Supplemental Digital Content is available in the text
Keywords: α2δ ligands, long-term extension, mirogabalin, peripheral neuropathic pain, postherpetic neuralgia
Abstract
Objective:
Postherpetic neuralgia (PHN) is a condition that results from nerve dysfunction following an episode of acute herpes zoster (shingles). Mirogabalin is a novel, selective oral α2δ ligand that demonstrated safety and efficacy in a multicenter, randomized, double-blind, placebo-controlled 14-week study in Asian patients with PHN. This 52-week, open-label extension study investigated the long-term safety and efficacy of flexible-dosage mirogabalin in Asian patients with PHN.
Methods:
This open-label extension study enrolled patients who completed the placebo-controlled study. Patients started with a dose of 5 mg mirogabalin twice daily (BID), which was followed by a flexible dose of 10 or 15 mg BID. During the study, patients assessed their pain using the Short-Form McGill Pain Questionnaire (SF-MPQ). Adverse events were monitored throughout the study.
Results:
Of 239 enrolled patients, 184 (77.0%) completed the study and 185 patients (77.4%) received the 15 mg BID dose most during the treatment duration. Most treatment-emergent adverse events (TEAEs) were mild or moderate. The most common TEAEs were nasopharyngitis, somnolence, dizziness, weight increased, and edema. All SF-MPQ scales decreased from baseline to week 52.
Conclusions:
This study showed the safety and stable pain management of a long-term flexible dosing regimen of mirogabalin 10 or 15 mg twice daily for 52 weeks in patients with PHN.
Clinical Trial Registered at ClinicalTrials.gov:
Summary for Table of Contents:
Mirogabalin—a novel α2δ oral ligand—was shown to be effective and well tolerated for treating postherpetic neuralgia (PHN) in an Asian multicenter, randomized, double-blind, placebo-controlled, 14-week study. This open-label, 52-week study was conducted as an extension of the double-blind study to demonstrate long-term safety and efficacy of mirogabalin.
1. Introduction
Postherpetic neuralgia (PHN) is a condition that is initiated by a dysfunction in the nervous system caused by acute herpes zoster (shingles). It is characterized by chronic peripheral neuropathic pain persisting for more than 3 months after the acute phase of herpes zoster, which is the result of reactivation of a latent varicella zoster virus.[1] Herpes zoster patients who are older or immunocompromised are more likely to develop PHN.[1] In the UK, 5.8% of patients with herpes zoster develop PHN, while approximately 20% of Japanese adults 50 years or older with herpes zoster develop PHN.[1,2] Patients experiencing PHN often report sensations of itching, burning, throbbing, or stabbing. The pain is often discretely localized, intermittent, unilateral, and intense enough to cause sleep interference.[3] These symptoms have a significant negative impact on patient quality of life as assessed by physical and mental health measurements.[4,5]
Peripheral neuropathic pain has been linked to the upregulation of the α2δ-1 subunit of voltage-gated calcium channels in the nervous system.[6–8] The α2δ-1 ligands are thought to exert analgesic effects by preventing the trafficking of the α2δ-1 subunit to presynaptic terminals, decreasing presynaptic calcium influx, and consequently, reducing excitatory neurotransmitter release (e.g., glutamate).[7–9]
Mirogabalin monobenzenesulfonate (herein referred to as mirogabalin, Daiichi Sankyo, Ltd., Tokyo, Japan) is an oral selective α2δ ligand that has demonstrated efficacy in patients with PHN. When compared with placebo, mirogabalin improved the average daily pain score in Asian patients with PHN in a 14-week, phase 3, randomized, double-blind, placebo-controlled, parallel-group study.[10] However, the long-term efficacy and safety of mirogabalin remained unclear.
This 52-week, open-label extension of the phase 3 study investigated the long-term safety and efficacy of flexible-dosage mirogabalin in Asian patients with PHN.
2. Methods
2.1. Overview of study design
This is a multinational (Asian), open-label, 52-week, extension study followed the randomized, double-blind, placebo-controlled, 14-week phase 3 study for patients with PHN (NCT02318706).[10] This extension study was conducted between May 1, 2015, and May 25, 2017, in approximately 200 study sites in Japan, Korea, Taiwan, Singapore, Malaysia, and Thailand. The study was approved by the institutional review board, or equivalent, for each site before beginning. Before enrollment, informed consent was obtained from all patients. Safety was periodically monitored by an independent Data Safety Monitoring Board.
