Abstract
Background:
Somatostatin analog therapies showed great potential for patients suffering advanced neuroendocrine tumors (NETs). This study was aimed to evaluate the therapeutic efficacy of 177Lu-DOTATATE/DOTATOC (177Lu-octreotate/octreotide) peptide receptor radionuclide therapy (PRRT) in advanced or inoperable NETs patients.
Methods:
Pubmed, Web of Science, Embase and Cochrane Library were searched from 1950 to April 2019. Eligible studies should include randomized or nonrandomized controlled trials (RCTs)-based investigations of 177Lu-octreotate/octreotide PRRT for NETs. All these studies were assessed with Response Evaluation Criteria in Solid Tumors (RECIST), RECIST 1.1, Southwest Oncology Group (SWOG) criteria or World Health Organization (WHO) criteria. Disease response rates (DRRs) and disease control rates (DCRs) were calculated according to each response criteria group. DRRs were defined as the percentages of patients with complete response (CR) + partial response (PR), while DCRs represented the percentages of patients with CR+ PR+ stable disease (SD). The pooled proportions were calculated with either a fixed-effects model or a random-effects model depending on the test for heterogeneity.
Results:
A total of 22 studies (1758 patients) were included in this meta-analysis: 8 studies with 478 patients met RECIST criteria, 10 studies with 1127 patients met RECIST 1.1 criteria, 5 studies with 459 patients met SWOG criteria, and 1 study with 40 patients met WHO criteria, and among these articles 1 study met both RECIST and RECIST 1.1 criteria and 1 met both RECIST 1.1 and SWOG criteria. The pooled DRRs were 33.0% (95% CI: 25.0%-42.0%, I2 = 65%), 35.0% (95% CI: 26.0%-45.0%, I2 = 91%) and 25.0% (95% CI: 14.0%-36.0%, I2 = 84%) according to RECIST, RECIST 1.1 and SWOG criteria, respectively. The pooled DCRs were 79.0% (95% CI: 75.0%-83.0%, I2 = 97%), 83.0% (95% CI: 78.0%-88.0%, I2 = 0) and 82.0% (95% CI: 75.0%-89.0%, I2 = 91%), respectively.
Conclusion:
In advanced NETs patients, DRRs and DCRs were significantly elevated after initial treatment with 177Lu-DOTATATE PRRT, which shows that this treatment would be beneficial and promising for advanced or inoperable NETs patients.
Keywords: 177Lu-DOTATATE/DOTATOC, advanced neuroendocrine tumors, meta-analysis, neuroimaging, positron emission tomography
1. Introduction
Heterogeneity and slow-growth are the main characteristics of neuroendocrine tumors (NETs).[1,2] They used to be defined as rare malignancies, but in the past 3 decades, the prevalence of NETs raised approximately 5 folds in the United States.[3] A study in 2008 based on the data of Surveillance, Epidemiology, and End Results (SEER) program registries showed that the incidence of NETs elevated from 1.09/100000 to 5.25/100000 from 1973 to 2005.[4] As a result, the perception and treatment of NETs drew oncologists and researchers’ great attention in the past 15 years.[5] As for NETs patients with operable and localized focus, the first choice is surgical resection. However, tumors of this type are usually diagnosed in the late-phase due to the slow-growing nature and the nonspecific signs which make surgical resection impossible.[6]
Most NETs cell membrane overexpressed somatostatin receptor which was emphasized as the target for peptide receptor radionuclide therapy (PRRT) with radiolabeled somatostatin.[7] In 1990 s, radiolabeled somatostatin analogeus 111In-octreotide, was first applied in NETs therapy.[8] Since then, more radionuclide tracers have been used for NETs treatment such as 90Y and 177Lu.[9,10]177Lu-octreotate/octreotide (DOTATATE/DOTATOC) PRRT, generated from ytterbium (Yb), is capable of delivering precise low dosages of β energies between 0.149 and 0.479 MeV with ranges of tissue penetration between 0.5 to 2.0 mm, and this property endows 177Lu-octreotate/octreotide with limited collateral damage to the normal tissues compared with 90Y. In addition, 177Lu has 2 main γ-emission energies, 0.113 MeV (relative abundance 6%) and 0.208 MeV (11%), thereby being provided as the adequate radiotracer for scintigraphic imaging during and after therapy, biodistribution, and dosimetry studies.[11] In 2015, Kim et al reviewed the efficacy of 177Lu-octreotate/octreotide PRRT and found that this treatment was very effective in inoperable or metastatic NETs patients.[12] Nevertheless, only single center trials were enrolled in the review. By far, there have been more single center trials and several multicentre randomized trials such as NETTER-1 about 177Lu-octreotate/octreotide PRRT in NETs. In this article we addressed and analyzed the efficacy and benefit of 177Lu-labeled PRRT for advanced NETs in recent years.
