Chen et al. show that Japanese encephalitis virus (JEV)–elicited CD8+ T cells and antibodies play protective and pathogenic roles in Zika virus–infected wild-type C57BL/6 mice, respectively, and JEV-elicited CD8+ T cells abrogate anti-JEV antibody–mediated enhancement of Zika virus infection in mice.
Abstract
Cross-reactive anti-flaviviral immunity can influence the outcome of infections with heterologous flaviviruses. However, it is unclear how the interplay between cross-reactive antibodies and T cells tilts the balance toward pathogenesis versus protection during secondary Zika virus (ZIKV) and Japanese encephalitis virus (JEV) infections. We show that sera and IgG from JEV-vaccinated humans and JEV-inoculated mice cross-reacted with ZIKV, exacerbated lethal ZIKV infection upon transfer to mice, and promoted viral replication and mortality upon ZIKV infection of the neonates born to immune mothers. In contrast, transfer of CD8+ T cells from JEV-exposed mice was protective, reducing the viral burden and mortality of ZIKV-infected mice and abrogating the lethal effects of antibody-mediated enhancement of ZIKV infection in mice. Conversely, cross-reactive anti-ZIKV antibodies or CD8+ T cells displayed the same pathogenic or protective effects upon JEV infection, with the exception that maternally acquired anti-ZIKV antibodies had no effect on JEV infection of the neonates. These results provide clues for developing safe anti-JEV/ZIKV vaccines.
Graphical Abstract
Introduction
Zika virus (ZIKV), a member of the Flaviviridae family, Flavivirus genus, shares a high degree of amino acid similarity with other flaviviruses, including yellow fever virus (YFV), dengue virus (DENV), Japanese encephalitis virus (JEV), and West Nile virus (WNV). ZIKV was initially isolated from a rhesus monkey in Uganda in 1947, and subsequently caused large outbreaks in French Polynesia (2013–2014) and South America (2015–2016). By early 2017, ZIKV had been reported in 84 countries or territories worldwide (World Health Organization, 2017). Most ZIKV infections cause mild symptoms of fever and headache but can also induce the neurological autoimmune disease Guillain–Barré syndrome (Monsalve et al., 2017). Moreover, infection of pregnant women has been linked to severe fetal defects, including microcephaly (Li et al., 2016a; Mlakar et al., 2016). JEV circulates mainly in Western Pacific, East Asian, Southeast Asian, and South Asian countries (Centers for Disease Control and Prevention (CDC), 2013). Like ZIKV infection, JEV predominantly causes mild or no symptoms, but ∼67,900 cases annually progress to Japanese encephalitis, which has a case fatality rate of 20 to 30% (Campbell et al., 2011; Centers for Disease Control and Prevention (CDC), 2013).
Current evidence suggests that exposure to one flavivirus can either protect against or exacerbate secondary infections with a heterotypic serotype or flavivirus (Bardina et al., 2017; Dejnirattisai et al., 2010, 2016; Fowler et al., 2018; George et al., 2017; Ngono and Shresta, 2018; Tesh et al., 2002; Vázquez-Calvo et al., 2017). The mechanisms by which flavivirus cross-reactive immune responses contribute to protection or pathogenesis are not fully understood but may be influenced by the degree of sequence homology, the sequence of infections, and the interval between infections (Elong Ngono and Shresta, 2019; Ngono and Shresta, 2018). Given that many countries routinely vaccinate against JEV (Campbell et al., 2011) and that ZIKV is rapidly spreading to JEV-endemic regions, including heavily populated countries such as China and India (Khaiboullina et al., 2018; Kutsuna et al., 2014; Quyen et al., 2017; Ruchusatsawat et al., 2019; World Health Organization, 2017; Zhang et al., 2016), there is an urgent need to understand the effects of prior immunity to JEV on the outcomes of ZIKV infection.
