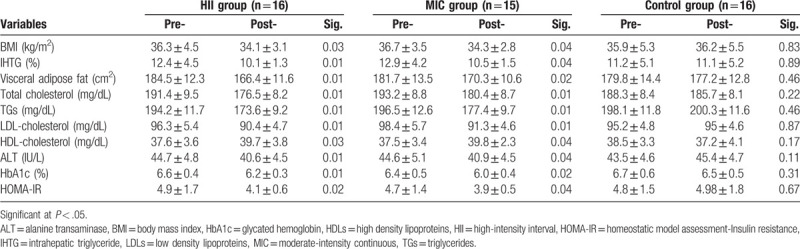
Table 3.
Differences between HII and MIC groups after intervention.
5. Discussion
This randomized controlled trial aimed to assess the effects of HII versus MIC on hepatic fat content and visceral lipids in diabetic obese patients with NAFLD. The study outcomes approved that both HII and MIC exercise programs reduce BMI, IHTG, visceral lipids, insulin resistance, and HbA1c in those patients.
Regarding to the findings of the current study, both of HII exercise program, 50 and 80% to 85% HRmax, 3 sessions/wk, for 8 weeks and MIC exercise program, 60% to 70% HRmax, 3 sessions/wk for 8 weeks showed significant reduction of IHTG, visceral lipids. These findings match the results of previous studies.[24–28]
Reduction of IHTG with both HII and MIC exercises is mechanically associated with reduction of circulating lipids and insulin resistance. This study emphasizes the importance of HII and MIC aerobic exercises in individuals with high hepatic fat content. High visceral lipids is increased through the high circulating fatty acids and secretions of adipocytokines, which decrease insulin sensitivity and intrahepatic lipids[32] while the physiological relation between liver metabolism and visceral lipids remains unclear. Controlling metabolism in the present study is alarming assumed the hepatic lipid reduction and the forceful link between hepatic insulin resistance and intrahepatic lipids.[33] Documents explained that the reduction of IHTG is importantly required to lower insulin resistance and blood glucose level.[33,34]
Similar to our study outcomes, Aoi et al, concluded that 20-minutes submaximal heart rate cycling or running exercise aspired to 20-minutes warm-up/cool-down 3 sessions per week for 4 weeks results in a reduction of insulin resistance and blood glucose level in patients with T2DM.[35]
Many documents studied the proper exercise intensity to improve basic and comprehensive metabolic panels. O’Donovan et al investigated the influences of moderate-intensity exercise (cycling exercise at 60% VO2max 3 times per week for 24 weeks) and high-intensity exercise (cycling exercise at 80% VO2max 3 times per week for 24 weeks) on blood glucose level and insulin sensitivity. Aerobic exercise at intensity of 60% and 80% VO2max was sufficient to increase insulin sensitivity and decrease plasma glucose level.[36]
As well, Benatti et al found that 60-minutes treadmill aerobic exercise daily for 12 weeks at 70% VO2max (80% max HR) leads to definitive decrease of body weight, insulin resistance, visceral lipids, and abdominal obesity.[37] Also, this study approved that aerobic exercise without reduction of body weight could reduce visceral and abdominal lipids.
Previous research approved that 50 to 60 minutes of daily aerobic exercise for 4 weeks (beginning with 60%–65% max HR and ending by 80%–85% max HR) cause insulin sensitivity improvement, glucose oxidation, and reduction of visceral lipids.[38]
Our study showed that the IHTG reduction following HII and MIC aerobic exercises for 8 weeks is combined with ALT reduction. Regardless of ALT increase is a usual prediction of hepatic dysfunction,[39] changes of plasma ALT are not a predictor of hepatic histological changes.[40] As well, our study found a remarkable decrease of plasma ALT in the exercise groups and approved beneficial clinical practice of aerobic exercise in diabetic obese patients with NAFLD.
The present study establishes strong evidence for accenting the important role of aerobic exercise in diabetic obese patients with NAFLD. Also, it approves that exercise training reduces hepatic fat content, visceral lipids, plasma ALT, plasma glucose level, and improves insulin sensitivity in diabetic obese patients with NAFLD. A appropriate control of fatty liver disease has to commence with exercise adherence consequently as HII and MIC exercises modulate insulin sensitivity by improving metabolism of free fatty acids in the exercised skeletal muscles. Therefore, free fatty acids oxidation and insulin sensitivity result in increasing of glucose-lipid metabolism. As well, regular exercise training results in expressive decrease of hepatic fat content by increase of energy expenditure, skeletal fat oxidation, and decrease of visceral lipids.