2. Materials and methods
2.1. Statement
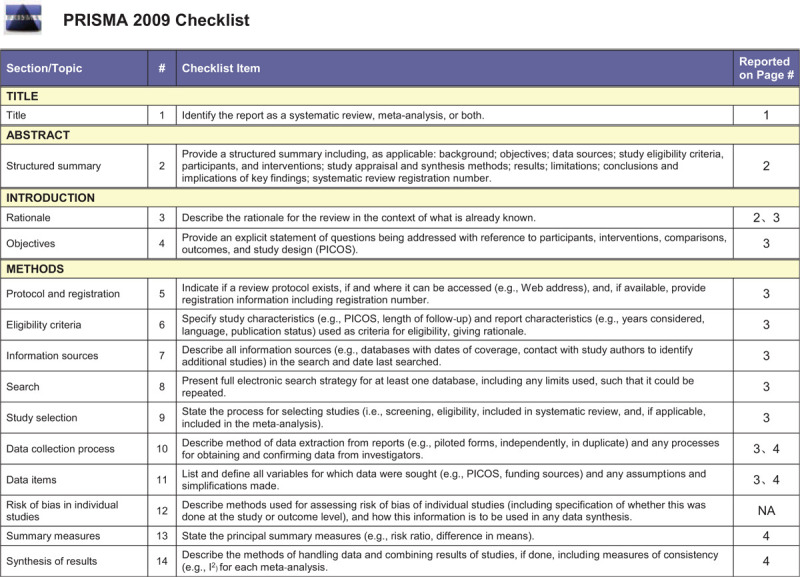
This meta-analysis was based entirely on previous published studies which had declared ethical approvals and no original clinical raw data was collected or utilized, thereby ethical approval was not conducted for this study. This review was conducted on the basis of the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA),[13] and the PRISMA checklist is shown in Table 1 .
Table 1.
PRISMA checklist.
2.2. Search and selection strategy
An independent review of the PubMed, Cochrane Library, Web of Science, and Embase data bases was performed from Jan 1, 1950, to Apr 30, 2019. The search was implemented by using the following keywords
“Neuroendocrine Tumors” and “177Lu-DOTATATE/DOTATOC”. The complete search phrases used for PubMed were: (“DOTA” AND (“177Lu” OR “Lu177” OR “Lu-177” OR “177-Lu”) AND (“neuroendocrine tumors”[MeSH Terms] OR “Neuroendocrine neoplasm”[Text Word] OR “Neuroendocrine Tumor”[Text Word] OR “Neuroendocrine carcinoma”[Text Word]). The searched articles were screened by Lin Lin and Meng-jiao Wang independently. Full texts were retrieved if they were confirmed to the eligibility criteria. If there were duplicates (patients’ data from the same trial or institution), only the most complete, recent and relevant study was selected.
2.3. Inclusion and exclusion criteria
Inclusion criteria were as follows: randomised clinical trials (patients >10) with the utilityof 177Lu-DOTATATE/DOTATOC (177Lu-octreotate/octreotide) PRRT in adults with NETs. Exclusion criteria: randomised clinical trials (patients < 10), case reports, review articles, meetings, news, conferences, abstracts and editorials.
2.4. Data extraction and primary outcomes
The data were extracted by 2 reviewers (Lin Lin and Meng-jiao Wang), independently. The following information was collected from each trial: first author, number of patients, treatment compound, dosages of radiopharmaceuticals, treatment cycles, radiopharmaceuticals’ cumulative activities and response criteria. The primary outcomes were disease response rates (DRRs) and disease control rates (DCRs). The definitions of DRRs and DCRs were described previously (DRRs = proportions of patients with complete response (CR) + partial response (PR), DCRs = proportions of patients with CR+ PR+ stable disease (SD).[12] Overall survival (OS) and progression free survival (PFS) were not assessed because most of the trials were single-arm trials.
2.5. Statistical analysis
We used the Review Manager (version 5.3) for statistical analyses. The efficacy of 177Lu-octreotate/octreotide treatment was assessed depending on 2 indicators: DRRs and DCRs. A Cochran Q test was used to assess heterogeneity between studies and I2 statistic was used to show the magnitude of the heterogeneity. For categorical variables, the pooled estimation of effects was calculated with a random-effects model or a fixed-effects model. If I2 value >50%, a random-effects model was used, otherwise we use a fixed-effects model. Funnel plots were performed to assess the potential publication bias. A 2-tailed P value of less than .05 was considered statistically significant.
3. Results
3.1. Study characteristics
We identified a total of 716 articles, and thereof 148 conference reports/editorials/meetings/news and 445 reviews/case reports (patients < 10)/comments/abstracts were excluded. The remaining 123 potentially relevant publications were retrieved for detailed assessment,and 64 studies were excluded because the research subjects were irrelevant. After a further detailed review of the remained 59 articles, 37 articles were excluded for inadequate data or duplicated data, and 22 studies including 1758 patients were eligible for inclusion criteria. The flow chart is shown in Figure 1. For each selected study, data quantification was completely assessed.