Antibody (Ab)-dependent enhancement (ADE) of infection can influence the severity of illness following flavivirus infections (Ngono and Shresta, 2018). ADE describes a phenomenon whereby cross-reactive, sub-neutralizing Abs induced during infection with one flavivirus promote infection of Fcγ receptor–bearing cells upon secondary infection by a heterotypic virus, thereby exacerbating the disease (Katzelnick et al., 2017; Salje et al., 2018). ADE was first experimentally characterized for DENV in studies showing that passive transfer of DENV-immune sera can enhance subsequent DENV infection and disease severity in naive mice (Balsitis et al., 2010; Zellweger et al., 2010). A growing body of evidence suggests that prior infection with DENV may have both positive and negative implications for the clinical consequences of ZIKV infection, depending on the context and balance of humoral and cellular immunity (Elong Ngono and Shresta, 2019; Wen and Shresta, 2019). For instance, recent studies using mice and human placental explants have demonstrated that DENV-specific Abs can mediate ADE of ZIKV infection and pathogenesis (Bardina et al., 2017; Brown et al., 2019; Rathore et al., 2019; Zimmerman et al., 2018). Although preexisting anti-DENV Abs may exacerbate ZIKV infection via ADE, cross-reactive anti-DENV cellular immunity appears to play a protective role during ZIKV infection. Mouse models of sequential DENV-ZIKV infection have revealed that DENV-elicited CD8+ T cells mediate short-term cross-protection against subsequent ZIKV infection in both nonpregnant and pregnant mice (Regla-Nava et al., 2018; Wen et al., 2017a, b). Consistent with these findings in mice, recent epidemiological studies indicate that prior DENV immunity confers cross-protection against ZIKV infection in humans (Gordon et al., 2019; Pedroso et al., 2019; Rodriguez-Barraquer et al., 2019). Thus, interplay between preexisting cross-reactive Ab and T cell responses likely determines the outcome of a subsequent ZIKV infection.
In contrast to sequential DENV-ZIKV and ZIKV-DENV infections, no studies have yet examined the impact of interplay between prior JEV humoral and cellular immunity on ZIKV infection, or vice versa. In hamsters and mice, immunization with a live-attenuated vaccine strain of JEV confers protection against encephalitis and/or death upon WNV and DENV challenge (Li et al., 2016c; Tesh et al., 2002). In addition, sera from JEV-vaccinated individuals can mediate ADE of DENV infection in Fcγ receptor–positive baby hamster kidney (BHK)–21 cells (Saito et al., 2016), and preexisting JEV Abs have been shown to increase the severity of symptomatic dengue illness in children and adults (Anderson et al., 2011; Sato et al., 2015). Pre-existing JEV Abs also enhance subsequent infection with live-attenuated YFV, thereby improving its immunogenicity (Chan et al., 2016). Thus, while the prevailing evidence suggests that coexisting JEV-elicited cross-reactive Abs and T cells are likely to affect ZIKV infection, this has not yet been investigated.
In the present study, we sought to address these knowledge gaps by modeling sequential infections with JEV and ZIKV or vice versa. We determined how transfer of serum, serum-derived IgG, and CD8+ T cells from mice or humans exposed to one flavivirus affects the development and outcome of a subsequent infection with the heterologous flavivirus in mice. We also assessed the effect of maternally acquired anti-JEV Abs on ZIKV infection in neonatal mice, and vice versa. We show that (i) JEV-immune human and mouse sera mediate ADE of ZIKV infection in vitro and in vivo and decrease the survival of recipient mice following ZIKV infection; (ii) maternally acquired anti-JEV Abs increase the mortality of neonatal mice challenged with ZIKV; (iii) CD8+ T cells from JEV-primed mice cross-react with immunodominant ZIKV epitopes, and transfer of such T cells protects against lethal ZIKV infection; and (iv) cotransfer of JEV-primed CD8+ T cells with JEV-immune serum to naive mice abrogates ADE of ZIKV infection. Importantly, most, but not all, of these results are also observed in the context of preexisting ZIKV immunity and subsequent JEV infection. Collectively, these results suggest that cross-reactive CD8+ T cells can protect against ADE of heterologous flaviviral infections, and thus imply that a precise balance between cross-reactive Ab and T cell responses determines the outcome of subsequent infection with a heterologous flavivirus.
Results
Sera from JEV- and ZIKV-immune mice and humans cross-react with heterologous flaviviral EDIII proteins
The amino acid homology between ZIKV and JEV ranges from ∼38 to 55% in the structural proteins (capsid [C], premembrane/membrane [prM/M], and envelope [E]) and ∼36 to 68% in the nonstructural (NS) proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5; BLAST search; data not shown). Flaviviral protein E contains three extracellular domains, I, II, and III, and is the main target for Ab responses to flaviviruses (Luca et al., 2012; Sirohi et al., 2016). E protein domain III (EDIII) encodes epitopes that bind to host cellular receptors and can generate potently neutralizing Abs (Luca et al., 2012). Notably, JEV and ZIKV EDIIIs are ∼54% identical at the amino acid level (BLAST search; data not shown).