The design of the double-blind phase 3 study has been described elsewhere and is briefly described here.[10] In the double-blind study, 765 patients were randomized 2:1:1:1 to placebo or mirogabalin 15, 20, or 30 mg/day. The initial 14-week study included a 1-to-2-week titration period and a 12-to-13-week fixed-dosage period.
At the end of week 14 of the double-blind study, patients who completed the study, met eligibility criteria, and obtained written informed consent entered this 52-week extension study, which consisted of a 4-week titration period, a 48-week dosage adjustment period, and a 1-week follow-up (Fig. 1). Patients who met eligibility criteria were enrolled in the extension regardless of their assigned treatment in the double-blind study, including patients who had previously been treated with placebo. Mirogabalin was administered orally as a tablet twice daily (in the morning and at bedtime in the same manner as in the phase 3 double-blind study). During the titration period, the dosage was 5 mg twice daily for the first 2 weeks and 10 mg twice daily for the second 2 weeks. From the fifth week, the dosage was increased to 15 mg twice daily if there were no safety issues. For the remainder of the study, the dosage could be changed to either 10 or 15 mg twice daily depending on the safety findings at each visit.
Figure 1.
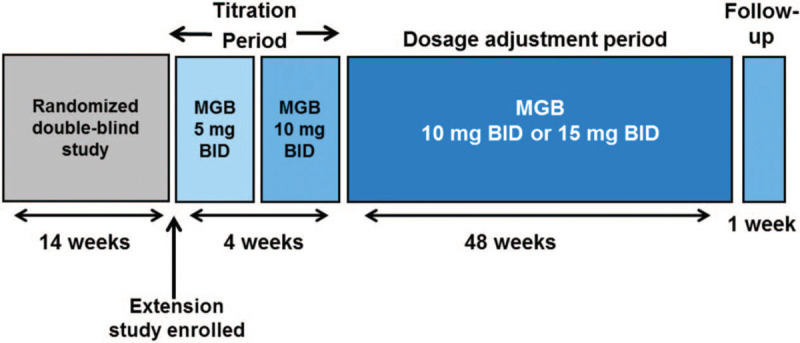
Study design. BID = twice daily, MGB = mirogabalin.
Any concomitant medications or therapies administered to patients during the study were documented regardless of whether they were permitted. Therapies prohibited from the titration period visit (week 1) to the post-treatment follow-up visit (week 53) included pregabalin, gabapentin, and drugs that could cause irreversible retinal degeneration (phenothiazine antipsychotics, deferoxamine, quinine, quinidine, ethambutol, voriconazole, etc.).
This extension study complies with the Declaration of Helsinki and Good Clinical Practice Guidelines as described by the International Council for Harmonisation. Local regulatory requirements were followed when applicable. Written informed consent was obtained from each patient prior to study participation.
2.2. Study population
This study included Asian patients with postherpetic neuralgia who completed 14 weeks of study drug administration in the double-blind study. Patients needed to be able to provide written informed consent, understand study procedures, and adequately complete patient-reported questionnaires for inclusion in the study. Patients were excluded if they had <80% drug compliance during the double-blind study, had creatinine clearance (CrCl; using the Cockcroft-Gault equation) <60 ml/minutes at the end of the double-blind study, or experienced a critical safety issue in the double-blind study. Exclusion criteria also included a known history of positive hepatitis B antigen or hepatitis C antibody, or prior drug treatment that could cause irreversible retinal degeneration. Women were excluded if they were pregnant, nursing, or unwilling to take reliable contraceptive measures throughout the study and for 4 weeks after study completion. Patients could also be excluded at investigator discretion if they were considered inappropriate for the study.
2.3. Safety assessments
Adverse events (AEs) were coded according to the Medical Dictionary for Regulatory Activities version 17.1 and were monitored throughout the study. Treatment-emergent AEs (TEAEs) were used for statistical analyses; these were defined as any AEs that emerged on or after the first dosing of the study and during the study treatment duration (having been absent prior to treatment) or worsened relative to the pretreatment state. Assessments of clinical vital signs, 12-lead electrocardiogram, and laboratory evaluations were performed. Additional safety endpoints included body weight, physical examinations, edema evaluation, neurological examination, ophthalmologic examination, Columbia-Suicide Severity Rating Scale (C-SSRS), Hospital Anxiety and Depression Scale (HADS).