Figure 1.
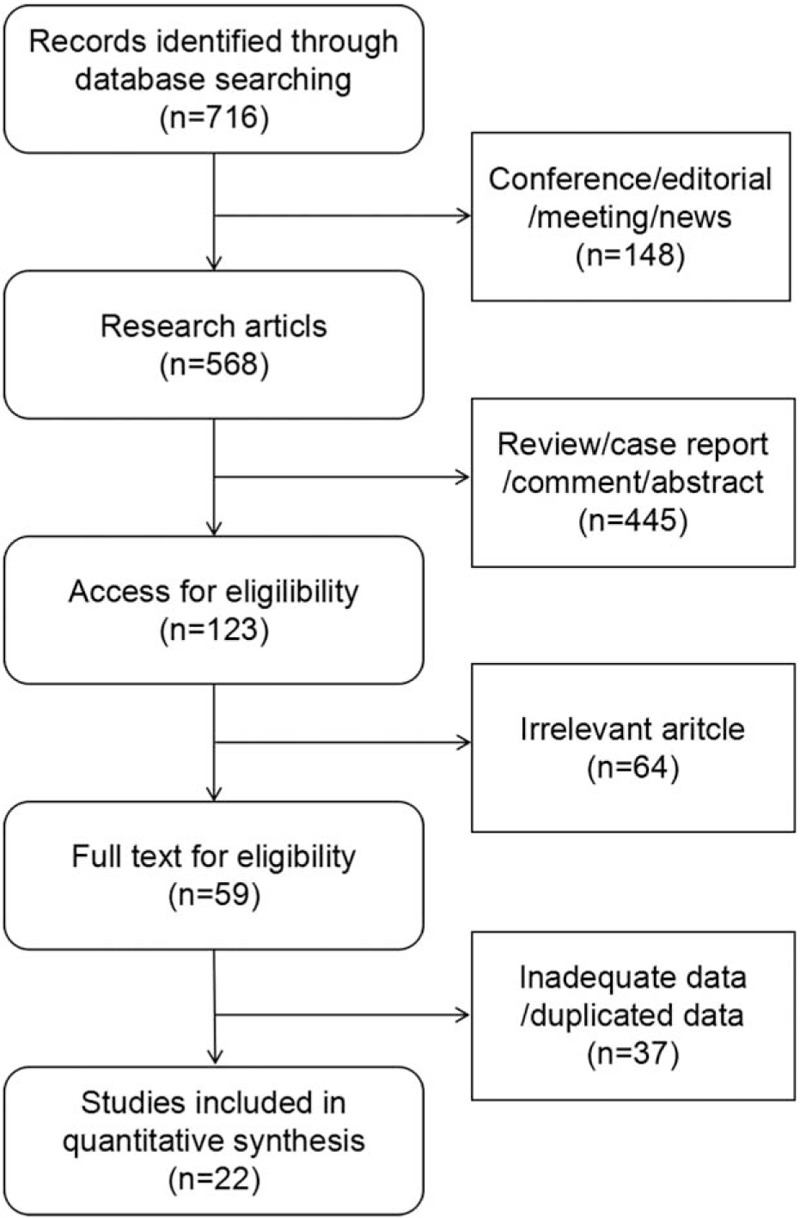
Selection of studies included in the meta-analysis.
In this meta-analysis, tumor response was evaluated by Response Evaluation Criteria in Solid Tumors (RECIST), RECIST 1.1, Southwest Oncology Group (SWOG) criteria, World Health Organization (WHO) criteria, or more than one criterion. 8 studies were evaluated by RECIST. 10 trials were evaluated by RECIST 1.1. Five trials were based on SWOG criteria. One trial was assessed by WHO criteria. There were 2 articles evaluated by 2 criteria, of them 1 by RECIST and RECIST 1.1, the other by RECIST 1.1 and SWOG.[14] The characteristics of these trials are shown in Table 2.