The present study has some limitations. First, lack of intermediate and long-term assessment. Second, home-based exercise and dietary intake were not supervised. Further researches have to include large sample size to evaluate different exercise intensities on diabetic obese patients with NAFLD.
6. Conclusions
Exercise training whether HII or MIC aerobic exercise reduces IHGT and visceral lipids in diabetic obese patients with NAFLD. No differences were observed between the effects of both exercise programs on diabetic obese patients with NAFLD. Clinical guidelines have to be recommended to adhere HII and MIC aerobic exercise programs among diabetic obese patients, particularly with NAFLD.
Acknowledgments
The authors would like to acknowledge all individuals who participated in this trial. This publication was supported by the Deanship of Scientific Research at Prince Sattam bin Abdulaziz University.
Author contributions
Conceptualization: Walid Kamal Abdelbasset, Sayed A. Tantawy, Dalia M. Kamel.
Data curation: Walid Kamal Abdelbasset, Sayed A. Tantawy, Dalia M. Kamel, Ahmed A. Ibrahim.
Formal analysis: Walid Kamal Abdelbasset, Dalia M. Kamel, Bader A. Alqahtani, Tamer E. Elnegamy, Ahmed A. Ibrahim.
Funding acquisition: Bader A. Alqahtani, Tamer E. Elnegamy.
Investigation: Walid Kamal Abdelbasset, Sayed A. Tantawy, Dalia M. Kamel, Gaber S. Soliman.
Methodology: Walid Kamal Abdelbasset, Sayed A. Tantawy, Dalia M. Kamel, Ahmed A. Ibrahim.
Project administration: Walid Kamal Abdelbasset.
Resources: Tamer E. Elnegamy, Gaber S. Soliman.
Software: Bader A. Alqahtani, Tamer E. Elnegamy.
Validation: Walid Kamal Abdelbasset, Gaber S. Soliman.
Visualization: Walid Kamal Abdelbasset, Gaber S. Soliman, Ahmed A. Ibrahim.
Writing – original draft: Walid Kamal Abdelbasset, Tamer E. Elnegamy, Gaber S. Soliman.
Writing – review and editing: Walid Kamal Abdelbasset, Sayed A. Tantawy, Dalia M. Kamel, Bader A. Alqahtani, Tamer E. Elnegamy, Ahmed A. Ibrahim.
Walid Kamal Abdelbasset orcid: 0000-0003-4703-661X.
Footnotes
Abbreviations: ALT = alanine-transaminase, HbA1c = glycated hemoglobin, HDLs = high-density lipoproteins, IHTG = intrahepatic triglyceride, LDLs = low-density lipoproteins, NAFLD = nonalcoholic fatty liver disease, T2DM = type 2 diabetes mellitus, TGs = total triglycerides, VO2peak = maximal oxygen uptake.
How to cite this article: Abdelbasset WK, Tantawy SA, Kamel DM, Alqahtani BA, Elnegamy TE, Soliman GS, Ibrahim AA. Effects of high-intensity interval and moderate-intensity continuous aerobic exercise on diabetic obese patients with nonalcoholic fatty liver disease: A comparative randomized controlled trial. Medicine. 2020;99:10(e19471).
The authors have no funding and conflicts of interest to disclose.