Of approximately 400 subjects expected to enroll in the long-term study, over 150 subjects were expected to complete 1-year treatment with mirogabalin. This meets the recommendations generally agreed upon in the International Conference on Harmonisation E1 guideline (100 subjects exposed for a minimum of 1 year).[11]
2.4. Efficacy assessments
Patients used the Short-Form McGill Pain Questionnaire (SF-MPQ) to self-assess their pain from the baseline to the end of treatment/early termination visit.[12] Assessments were recorded every 2 weeks during the titration period, and every 4 weeks during the dosage adjustment period. Assessments from the SF-MPQ included the sensory score, affective score, total score, visual analogue scale (VAS), and the present pain intensity index. The baseline value was defined as the last non-missing available value prior to first dose of the study drug in the open-label extension study.
2.5. Statistical analysis
All safety analyses and efficacy analyses were conducted using the safety and efficacy analyses set, respectively, which were defined as all patients who received at least 1 dose of the study medication. As a safety analysis, TEAEs were summarized using a frequency table. For efficacy analysis, mean and change from baseline for the sensory score, affective score, total score, and VAS in SF-MPQ were summarized at each scheduled visit. At Week 52, the summaries using the last observation carried forward imputation were also calculated. Baseline value is defined as the last available non-missing value prior to first dose of the extension study. All statistical analyses were performed using Statistical Analysis System software version 9.3 or higher (SAS Institute, Cary, NC, USA).
3. Results
3.1. Patients
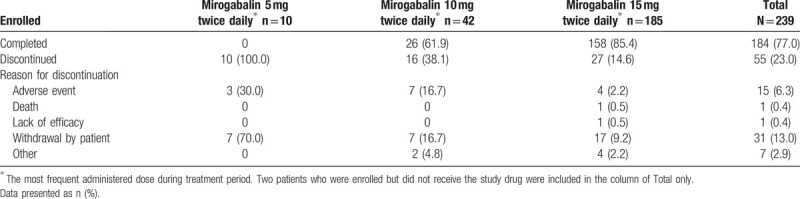
Of the 239 enrolled patients (all of whom provided informed consent), 237 received at least 1 study drug dose and were included in the safety and efficacy analysis sets, 184 patients (77.0%) completed the study and 55 patients (23.0%) discontinued the study (Supplemental Fig. 1). The reasons for study discontinuation were withdrawal by patient (31 patients [13.0%]), AE (15 patients [6.3%]), other (7 patients [2.9%]), death (1 patient [0.4%]), and lack of efficacy (1 patient [0.4%]) (Table 1, Supplemental Fig. 1). Patients who received 15 mg BID most frequently during their treatment duration were 77.4% of the enrolled patients.
Table 1.
Patient disposition.