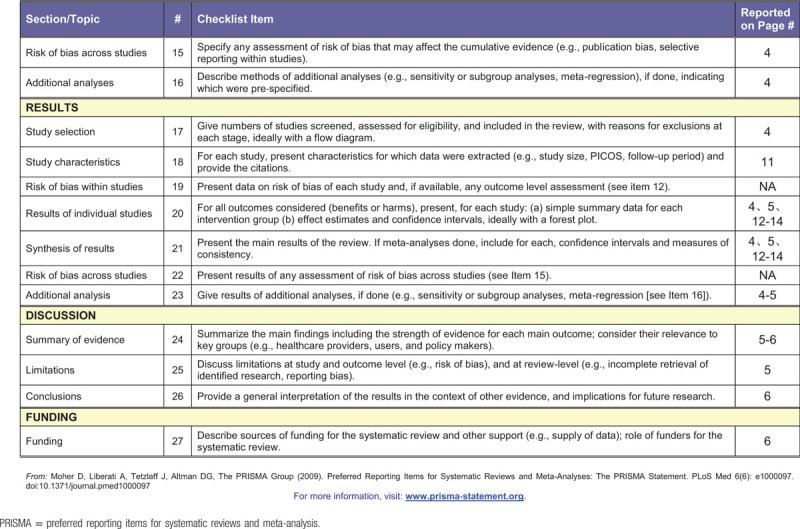
Table 1 (Continued).
PRISMA checklist.
3.2. DRRs and DCRs
Detailed data of these selected studies data, DRRs and DCRs are demonstrated in Table 3. The pooled rates were presented with a random-effects model or a fixed-effects model on the basis of magnitude of the heterogeneity. There was only 1 article evaluated by WHO criteria, so it was not included in the following assessment.
Table 2.
List of study characteristics.
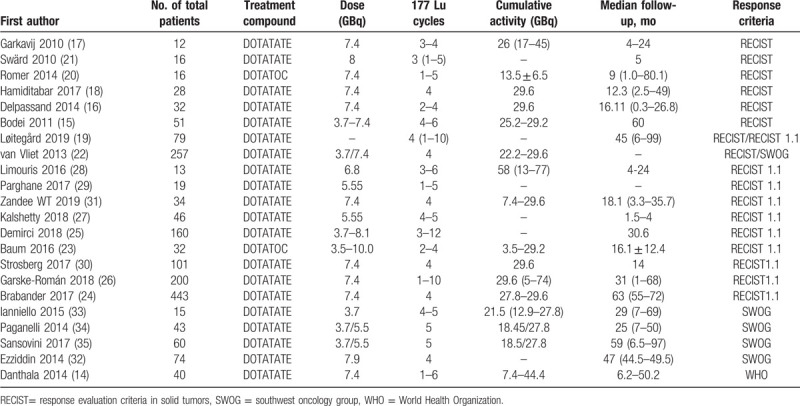
Table 3.
Disease response and control rates of 177Lu-labelled PRRT.
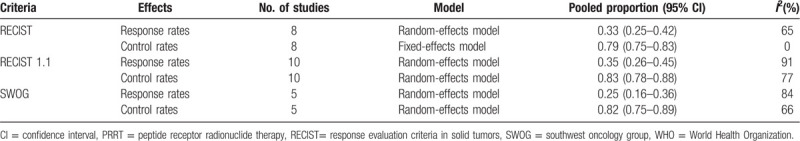
3.2.1. RECIST criteria group
For RECIST criteria, 8 studies with 478 patients were analyzed.[15–22] As shown in Figure 2, the test for heterogeneity showed heterogeneity for DRRs (I2 = 65%, P = .006). DRRs ranged between 16.7% and 53.0%. The random-effects model showed a pooled effect of 33.0% (95% CI: 25.0%-42.0%) for DRRs. As for DCRs, the test for heterogeneity performed no statistical significance (I2 = 0%, P = .62). DCRs ranged from 72.0% to 100%. The pooled effect was 79.0% (95% CI: 75.0%-83.0%) for DCRs according to the fixed-effects model.
Figure 2.
Forest plots of proportions of disease response rates (A) and disease control rates (B) in RECIST criteria group. RECIST= response evaluation criteria in solid tumors.
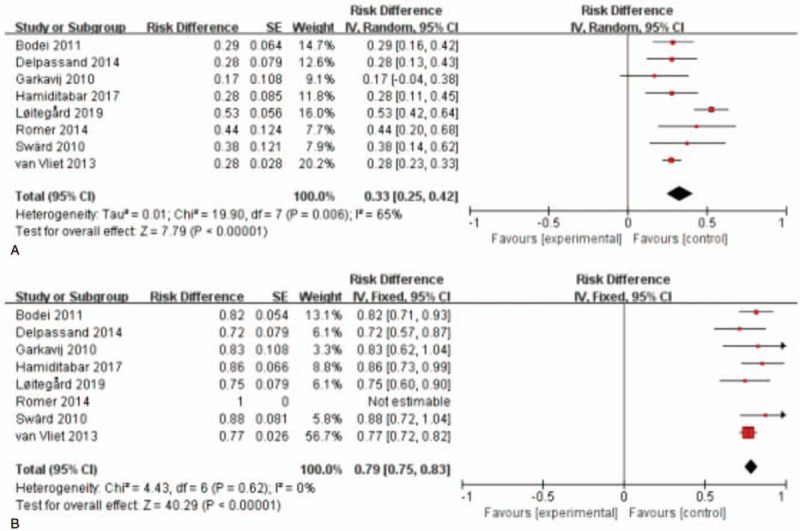
3.2.2. RECIST 1.1 criteria group
For RECIST1.1 criteria, 10 studies with 1127 patients were analyzed.[19,23–31] As shown in Figure 3, the test for heterogeneity showed heterogeneity for DRRs (I2 = 91%, P < .001). DRRs ranged between 10.0% and 69.0%. The random-effects model showed a pooled effect of 35.0% (95% CI: 26.0%-45.0%) for DRRs. As for DCRs, the test for heterogeneity presented statistical significance (I2 = 77%, P < .001) among these articles. DCRs ranged from 68.0% to 93.8%. The pooled effect was 83.0% (95% CI: 78.0%-88.0%) for DCRs according to the random-effects model.