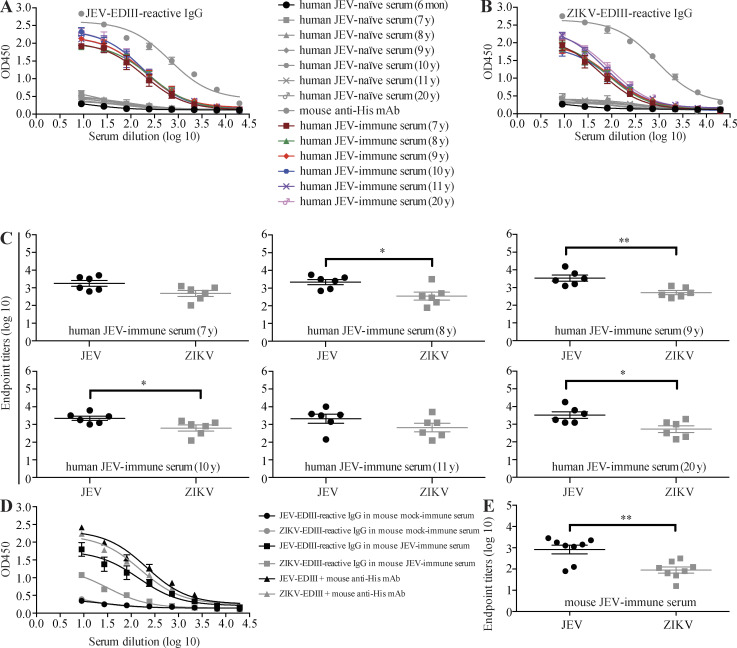
We first tested sera from JEV-naive infants or JEV-naive, JEV-vaccinated children and young adults (aged 7–20 yr, with no history of DENV, ZIKV, or YFV infection or vaccination) for their ability to bind recombinant ZIKV-EDIII or JEV-EDIII using indirect ELISAs. As shown in Fig. 1, sera from JEV-vaccinated individuals contained comparably high titers of IgG to both JEV-EDIII and ZIKV-EDIII, suggesting strong antigenic cross-reactivity between the two proteins (Fig. 1, A–C). Moreover, the anti–JEV-EDIII and anti–ZIKV-EDIII titers did not differ markedly between the sera from JEV-immune individuals of different ages (Fig. 1, A and B), suggesting that JEV vaccination induced long-lasting Ab responses to both viruses. A cross-reactive IgG response to JEV-EDIII and ZIKV-EDIII was also observed in sera from WT C57BL/6 mice inoculated with JEV (Fig. 1, D and E). Analysis of sera from ZIKV-inoculated WT mice gave similar results, with high IgG titers to both ZIKV-EDIII and JEV-EDIII (Fig. 2, A and B). These data indicate that exposure of humans or mice to JEV or ZIKV elicits an Ab response that strongly cross-reacts with ZIKV or JEV, respectively.
Figure 1.
Sera from JEV-exposed humans and mice contain JEV-EDIII- and ZIKV-EDIII-reactive IgG. (A–C) Human serum samples were prepared from JEV-naive 6-mo-old infants (n = 8) or JEV-naive, JEV-vaccinated children or young adults aged 7 (n = 6), 8 (n = 6), 9 (n = 6), 10 (n = 6), 11 (n = 6), and 20 (n = 6) yr. (D and E) Mouse serum samples were prepared from JEV-naive (mock-immune, n = 8) and JEV-immune (n = 8) mice. Mouse anti-His mAb was used as positive control (n = 6). JEV-EDIII– and ZIKV-EDIII–reactive IgG levels were measured by indirect ELISA. Data are presented as the mean ± SEM. *, P < 0.05; **, P < 0.01 by two-tailed Mann–Whitney U test.
Figure 2.
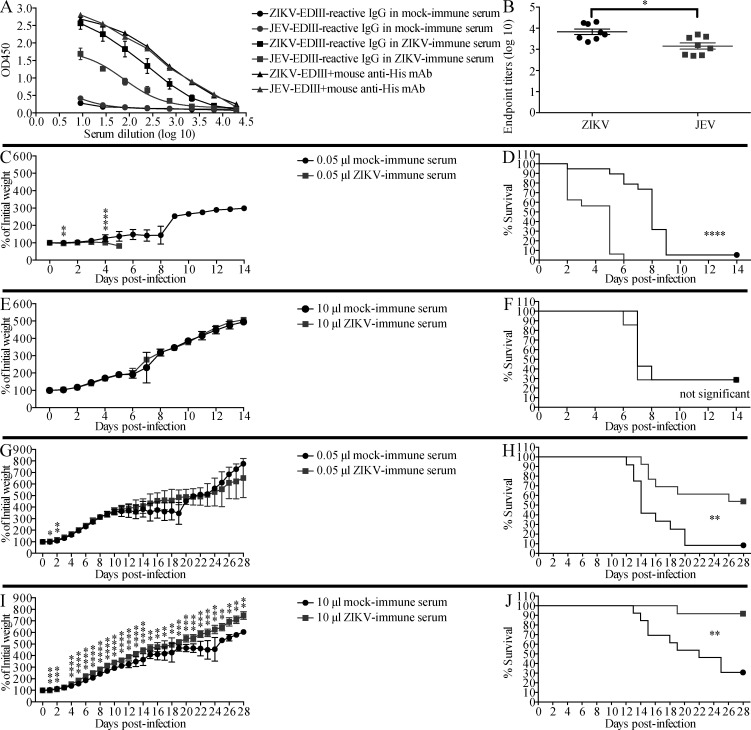
Transfer of ZIKV-immune mouse sera increases the survival of ZIKV-infected mice but decreases that of JEV-infected mice. (A and B) Mouse serum samples were prepared from ZIKV-naive (mock-immune, n = 6) and ZIKV-immune (n = 6) mice. ZIKV-EDIII– and JEV-EDIII–reactive IgG levels were measured by indirect ELISA. Mouse anti-His mAb was used as positive control. Data are presented as the mean ± SEM. (C–F) 1-d-old naive C57BL/6 mice were injected s.c. with 0.05 µl (C and D) or 10 µl (E and F) of mock-immune (n = 19, n = 7, respectively) or ZIKV-immune (n = 16, n = 7, respectively) mouse sera, and 6 h later, the mice were injected s.c. with JEV (12 PFU). Mouse weights and survival were recorded daily for 14 d. Data are presented as the mean ± SD and are pooled from two experiments, each with 3–10 mice per group. (G–J) 1-d-old naive C57BL/6 mice were injected s.c. with 0.05 µl (G and H) or 10 µl (I and J) of serum from mock-immune (n = 12, n = 13, respectively) or ZIKV-immune (n = 13, n = 12, respectively) mice, and injected s.c. with ZIKV (102 FFU) 6 h later. Weights and survival were recorded daily for 28 d. Data are presented as the mean ± SD and are pooled from two experiments, each with six or seven mice/group. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001 by two-tailed Mann–Whitney U test (B, C, E, G, and I) or log-rank test (D, F, H, and J).
Sera from JEV-immune mice and humans contain modest neutralizing activity and can enhance ZIKV infection in vitro
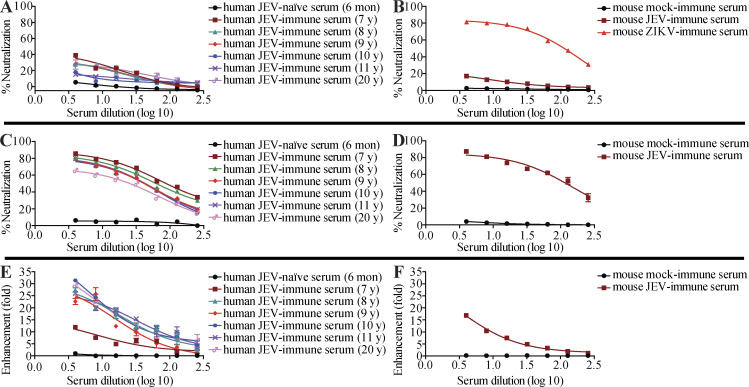
The ADE paradigm of viral infection proposes that failure to elicit a fully neutralizing Ab response during a primary viral infection can render the host susceptible to enhancement of subsequent infections. Therefore, we determined the ability of JEV-elicited ZIKV-reactive Abs to mediate protection vs. enhancement of ZIKV infection. We first performed a microneutralization assay to assess the ability of JEV-immune sera to block ZIKV infection of Vero cells in vitro. Naive mouse and human sera failed to neutralize ZIKV infection, as expected; however, mouse and human JEV-immune sera had only modest neutralizing activity (Fig. S1, A and B). As expected, both mouse and human JEV-immune sera efficiently neutralized JEV (Fig. S1, C and D). The 50% neutralizing titers for mouse and human JEV-immune sera were 1:112 and 1:25–1:79, respectively. To determine whether JEV-immune sera could mediate ADE of ZIKV infection in vitro, we performed infection enhancement assays with the human macrophage cell line U937, which is relatively resistant to infection by ZIKV without ADE (Dejnirattisai et al., 2016). Interestingly, sera from JEV-immune humans or mice caused significant enhancement of ZIKV infection of cells (12–31-fold and 17-fold, respectively) compared with JEV-naive sera (Fig. S1, E and F). Thus, JEV-elicited human and mouse sera with modest ZIKV-neutralizing activity are able to enhance ZIKV infection of cells in vitro.
Figure S1.
Modest neutralization but efficient enhancement of ZIKV infection in vitro by JEV-neutralizing, JEV-immune sera from humans and mice. (A–D) Human serum samples isolated from JEV-naive infants (6 mo of age, n = 6) or JEV-immune subjects (n = 6 for all age groups), and mouse serum samples (n = 6 for all groups) were tested for neutralization of ZIKV (A and B) or JEV (C and D) infection of Vero cells. Mouse ZIKV-immune serum was used as the positive control. (E and F) Enhancement of ZIKV infection by JEV-immune sera from humans and mice was evaluated in U937 cells. Data are expressed as the mean ± SEM.