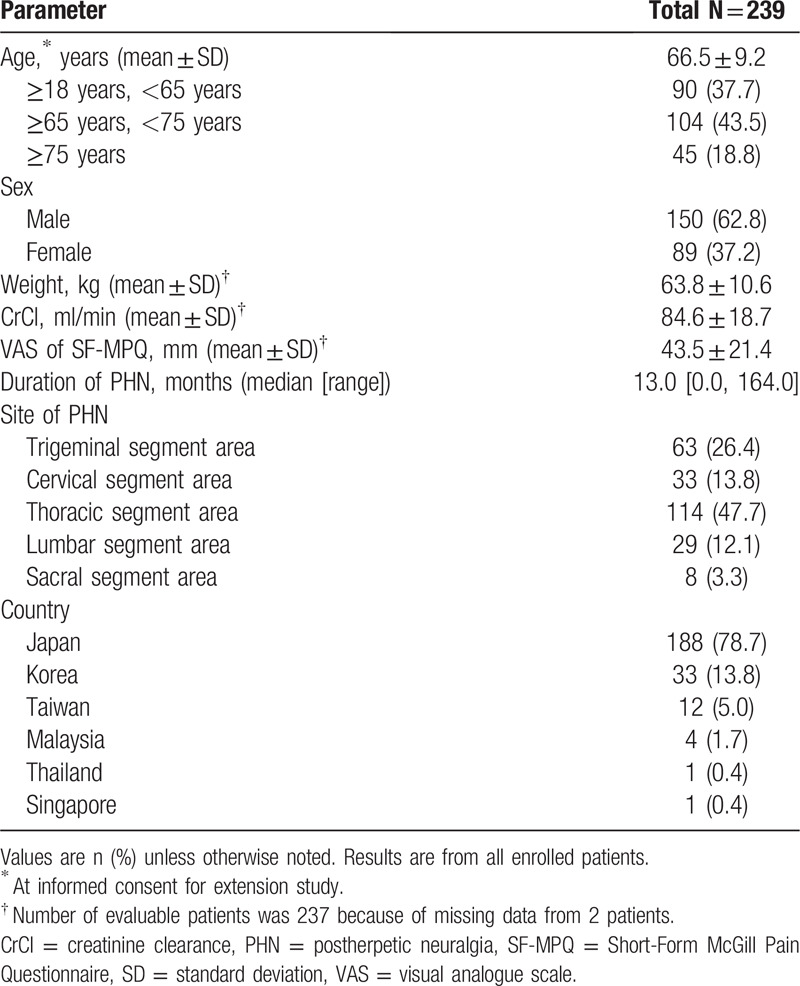
Demographics and baseline characteristics for enrolled patients are shown in Table 2. Most patients were male (150/239, 62.8%) and enrolled in Japan (188/239, 78.7%). The mean (standard deviation [SD]) age at the time of informed consent was 66.5 (9.2) years. At baseline, the mean (SD) body weight was 63.8 (10.6) kg, and body mass index was 24.26 (3.1) kg/m2. The mean baseline pain score in VAS was 43.5 mm, and median (range) duration of PHN at randomization of the previous double-blind study was 13.0 (0–164) months.
Table 2.
Demographics and baseline characteristics.
3.2. Safety
Patients received treatment for mean (SD; median) of 313.4 (106.7; 365.0) days. Overall, 85.7% of patients experienced at least 1 TEAE. The most common TEAEs were nasopharyngitis (16.5%), somnolence (15.2%), dizziness (11.0%), weight increased (9.3%), and edema (5.9%) (Table 3). Most TEAEs were mild or moderate and resolved without any treatment. There were 10 severe TEAEs reported (4.2%) for 1 patient each: pneumonia, breast cancer, hyperglycemia, dizziness, acute myocardial infarction, gastric ulcer hemorrhage, blood triglycerides increased, femur fracture, laceration, and road traffic accident. Serious TEAEs were reported in 8.4% of patients; all were considered unrelated to the study drug. One death due to AE of completed suicide was reported. This incident was not handled as a TEAE, because it occurred 42 days after the last dose (15 mg BID) of study drug, and was reported after the completion of the TEAE evaluation period. TEAEs leading to treatment discontinuation occurred in 8.4% of patients; most of them were mild or moderate, and were resolved or are resolving without any treatment.
Table 3.
Most frequent (≥2%) TEAEs.