Figure 3.
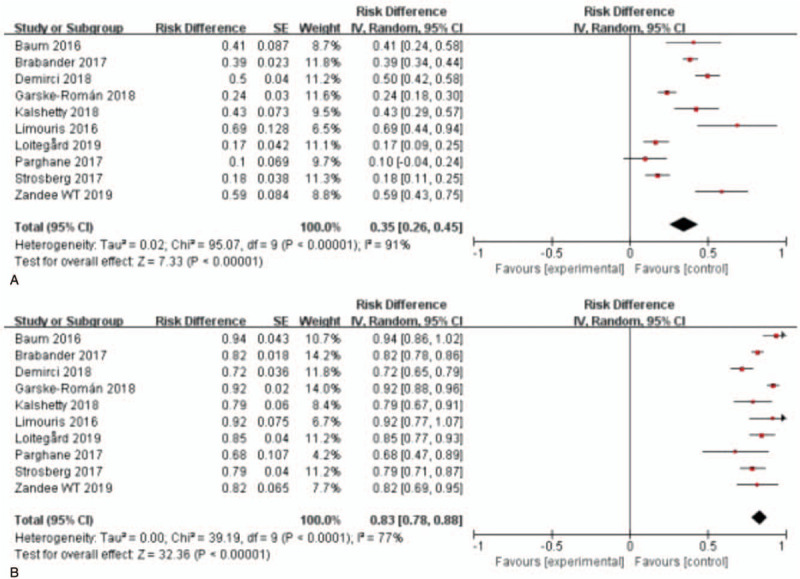
Forest plots of proportions of disease response rates (A) and disease control rates (B) in RECIST 1.1 criteria group. RECIST= response evaluation criteria in solid tumors.
3.2.3. SWOG criteria group
For SWOG criteria, 5 studies with 459 patients were analyzed.[22,32–35] As shown in Figure 4, the test for heterogeneity showed heterogeneity for DRRs (I2 = 84%, P < .001). DRRs ranged between 7% and 36.5%. The random-effects model showed a pooled effect of 25.0% (95% CI: 14.0%-36.0%) for DRRs. For DCRs, the test for heterogeneity showed some significance (I2 = 66%, P = .02) among these articles. DCRs ranged from 74.0% to 89.2%. The pooled effect was 82.0% (95% CI: 75.0%-89.0%) for DCRs according to the random-effects model.
Figure 4.
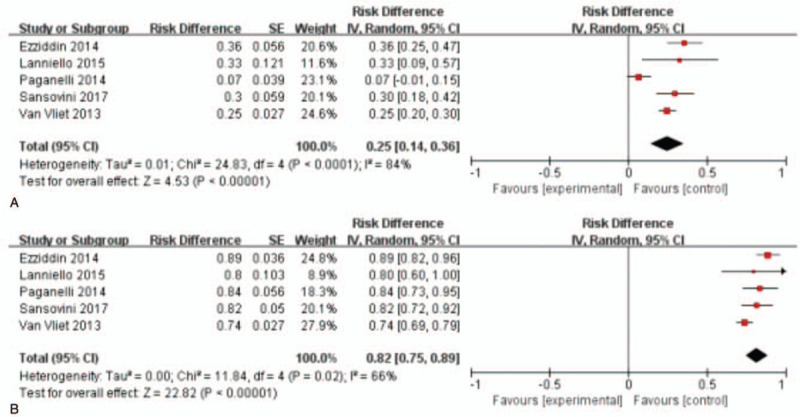
Forest plots of proportions of disease response rates (A) and disease control rates (B) in SWOG criteria group. SWOG = southwest oncology group.