References
- [1].Dunn W, Xu R, Wingard DL, et al. Suspected nonalcoholic fatty liver disease and mortality risk in a population-based cohort study. Am J Gastroenterol 2008;103:2263–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA 2002;287:356–9. [DOI] [PubMed] [Google Scholar]
- [3].McMillan KP, Kuk JL, Church TS, et al. Independent associations between liver fat, visceral adipose tissue, and metabolic risk factors in men. Appl Physiol Nutr Metab 2007;32:265–72. [DOI] [PubMed] [Google Scholar]
- [4].Younossi ZM, Gramlich T, Matteoni CA, et al. Nonalcoholic fatty liver disease in patients with type 2 diabetes. Clin Gastroenterol Hepatol 2004;2:262–5. [DOI] [PubMed] [Google Scholar]
- [5].Church TS, Kuk JL, Ross R, et al. Association of cardiorespiratory fitness, body mass index, and waist circumference to nonalcoholic fatty liver disease. Gastroenterology 2006;130:2023–30. [DOI] [PubMed] [Google Scholar]
- [6].Bays H, Dujovne CA. Adiposopathy is a more rational treatment target for metabolic disease than obesity alone. Curr Atheroscler Rep 2006;8:144–56. [DOI] [PubMed] [Google Scholar]
- [7].Hwang JH, Stein DT, Barzilai N, et al. Increased intrahepatic triglyceride is associated with peripheral insulin resistance: in vivo MR imaging and spectroscopy studies. Am J Physiol Endocrinol Metab 2007;293:E1663–9. [DOI] [PubMed] [Google Scholar]
- [8].Petersen KF, Dufour S, Befroy D, et al. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes 2005;54:603–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sass DA, Chang P, Chopra KB. Nonalcoholic fatty liver disease: a clinical review. Dig Dis Sci 2005;50:171–80. [DOI] [PubMed] [Google Scholar]
- [10].Fabbrini E, Mohammed BS, Magkos F, et al. Alterations in adipose tissue and hepatic lipid kinetics in obese men and women with nonalcoholic fatty liver disease. Gastroenterology 2008;134:424–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Verrijen A, Francque S, van Gaal L. The role of visceral adipose tissue in the pathogenesis of non-alcoholic fatty liver disease. Eur Endocrinol 2011;7:96–103. [Google Scholar]
- [12].Liu Q, Bengmark S, Qu S. The role of hepatic fat accumulation in pathogenesis of non-alcoholic fatty liver disease (NAFLD). Lipids Health Dis 2010;9:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lazo M, Solga SF, Horska A, et al. Effect of a 12-month intensive lifestyle intervention on hepatic steatosis in adults with type 2 diabetes. Diabetes Care 2010;33:2156–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kantartzis K, Machann J, Schick F, et al. Effects of a lifestyle intervention in metabolically benign and malign obesity. Diabetologia 2011;54:864–8. [DOI] [PubMed] [Google Scholar]
- [15].Koot BG, van der Baan-Slootweg OH, Tamminga-Smeulders CL, et al. Lifestyle intervention for non-alcoholic fatty liver disease: prospective cohort study of its efficacy and factors related to improvement. Arch Dis Child 2011;96:669–74. [DOI] [PubMed] [Google Scholar]
- [16].Abdelbasset WK, Badr NM, Elsayed SH, et al. Outcomes of resisted exercise on serum liver transaminases in hepatic patients with diabesity. Med J Cairo Univ 2014;82:9–16. [Google Scholar]
- [17].Krasnoff JB, Painter PL, Wallace JP, et al. Health-related fitness and physical activity in patients with nonalcoholic fatty liver disease. Hepatology 2008;47:1158–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hallsworth K, Fattakhova G, Hollingsworth KG, et al. Resistance exercise reduces liver fat and its mediators in non-alcoholic fatty liver disease independent of weight loss. Gut 2011;60:1278–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kim HK, Park JY, Lee KU, et al. Effect of body weight and lifestyle changes on long-term course of nonalcoholic fatty liver disease in Koreans. Am J Med Sci 2009;337:98–102. [DOI] [PubMed] [Google Scholar]
- [20].George St, Bauman A, Johnston A, et al. The independent effects of physical activity in patients with non-alcoholic fatty liver disease. Hepatology 2009;50:68–76. [DOI] [PubMed] [Google Scholar]
- [21].Oh S, Shida T, Yamagishi K, et al. Moderate to vigorous physical activity volume is an important factor for managing nonalcoholic fatty liver disease: a retrospective study. Hepatology 2015;61:1205–15. [DOI] [PubMed] [Google Scholar]