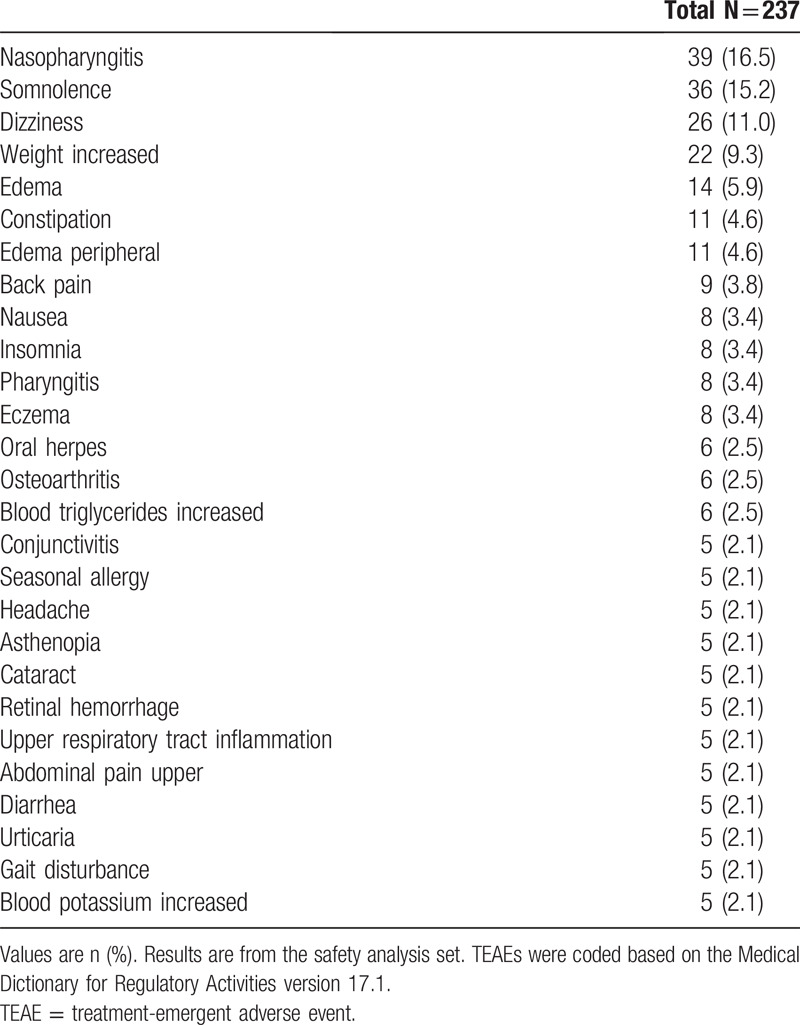
TEAEs related to the study drug occurred in 39.7% of patients analyzed. The most common (reported for ≥2% of patients) were somnolence (13.5%), dizziness (10.1%), weight increased (7.2%), edema (4.2%), and peripheral edema (2.5%). With 1 exception (a severe AE of dizziness), all TEAEs related to the study drug were mild or moderate.
No notable changes were found in hematology/blood chemistry parameters, urinalysis findings, vital signs, blood pressure, or pulse rate over time. Clinically significant 12-lead electrocardiogram abnormalities were found at week 52 in 2 patients: atrial flutter and acute myocardial infarction in 1 patient each. No affirmative answers were recorded to any of the questions in the C-SSRS regarding suicidal behavior and ideation. No patients met the protocol-defined criterion for the “suicidal behavior and ideation” TEAE of special interest. The changes from baseline in the HADS subscales of both depression and anxiety at week 52 of the extension study showed improvement overall.
3.3. Efficacy
Table 4 shows the mean changes from baseline to week 52 in the SF-MPQ. For VAS, the mean (SD) change from baseline at week 52 in the extension study was −12.4 (16.1). The VAS gradually decreased from baseline through week 8 of the extension study and was stable thereafter (Fig. 2). Other subscales of the SF-MPQ (sensory score, affective score, total score, and present pain intensity) decreased from baseline to week 52 of the extension study (Table 4).
Table 4.
Short-form McGill Pain Questionnaire.
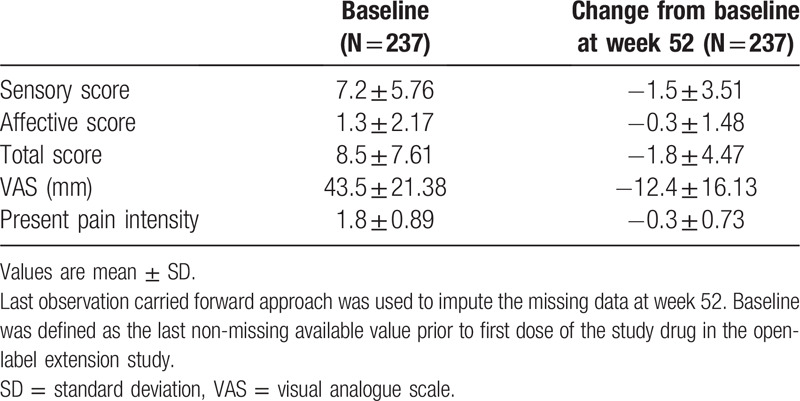
Figure 2.