4. Discussion
In this meta-analysis, 22 high-quality published articles containing 1758 inoperable or metastatic NETs patients who adopted 177Lu-labelled PRRT were included. The evaluation of treatment efficacy was performed by RECIST or RECIST 1.1 or SWOG. The results showed that the pooled effects of DRRs were 33.0% (95% CI: 25.0%-42.0%) by RECIST, 35.0% (95% CI: 26.0%-45.0%) by RECIST 1.1 and 25.0% (95% CI: 14.0%-36.0%) by SWOG, while the DCRs were 79.0% (95% CI: 75.0%-83.0%) by RECIST, 83.0% (95% CI: 78.0%-88.0%) by RECIST 1.1 and 82.0% (95% CI: 75.0%-89.0%) by SWOG. Based on these results, we concluded that 177Lu-labelled PRRT displayed encouraging treatment efficiency for advanced NETs.
Meanwhile, I2 statistical test demonstrated significant heterogeneity among the studies in different criteria groups with an exception of the analysis of DCRs in RECIST criteria group. The heterogeneity may be attributed to differences in basic characteristics of the study populations, locations of the study, drug compliance in each study, batch of drug and correction of relevant factors. Due to the limited information in individual studies, subgroup analysis or meta-regression were not applicable to assay the sources of heterogeneity in this meta-analysis. In consequence, the results of this analysis should be interpreted with caution especially when extrapolation was considered.
Recently, a phase III clinical trial (NETTER-1) designed for evaluating the efficacy and safety of 177Lu-DOTATATE PRRT in patients with advanced, SSTR positive and G1/G2 midgut NET has published their stage results.[30] At the data cutoff date for the cohort, compared to the control group (high-dose octreotide long-acting repeatable group), 177Lu-DOTATATE group had more patients survived more than 20 months (65.2% vs 10.8%). The DRR, evaluated by RECIST 1.1, was 18% in the 177Lu-DOTATATE group while in the control group it was 3% (P < .001). Another large clinical trial with over 200 patients treated with 177Lu-DOTATATEPRRT showed that the quality of life and symptoms were improved in 40% to 70% of cases depending on the preexistence of a certain condition.[36] Strosberg et al found that compared with the control group (high-dose octreotide long-acting repeatable group), 177Lu-DOTATATE PRRT group demonstrated a longer PFS (28.4 months vs 8.5 months).[37] Altogether, repeated cycles of 177Lu-DOTATATE PRRT provided an obvious improvement of the quality of life and prolonged the patients’ survival time.
There was no obvious acute toxicity during or immediately after the 177Lu-DOTATATE treatment. The maximum toleration of this treatment was up to 29 GBq cumulative activity (up to 7.4 GBq/cycle) with minimal hematological or renal damage.[15,25] In a study by Danthala et al, there was no significant impact on white blood cells or platelets and no renal toxicity was observed during PRRT with 177Lu-DOTATOC and 24 months after treatment.[14] Nausea and vomiting were the most common side effects, followed by transient skin redness.[16] Delpassand et al. found that hematological toxicity and bone metastasis may occur after the repeated cycles of 177Lu-DOTATATE PRRT, and these side effects were associated with the prior history of chemotherapy treatment.[16]
5. Conclusion
This meta-analysis demonstrated that the curative effect of repeated treatment with 177Lu-octreotate/octreotide PRRT was promising in advanced NETs patients. Up to date, there are still several clinical studies in progression, so we will get more information to validate this therapeutic modality.
Author contributions
Data curation: Lin Lin, Meng-jiao Wang.
Formal analysis: Lin Lin, Meng-jiao Wang.
Writing – original draft: Li-fan Wang.
Writing – review and editing: Yong Li.
Footnotes
Abbreviations: 177Lu-DOTATATE/DOTATOC = 177Lu-octreotate/octreotide, DCRs = disease control rates, DRRs = disease response rates, NETs = neuroendocrine tumors, PFS = progression free survival, PRISMA = preferred reporting items for systematic reviews and meta-analysis, PRRT = peptide receptor radionuclide therapy, RECIST = response evaluation criteria in solid tumors, SWOG = Southwest Oncology Group, WHO = World Health Organization.
How to cite this article: Wang Lf, Lin L, Wang Mj, Li Y. The therapeutic efficacy of 177Lu-DOTATATE/DOTATOC in advanced neuroendocrine tumors: A meta-analysis. Medicine. 2020;99:10(e19304).
The project described was supported by the National Natural Science Foundation of China (NO. 81430088).
The authors have no conflicts of interest to disclose.