Sera from JEV- or ZIKV-immune mice and humans promote lethal infection with the heterologous virus
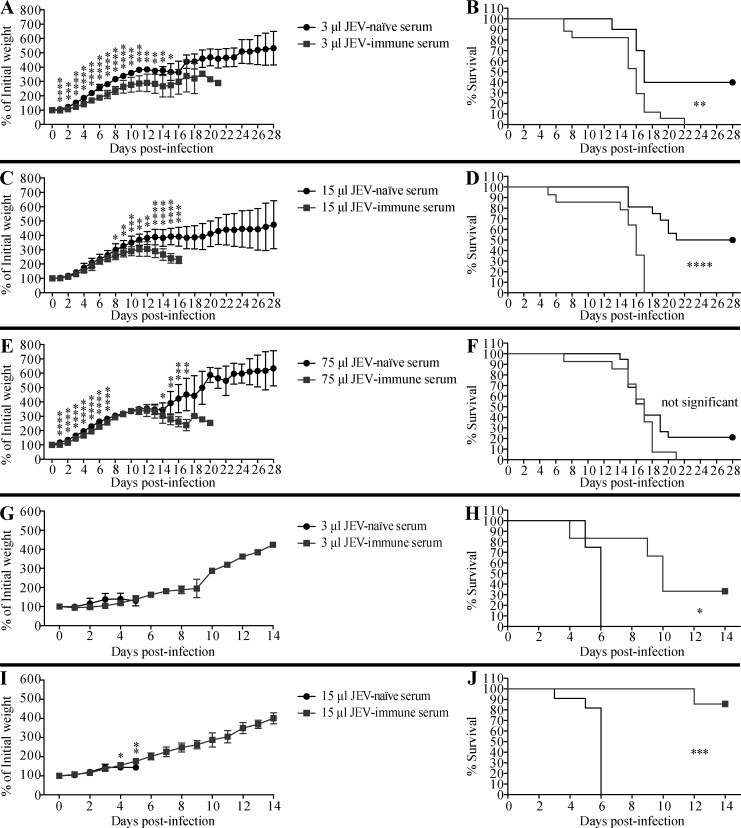
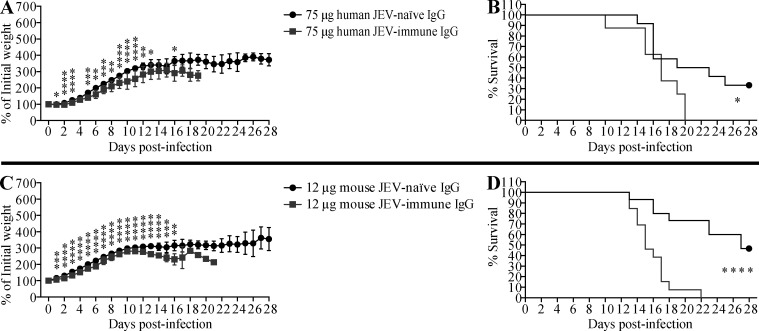
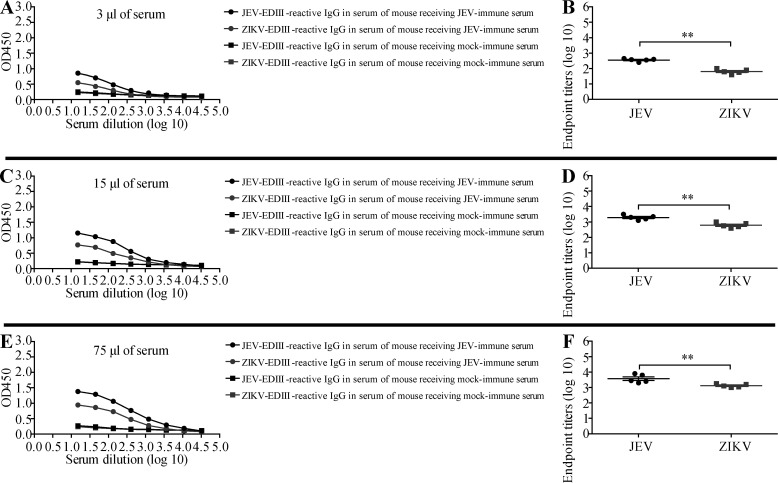
Next, we examined whether JEV-immune sera could promote ADE of ZIKV infection in vivo as well as in vitro. For these experiments, we selected 1-d-old WT mice because ZIKV infection is lethal in neonates but not in adults (Li et al., 2018; Manangeeswaran et al., 2016). Although the precise reasons for this differential susceptibility are unclear, it may be due to the immaturity of the neonatal immune system, including the antiviral type I IFN response, as shown for herpes simplex virus and respiratory syncytial virus infections (Remot et al., 2016; Wilcox et al., 2015), and the antiviral CD8+ T cell response, as shown for Hantaan virus (Araki et al., 2004). In the present study, 1-d-old mice were injected s.c. with 3, 15, or 75 µl of pooled JEV-naive or JEV-immune human sera, and then challenged with ZIKV (102 focus-forming units [FFU] s.c.) 6 h later. Mice that received 3 or 15 µl of JEV-immune serum lost more weight (Fig. 3, A and C) and succumbed more rapidly to ZIKV infection than mice receiving JEV-naive serum (100% vs. 50–60% death, respectively; Fig. 3, B and D). A higher volume of sera (75 µl) was not markedly more pathogenic than 15 µl (Fig. 3, E and F). Similar results were observed when mice were injected with JEV-immune or mock-immune mouse serum before ZIKV infection; in this case, mortality was 100%, 100%, and 60% for mice receiving 3, 15, and 75 µl JEV-immune mouse serum and 