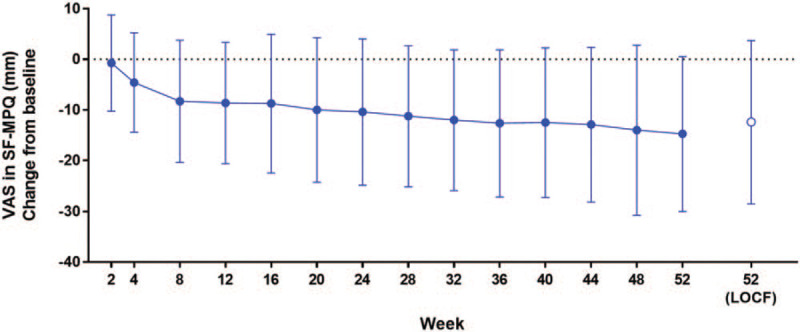
Change from baseline in pain based on short-form McGill Pain Questionnaire Data is shown as mean ± standard deviation. LOCF = last observation carried forward, SF-MPQ = short-form McGill Pain Questionnaire, VAS = visual analogue scale.
4. Discussion
Upregulation of the α2δ-1 subunit of voltage-gated calcium channels has been linked with neuropathic pain, and this subunit is a target for α2δ ligands.[9,13] The analgesic effects of these ligands are believed to prevent trafficking of the subunit to presynaptic terminals, thus reducing neurotransmitter release by decreasing presynaptic calcium influx.[9]
Mirogabalin binds human and rat α2δ subunits with a potent, selective affinity, and a slower dissociation rate for α2δ-1 vs α2δ-2 subunit.[14] In rat models for neuropathic pain, mirogabalin exhibits potent, long-lasting analgesic effects with wider safety margins for nervous system side effects.[14] In addition, the randomized, double-blind, placebo-controlled, phase 3 study demonstrated that the mirogabalin was efficacious and well tolerated for PHN management in Asian patients in doses of 15 to 30 mg/day over a 14-week period.[15]
In this extension study, which enrolled a subset of patients who completed the double-blind phase 3 study, long-term safety and efficacy of mirogabalin were evaluated. The 85.7% of the patients analyzed experienced at least 1 TEAE. The most common TEAEs in the extension study were nasopharyngitis, somnolence, dizziness, weight increased, and edema. The percentage of patients who experienced at least 1 TEAE leading to treatment discontinuation was 8.4% in the extension study. Most of the TEAEs were mild or moderate, and resolved without any treatment. No notable safety concerns were observed in the extension study nor in the randomized double-blind, placebo-controlled phase 3 study. Overall, the TEAE profile observed in this extension study was similar to that in the randomized double-blind, placebo-controlled phase 3 study, in which mirogabalin was administered for short-term (14 weeks). For instance, the percentage of patients who experienced at least 1 TEAE leading to treatment discontinuation was 8.4% in the extension study (vs 6.3% in the double-blind study); serious TEAEs (8.4% vs 2.0%); and severe TEAEs (4.2% vs 2.0%) also had similar profiles in the extension study vs the double-blind study. Drug-related adverse events typical of this drug class include dizziness, fatigue, sedation, somnolence, and ataxia, with frequent reports of peripheral edema and increased weight gain.[16–20] In a 52-week, open-label phase 3 extension trial in Japanese PHN patients treated with pregabalin, a similar AE profile to that observed in this extension study was reported. The most common drug-related AEs in the pregabalin study included dizziness (28.6%), peripheral edema (16.7%), somnolence (15.1%), and increased weight (13.5%).[21]
In terms of efficacy, improvements from baseline in SF-MPQ subscales occurred over the 52-week extension period. This indicates long-term efficacy of mirogabalin for pain relief in PHN patients.
This study has several limitations to consider. First, the study results should be interpreted with caution as the study was open-label without any control arm and included patients who previously received mirogabalin and those who received placebo in the randomized, double-blind study. This limits the efficacy conclusions that can be drawn from this study alone. The nature of the study might bring more bias in safety and efficacy evaluations than the randomized double-blind study with a control arm. Second, this study enrolled a homogenous patient population, with all enrolled patients located in Asia (mostly Japan). Thus, study outcomes reported here may not be consistent in other ethnicities. However, as the pooled safety data from pregabalin (another α2δ ligand) in diabetic peripheral neuropathic pain and PHN patients indicates similar safety outcomes between Japanese and Western patients,[22] it is expected that there would not be large differences in safety profile between Japanese and Western patients. Third, mirogabalin is primarily excreted through renal elimination.[15] However, all patients enrolled in this study met the criteria of having CrCl ≥ 60 ml/minute. Therefore, safety and efficacy were not assessed in PHN patients with renal impairment. Finally, concomitant medications were restricted during the study, which may have also impacted patient outcomes.