References
- [1].Oronsky B, Ma PC, Morgensztern D, et al. Nothing but NET: a review of neuroendocrine tumors and carcinomas. Neoplasia 2017;19:991–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Guadagno E, De Caro Mdel B, Insabato L. An update on the pathology of neuroendocrine tumors. Front Biosci 2016;8:1–2. [DOI] [PubMed] [Google Scholar]
- [3].Dasari A, Shen C, Halperin D, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol 2017;3:1335–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008;26:3063–72. [DOI] [PubMed] [Google Scholar]
- [5].Chauhan A, Yu Q, Ray N, et al. Global burden of neuroendocrine tumors and changing incidence in Kentucky. Oncotarget 2018;9:19245–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Taal BG, Visser OJN. Epidemiology of neuroendocrine tumours. Neuroendocrinology 2004;80:3–7. [DOI] [PubMed] [Google Scholar]
- [7].Narayanan S, Kunz PL. Role of somatostatin analogues in the treatment of neuroendocrine tumors. Hematol Oncol Clin North Am 2016;30:163–77. [DOI] [PubMed] [Google Scholar]
- [8].Krenning EP, Kooij PP, Bakker WH, et al. Radiotherapy with a radiolabeled somatostatin analogue, [111In-DTPA-D-Phe1]-octreotide. A case history. Ann N Y Acad Sci 2010;733:496–506. [DOI] [PubMed] [Google Scholar]
- [9].Zlatko D, Jarrett R, Braat AJA, et al. The efficacy of hepatic 90Y resin radioembolization for metastatic neuroendocrine tumors: a meta-analysis. J Nucl Med 2014;55:1404–10. [DOI] [PubMed] [Google Scholar]
- [10].Seregni E, Maccauro M, Coliva A, et al. Treatment with tandem [90Y]DOTA-TATE and [177Lu] DOTA-TATE of neuroendocrine tumors refractory to conventional therapy: preliminary results. Q J Nucl Med Mol Imaging 2010;54:84–91. [PubMed] [Google Scholar]
- [11].Bodei L, Mueller-Brand J, Baum RP, et al. The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. Eur J Nucl Med Mol Imaging 2013;40:800–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kim SJ, Pak K, Koo PJ, et al. The efficacy of 177Lu-labelled peptide receptor radionuclide therapy in patients with neuroendocrine tumours: a meta-analysis. Eur J Nucl Med Mol Imaging 2015;42:1964–70. [DOI] [PubMed] [Google Scholar]
- [13].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010;8:336–41. [DOI] [PubMed] [Google Scholar]
- [14].Danthala M, Kallur KG, Prashant GR, et al. 177Lu-DOTATATE therapy in patients with neuroendocrine tumours: 5 years’ experience from a tertiary cancer care centre in India. Eur J Nucl Med Mol Imaging 2014;41:1319–26. [DOI] [PubMed] [Google Scholar]
- [15].Bodei L, Cremonesi M, Grana CM, et al. Peptide receptor radionuclide therapy with 177 Lu-DOTATATE: the IEO phase I-II study. Eur J Nucl Med Mol Imaging 2011;38:2125–35. [DOI] [PubMed] [Google Scholar]
- [16].Delpassand ES, Samarghandi A, Zamanian S, et al. Peptide receptor radionuclide therapy with 177Lu-DOTATATE for patients with somatostatin receptor-expressing neuroendocrine tumors: the first US phase 2 experience. Pancreas 2014;43:518–25. [DOI] [PubMed] [Google Scholar]
- [17].Michael G, Mattias N, Katarina SGG, et al. 177Lu-[DOTA0,Tyr3] octreotate therapy in patients with disseminated neuroendocrine tumors: analysis of dosimetry with impact on future therapeutic strategy. Cancer 2010;116(S4):1084–92. [DOI] [PubMed] [Google Scholar]
- [18].Hamiditabar M, Ali M, Roys J, et al. Peptide receptor radionuclide therapy with 177Lu-Octreotate in patients with somatostatin receptor expressing neuroendocrine tumors: six years’ assessment. Clin Nucl Med 2017;42:436–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Loitegard T, Berntzen DT, Thiis-Evensen E. The RECIST criteria compared to conventional response evaluation after peptide receptor radionuclide therapy in patients with neuroendocrine neoplasms. Ann Nucl Med 2019;33:147–52. [DOI] [PubMed] [Google Scholar]
- [20].Romer A, Seiler D, Marincek N, et al. Somatostatin-based radiopeptide therapy with [177Lu-DOTA]-TOC versus [90Y-DOTA]-TOC in neuroendocrine tumours. Eur J Nucl Med Mol Imaging 2014;41:214–22. [DOI] [PubMed] [Google Scholar]
- [21].Sward C, Bernhardt P, Ahlman H, et al. [177Lu-DOTA 0-Tyr 3]-octreotate treatment in patients with disseminated gastroenteropancreatic neuroendocrine tumors: the value of measuring absorbed dose to the kidney. World J Surg 2010;34:1368–72. [DOI] [PubMed] [Google Scholar]