70%, 75%, and 64% for mice receiving 3, 15, and 75 µl mock-immune mouse serum, respectively (Fig. 4). Taken together, these results suggest that preexisting JEV Abs may confer an increased risk of severe disease upon infection with ZIKV. To confirm that the pathogenic effects of JEV-immune sera were mediated by IgG, we injected 1-d-old mice s.c. with either 12 µg mouse IgG (purified from naive or JEV-immune mouse serum; equivalent to 3 µl of serum) or 75 µg of human IgG (purified from naive or JEV-immune human serum; equivalent to 15 µl of serum), and infected the mice 6 h later with ZIKV. As shown in Fig. S2, purified mouse and human IgG from JEV-immune sera had the same effect as the originating sera, accelerating both weight loss and mortality of ZIKV-infected mice. These data indicate that IgG mediates the lethal ADE of ZIKV pathogenesis induced by JEV-immune human and mouse sera.
Figure 3.
Transfer of JEV-immune human sera increases the survival of JEV-infected mice but decreases that of ZIKV-infected mice. (A–J) 1-d-old naive C57BL/6 mice were injected s.c. with the indicated volumes of JEV-naive or JEV-immune human sera. 6 h later, the mice were injected s.c. with (A–F) ZIKV (102 FFU) or (G–J) JEV (12 PFU). Mouse weights and survival were recorded daily for up to 28 d. Data are presented as the mean ± SD and are pooled from two experiments, each with 5–10 mice per group (A–F) or 3–6 mice/group (G–J). *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001 by two-tailed Mann–Whitney U test (A, C, E, G, and I) or log-rank test (B, D, F, H, and J).
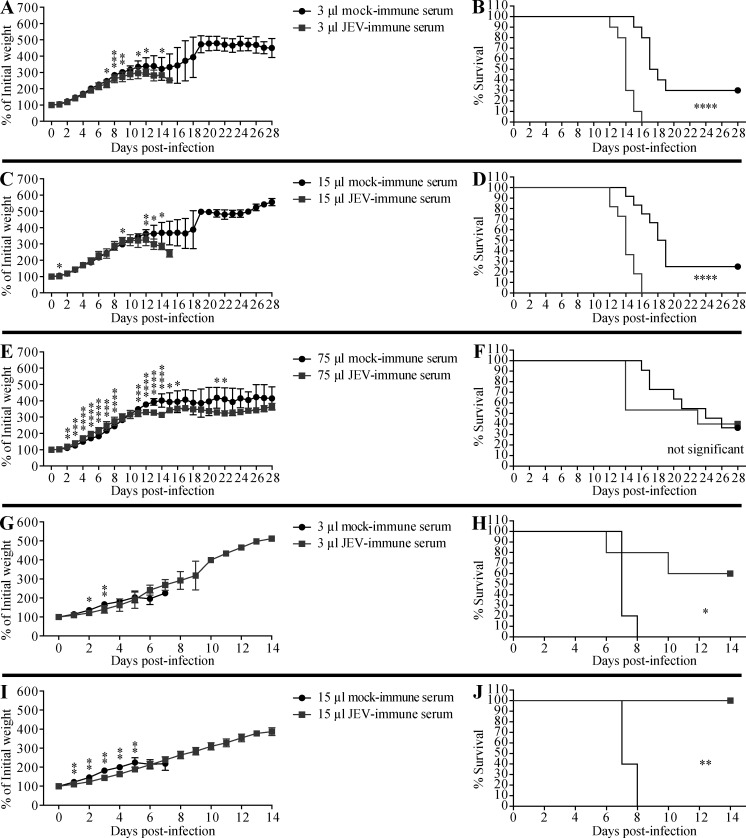
Figure 4.