In conclusion, the present long-term study showed the safety and stable pain management with a flexible dosing regimen of mirogabalin 10 mg or 15 mg twice daily in patients with PHN.
Acknowledgments
Writing and editorial support was provided by Claire Daniele, PhD, CMPP; and Alicia Salinero, PhD, of AlphaBioCom, LLC, King of Prussia, PA.
Supplementary Acknowledgments
Author contributions
All authors participated in designing and conducting the study, analyzed or interpreted the results, drafted and provided critical review or revision the manuscript, and approved the final draft of the manuscript.
Conceptualization: Jitsu Kato, Norimitsu Matsui, Yoshihiro Kakehi, Emiko Murayama, Shoichi Ohwada.
Data curation: Norimitsu Matsui, Yoshihiro Kakehi, Emiko Murayama.
Formal analysis: Shoichi Ohwada.
Investigation: Jitsu Kato, Norimitsu Matsui, Yoshihiro Kakehi, Emiko Murayama, Shoichi Ohwada.
Methodology: Norimitsu Matsui, Yoshihiro Kakehi, Emiko Murayama, Shoichi Ohwada.
Project administration: Norimitsu Matsui, Yoshihiro Kakehi, Emiko Murayama.
Supervision: Jitsu Kato, Norimitsu Matsui.
Validation: Shoichi Ohwada.
Writing – original draft: Jitsu Kato, Yoshihiro Kakehi, Norimitsu Matsui, Emiko Murayama, Shoichi Ohwada.
Writing – review & editing: Jitsu Kato, Yoshihiro Kakehi, Norimitsu Matsui, Yoshihiro Kakehi, Emiko Murayama, Shoichi Ohwada.
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: AE = adverse event, BID = twice daily, CrCl = creatinine clearance, C-SSRS = Columbia-Suicide Severity Rating Scale, HADS = Hospital Anxiety and Depression Scale, LOCF = last observation carried forward, MGB = mirogabalin, PHN = postherpetic neuralgia, SD = standard deviation, SF-MPQ = Short-Form McGill Pain Questionnaire, TEAE = treatment-emergent adverse event, VAS = visual analog scale.
How to cite this article: Kato J, Matsui N, Kakehi Y, Murayama E, Ohwada S. Long-term safety and efficacy of mirogabalin in Asian patients with postherpetic neuralgia: Results from an open-label extension of a multicenter randomized, double-blind, placebo-controlled trial. Medicine. 2020;99:36(e21976).
This study was funded by Daiichi Sankyo, Co., Ltd., Tokyo, Japan.
E.M., N.M., S.O., and Y.K. are employees of Daiichi Sankyo Co., Ltd. J.K. reports personal fees from Daiichi Sankyo Co., Ltd., during the conduct of the study; and personal fees from Pfizer Japan Inc.; Eisai Co., Ltd.; and Mochida Pharmaceutical Co., Ltd.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request at https://vivli.org/ourmember/daiichi-sankyo/.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
References
- [1].Forbes HJ, Bhaskaran K, Thomas SL, et al. Quantification of risk factors for postherpetic neuralgia in herpes zoster patients: a cohort study. Neurology 2016;87:94–102.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Takao Y, Miyazaki Y, Okeda M, et al. Incidences of herpes zoster and postherpetic neuralgia in japanese adults aged 50 years and older from a community-based prospective cohort study: the shez study. J Epidemiol 2015;25:617–25.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Nalamachu S, Morley-Forster P. Diagnosing and managing postherpetic neuralgia. Drugs Aging 2012;29:863–9.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Jensen MP, Chodroff MJ, Dworkin RH. The impact of neuropathic pain on health-related quality of life: review and implications. Neurology 2007;68:1178–82.. [DOI] [PubMed] [Google Scholar]