- [22].Vliet EI, Van, Krenning EP, et al. Comparison of response evaluation in patients with gastroenteropancreatic and thoracic neuroendocrine tumors after treatment with [177Lu-DOTA0,Tyr3]octreotate. J Nucl Med 2013;54:1689–96. [DOI] [PubMed] [Google Scholar]
- [23].Baum RP, Kluge AW, Harshad K, et al. [177Lu-DOTA]0-D-Phe1-Tyr3-Octreotide (177Lu-DOTATOC) for peptide receptor radiotherapy in patients with advanced neuroendocrine tumours: a phase-II study. Theranostics 2016;6:501–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Brabander T, Wa VDZ, Teunissen JJ, et al. Long-term efficacy, survival and safety of [177Lu-DOTA0,Tyr3]octreotate in patients with gastroenteropancreatic and bronchial neuroendocrine tumors. Clin Cancer Res 2017;23:4617–24. [DOI] [PubMed] [Google Scholar]
- [25].Demirci E, Kabasakal L, Toklu T, et al. 177Lu-DOTATATE therapy in patients with neuroendocrine tumours including high-grade (WHO G3) neuroendocrine tumours: response to treatment and long-term survival update. Nucl Med Commun 2018;39:789. [DOI] [PubMed] [Google Scholar]
- [26].Garskeromán U, Sandström M, Fröss KB, et al. Prospective observational study of 177Lu-DOTA-octreotate therapy in 200 patients with advanced metastasized neuroendocrine tumours (NETs): feasibility and impact of a dosimetry-guided study protocol on outcome and toxicity. Eur J Nucl Med Mol Imaging 2018;45:970–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kalshetty A, Ramaswamy A, Ostwal V, et al. Resistant functioning and/or progressive symptomatic metastatic gastroenteropancreatic neuroendocrine tumors: efficacy of 177Lu-DOTATATE peptide receptor radionuclide therapy in this setting. Nucl Med Commun 2018;39:39. [DOI] [PubMed] [Google Scholar]
- [28].Limouris GS, Poulantzas V, Trompoukis N, et al. Comparison of 111In-[DTPA0]Octreotide versus non carrier added 177Lu- [DOTA0,Tyr3]-octreotate efficacy in patients with GEP-NET treated intra-arterially for liver metastases. Clin Nucl Med 2016;41:194. [DOI] [PubMed] [Google Scholar]
- [29].Parghane RV, Talole S, Prabhash K, et al. Clinical response profile of metastatic/advanced pulmonary neuroendocrine tumors to peptide receptor radionuclide therapy with 177Lu-DOTATATE. Clin Nucl Med 2017;42:428–35. [DOI] [PubMed] [Google Scholar]
- [30].Strosberg J, El-Haddad G, Wolin E, et al. Phase 3 Trial of 177Lu-Dotatate for midgut neuroendocrine tumors. N Engl J Med 2017;376:125–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zandee WT, Brabander T, Blazevic A, et al. Symptomatic and radiological response to 177Lu-DOTATATE for the treatment of functioning pancreatic neuroendocrine tumors. J Clin Endocrinol Metab 2019;104:1336–44. [DOI] [PubMed] [Google Scholar]
- [32].Ezziddin S, Khalaf F, Vanezi M, et al. Outcome of peptide receptor radionuclide therapy with 177Lu-octreotate in advanced grade 1/2 pancreatic neuroendocrine tumours. Eur J Nucl Med Mol Imaging 2014;41:925–33. [DOI] [PubMed] [Google Scholar]
- [33].Ianniello A, Sansovini M, Severi S, et al. Peptide receptor radionuclide therapy with 177Lu-DOTATATE in advanced bronchial carcinoids: prognostic role of thyroid transcription factor 1 and 18F-FDG PET. Eur J Nucl Med Mol Imaging 2015;43:1040–6. [DOI] [PubMed] [Google Scholar]
- [34].Paganelli G, Sansovini M, Ambrosetti A, et al. 177 Lu-Dota-octreotate radionuclide therapy of advanced gastrointestinal neuroendocrine tumors: results from a phase II study. Eur J Nucl Med Mol Imaging 2014;41:1845–51. [DOI] [PubMed] [Google Scholar]
- [35].Sansovini M, Severi S, Ianniello A, et al. Long-term follow-up and role of FDG PET in advanced pancreatic neuroendocrine patients treated with 177Lu-D OTATATE. Eur J Nucl Med Mol Imaging 2016;44:1–0. [DOI] [PubMed] [Google Scholar]
- [36].Khan S, Krenning EP, Essen MV, et al. Quality of life in 265 patients with gastroenteropancreatic or bronchial neuroendocrine tumors treated with [177Lu-DOTA0,Tyr3]Octreotate. J Nucl Med 2011;52:1361. [DOI] [PubMed] [Google Scholar]
- [37].Strosberg J, Wolin E, Chasen B, et al. Health-related quality of life in patients with progressive midgut neuroendocrine tumors treated with 177Lu-Dotatate in the Phase III NETTER-1 trial. J Clin Oncol 2018;36:2578–84. [DOI] [PMC free article] [PubMed] [Google Scholar]