Transfer of JEV-immune mouse sera increases the survival of JEV-infected mice but decreases that of ZIKV-infected mice. (A–J) 1-d-old naive C57BL/6 mice were injected s.c. with the indicated volumes of mock- or JEV-immune mouse sera, and 6 h later, the mice were injected s.c. with (A–F) ZIKV (102 FFU) or (G–J) JEV (12 PFU). Mouse weights and survival were recorded daily for up to 28 d. Data are presented as the mean ± SD and are pooled from two experiments, each with five to eight mice per group (A–F) or two or three mice per group (G–J). *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001 by two-tailed Mann–Whitney U test (A, C, E, G, and I) or log-rank test (B, D, F, H, and J).
Figure S2.
Transfer of JEV-immune IgG exacerbates lethal ZIKV infection in mice. (A–D) 1-d-old naive C57BL/6 mice were injected s.c. with (A and B) 75 µg of IgG purified from JEV-naive (n = 12) or JEV-immune (n = 8) human sera or (C and D) 12 µg of IgG purified from mock-immune (n = 15) or JEV-immune (n = 13) mice and injected s.c. with ZIKV (102 FFU) 6 h later. Weights and survival were recorded daily for up to 28 d. Data are presented as the mean ± SD and are pooled from two experiments, each with four to eight mice/group. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001 by two-tailed Mann–Whitney U test (A and C) or log-rank test (B and D).
We then determined whether ZIKV-immune sera could accelerate the pathogenesis of JEV infection in vivo using the same experimental model. 1-d-old mice were injected s.c. with 0.05 or 10 µl of pooled mock-immune or ZIKV-immune mouse sera, and then challenged with JEV (12 PFU s.c.) 6 h later. Here, too, we observed that, compared with mock-immune sera, the smaller volume of ZIKV-immune serum induced greater weight loss and earlier death upon JEV infection (100% within 6 d vs. 95% within 9 d; Fig. 2, C and D). As also observed for anti-JEV effects on ZIKV infection, a larger volume of ZIKV-immune sera did not further increase the pathogenicity of JEV infection (Fig. 2, E and F).
Sera from JEV- or ZIKV-immune mice and humans protect against lethal infection with the homologous virus
Having shown that JEV- and ZIKV-immune sera enhanced the lethality of ZIKV and JEV infection, respectively, we next performed similar experiments to determine whether the sera could also mediate ADE of infection with the same virus. Mice receiving serum from JEV-naive humans (Fig. 3, G–J) or mock-immune mice (Fig. 4, G–J) died within 8 d of JEV infection, as expected. However, mice receiving JEV-immune human sera showed a dose-dependent and significant reduction in mortality, with ∼33% and ∼86% survival of mice receiving 3 and 15 µl sera, respectively (Fig. 3, G–J). Similarly, ∼60% and 100% of mice receiving 3 and 15 µl, respectively, of JEV-immune mouse sera survived a lethal JEV infection (Fig. 4, G–J). We observed the same pattern of protection by ZIKV-immune mouse sera against ZIKV infection, namely, reduced weight loss and protection against ZIKV-induced death in mice receiving ZIKV-immune compared with mock-immune sera (54–92% vs. 8–30% survival, respectively; Fig. 2, G–J). These results indicate that JEV- or ZIKV-elicited Abs protect against subsequent infection with the homologous virus, in striking contrast to the detrimental effect of the Abs on subsequent infection with the heterologous virus.