- [22].Abdelbasset WK, Badr NM, Elsayed SH, et al. Outcomes of resisted exercise on alkaline phosphatase and bilirubin in hepatic female patients with diabesity. Med J Cairo Univ 2014;82:167–74. [Google Scholar]
- [23].Abdelbasset WK, Abo Elyazid TI, Elsayed SH. Comparison of high intensity interval to moderate intensity continuous aerobic exercise on ventilatory markers in coronary heart disease patients: a randomized controlled study. Int J Physiother Res 2017;5:2013–8. [Google Scholar]
- [24].Hallsworth K, Thoma C, Hollingsworth KG, et al. Modified high-intensity interval training reduces liver fat and improves cardiac function in non-alcoholic fatty liver disease: a randomized controlled trial. Clin Sci 2015;129:1097–105. [DOI] [PubMed] [Google Scholar]
- [25].Cassidy S, Thoma C, Houghton D, et al. High-intensity interval training: a review of its impact on glucose control and cardiometabolic health. Diabetologia 2017;60:7–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Abdelbasset WK, Tantawy SA, Kamel DM, et al. A randomized controlled trial on the effectiveness of 8-week high-intensity interval exercise on intrahepatic triglycerides, visceral lipids, and health-related quality of life in diabetic obese patients with nonalcoholic fatty liver disease. Medicine 2018;98:e14918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zoppini G, Targher G, Zamboni C, et al. Effects of moderate-intensity exercise training on plasma biomarkers of inflammation and endothelial dysfunction in older patients with type 2 diabetes. Nutr Metab Cardiovas Dis 2016;16:543–9. [DOI] [PubMed] [Google Scholar]
- [28].van Dijk JW, Venema M, van Mechelen W, et al. Effect of moderate-intensity exercise versus activities of daily living on 24-hour blood glucose homeostasis in male patients with type 2 diabetes. Diabetes Care 2013;36:3448–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Farrell GC, Chitturi S, Lau GK, et al. Guidelines for the assessment and management of non-alcoholic fatty liver disease in the Asia-Pacific region: executive summary. J Gastroenterol Hepatol 2007;22:775–7. [DOI] [PubMed] [Google Scholar]
- [30].Tang A, Loomba R, Lavine JE, et al. Nonalcoholic fatty liver disease: MR imaging of liver proton density fat fraction to assess hepatic steatosis. Radiology 2013;267:422–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Johnson NA, Sachinwalla T, Walton DW, et al. Aerobic exercise training reduces hepatic and visceral lipids in obese individuals without weight loss. Hepatology 2009;50:1105–12. [DOI] [PubMed] [Google Scholar]
- [32].Van der Poorten D, Milner KL, Hui J, et al. Visceral fat: a key mediator of steatohepatitis in metabolic liver disease. Hepatology 2008;48:449–57. [DOI] [PubMed] [Google Scholar]
- [33].Samuel VT, Liu ZX, Qu X, et al. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J Biol Chem 2004;279:32345–53. [DOI] [PubMed] [Google Scholar]
- [34].Lim EL, Hollingsworth KG, Aribisala BS, et al. Reversal of type 2 diabetes: normalization of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia 2011;54:2506–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Aoi W, Naito Y, Yoshikawa T. Dietary exercise as a novel strategy for the prevention and treatment of metabolic syndrome: effects on skeletal muscle function. J Nutr Metab 2011;2011:676208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].O’Donovan G, Kearney EM, Nevill AM, et al. The effects of 24 weeks of moderate- or high-intensity exercise on insulin resistance. Eur J Appl Physiol 2005;95:522–8. [DOI] [PubMed] [Google Scholar]
- [37].Benatti FB, Lira FS, Oyama LM, et al. Strategies for reducing body fat mass: effects of liposuction and exercise on cardiovascular risk factors and adiposity. Diabetes Metab Syndr Obes 2011;4:141–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ray L, Lipton RB, Zimmerman ME, et al. Mechanisms of association between obesity and chronic pain in the elderly. Pain 2011;152:53–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Dixon JB, Bhathal PS, O’Brien PE. Nonalcoholic fatty liver disease:predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology 2001;121:91–100. [DOI] [PubMed] [Google Scholar]
- [40].Dixon JB, Bhathal PS, O’Brien PE. Weight loss and non-alcoholic fatty liver disease: falls in gamma-glutamyltransferase concentrations are associated with histologic improvement. Obesity Surg 2006;16:1278–86. [DOI] [PubMed] [Google Scholar]