- [5].Mizukami A, Sato K, Adachi K, et al. Impact of herpes zoster and post-herpetic neuralgia on health-related quality of life in Japanese adults aged 60 years or older: results from a prospective, observational cohort study. Clin Drug Investig 2018;38:29–37.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Field MJ, Cox PJ, Stott E, et al. Identification of the a2d-1 subunit of voltage-dependent calcium channels as a molecular target for pain mediating the analgesic actions of pregabalin. Proc Natl Acad Sci U S A 2006;103:17537–42.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Li CY, Zhang XL, Matthews EA, et al. Calcium channel α2δ–1 subunit mediates spinal hyperexcitability in pain modulation. Pain 2006;125:20–34.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Li KW, Yu YP, Zhou C, et al. Calcium channel alpha2delta1 proteins mediate trigeminal neuropathic pain states associated with aberrant excitatory synaptogenesis. J Biol Chem 2014;289:7025–37.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bauer CS, Nieto-Rostro M, Rahman W, et al. The increased trafficking of the calcium channel subunit alpha2delta-1 to presynaptic terminals in neuropathic pain is inhibited by the α2δ ligand pregabalin. J Neurosci 2009;29:4076–88.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kato J, Matsui N, Kakehi Y, et al. Mirogabalin for the management of postherpetic neuralgia: a randomized, double-blind, placebo-controlled phase 3 study in Asian patients. Pain 2019;160:1175–85.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH Harmonised Tripartite Guideline: The Extent of Population Exposure to Assess Clinical Safety for Drugs Intended for Long-term Treatment of Non-Life-Threatening Conditions E1; 1994. https://database.ich.org/sites/default/files/E1_Guideline.pdf. Accessed August 18, 2020. [Google Scholar]
- [12].Melzack R. The short-form McGill pain questionnaire. Pain 1987;30:191–7.. [DOI] [PubMed] [Google Scholar]
- [13].Chen J, Li L, Chen SR, et al. The alpha2delta-1-NMDA receptor complex is critically involved in neuropathic pain development and gabapentin therapeutic actions. Cell Rep 2018;22:2307–21.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Domon Y, Arakawa N, Inoue T, et al. Binding characteristics and analgesic effects of mirogabalin, a novel ligand for the alpha2delta subunit of voltage-gated calcium channels. J Pharmacol Exp Ther 2018;365:573–82.. [DOI] [PubMed] [Google Scholar]
- [15].Kato M, Tajima N, Shimizu T, et al. Pharmacokinetics and safety of a single oral dose of mirogabalin in japanese subjects with varying degrees of renal impairment. J Clin Pharmacol 2018;58:57–63.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Calandre EP, Rico-Villademoros F, Slim M. Alpha2delta ligands, gabapentin, pregabalin and mirogabalin: a review of their clinical pharmacology and therapeutic use. Expert Rev Neurother 2016;16:1263–77.. [DOI] [PubMed] [Google Scholar]
- [17].Derry S, Cording M, Wiffen PJ, et al. Pregabalin for pain in fibromyalgia in adults. Cochrane Database Syst Rev 2016;9:Cd011790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ohta H, Oka H, Usui C, et al. An open-label long-term phase III extension trial to evaluate the safety and efficacy of pregabalin in Japanese patients with fibromyalgia. Mod Rheumatol 2013;23:1108–15.. [DOI] [PubMed] [Google Scholar]
- [19].Onouchi K, Koga H, Yokoyama K, et al. An open-label, long-term study examining the safety and tolerability of pregabalin in Japanese patients with central neuropathic pain. J Pain Res 2014;7:439–47.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Satoh J, Yagihashi S, Baba M, et al. Efficacy and safety evaluation of pregabalin treatment over 52 weeks in patients with diabetic neuropathic pain extended after a double-blind placebo-controlled trial. J Diabetes Investig 2011;2:457–63.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ogawa S, Suzuki M, Arakawa A, et al. [Long-term efficacy and safety of pregabalin in patients with postherpetic neuralgia: results of a 52-week, open-label, flexible-dose study]. Masui 2010;59:961–70.. [PubMed] [Google Scholar]
- [22].Ogawa S, Satoh J, Arakawa A, et al. Pregabalin treatment for peripheral neuropathic pain: a review of safety data from randomized controlled trials conducted in Japan and in the west. Drug Saf 2012;35:793–806.. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