Maternally acquired anti-JEV Abs, but not anti-ZIKV Abs, promote lethal infection with the heterologous virus in neonates
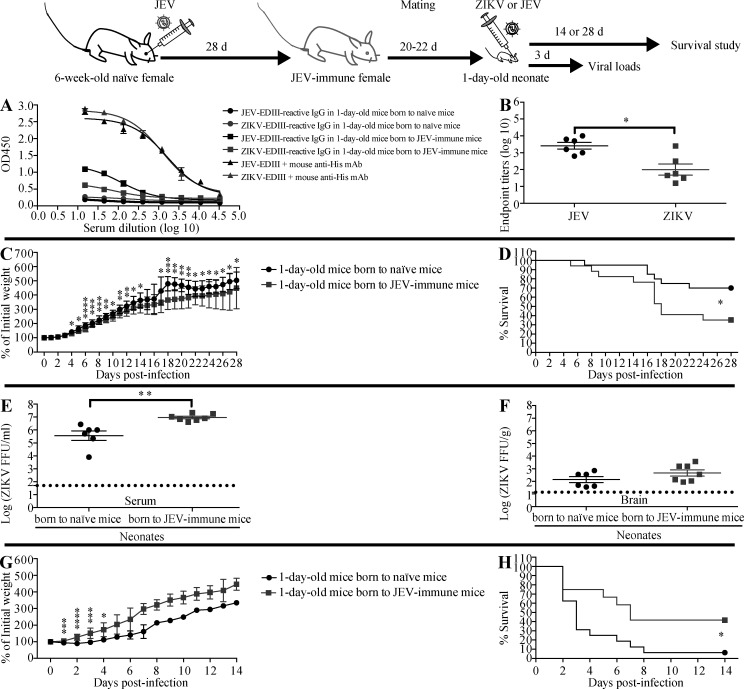
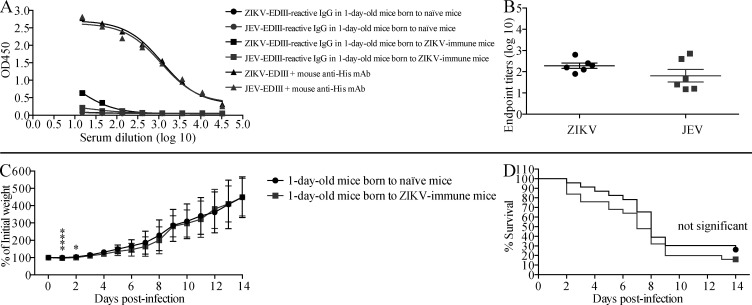
Because ADE of ZIKV infection in pregnant women could have devastating effects on the developing offspring, we next investigated whether maternally acquired anti-JEV Abs could enhance the severity of ZIKV infection in newborn mice. Sera from 1-d-old naive pups born to JEV-immune mothers contained moderate-to-high titers of IgG reactive to JEV-EDIII and cross-reactive with ZIKV-EDIII (Fig. 5, A and B). The titer of ZIKV-reactive IgG in 1-d-old pups born to JEV-immune mothers was similar to that in the serum of mice receiving 3 µl of mouse JEV-immune serum, and lower than that in mice receiving 75 µl of mouse JEV-immune serum (Fig. S3 and Fig. 5, A and B). ZIKV infection of 1-d-old mice was more lethal for pups born to JEV-immune mothers than pups born to naive mothers (65% vs. 30% mortality; Fig. 5, C and D), suggesting that the maternally acquired anti-JEV Abs could mediate ADE of ZIKV infection. Consistent with this, ZIKV levels at day 3 after infection were significantly higher in the serum (25-fold, P < 0.01) and slightly higher in the brain (threefold, P = 0.1667) of pups born to JEV-immune mothers compared with those born to naive mothers (Fig. 5, E and F). These data indicate that maternally acquired anti-JEV Abs mediate ADE of ZIKV infection and increase the severity of disease in 1-d-old mouse pups. We performed similar experiments to determine whether the maternally acquired anti-JEV Abs similarly increased the lethality of JEV infection in neonates. However, in this case, pups born to JEV-immune mothers were protected against JEV infection (Fig. 5, G and H). Thus, whether passively transferred or maternally acquired, anti-JEV Abs protect against JEV infection but exacerbate ZIKV infection.
Figure 5.
Pups born to JEV-immune mothers have decreased survival and increased viral burden upon ZIKV challenge compared with pups born to naive mothers. 6-wk-old female C57BL/6 mice were i.p. injected with 1 mg anti-Ifnar1 mAb (MAR1-5A3) 1 d before r.o. injection with JEV (102 PFU). 4 wk later, naive or JEV-immune females were mated with 10-wk-old naive male mice. (A and B) 1-d-old C57BL/6 pups were sacrificed, and sera were prepared. JEV-EDIII and ZIKV-EDIII–reactive IgG were measured by indirect ELISA. Mouse anti-His mAb was used as positive control. Data are presented as the mean ± SEM. (C–H) 1-d-old pups were injected s.c. with (C–F) ZIKV (102 FFU) or (G and H) JEV (12 PFU). (C, D, G, and H) Mouse weights and survival were recorded daily for up to 28 d. Data are presented as the mean ± SD and are pooled from three experiments totaling 20 infected mice born to 3 naive mothers and 17 infected mice born to 3 JEV-immune mothers (C and D), or two experiments totaling 16 infected mice born to 2 naive mothers and 12 infected mice born to 2 JEV-immune mothers (G and H). (E and F) Mice were sacrificed 3 d after ZIKV infection, and blood and brains were collected for measurement of viral burden using the FFA. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001 by two-tailed Mann–Whitney U test (B, C, E, and G) or log-rank test (D and H).
Figure S3.
JEV- and ZIKV-reactive IgG in the serum of mice 6 h after transfer of mouse JEV-immune serum. (A–F) 1-d-old naive C57BL/6 mice were injected s.c. with 3, 15, or 75 µl of mock- or JEV-immune mouse sera (n = 5 for all groups). 6 h later, the mice were sacrificed, and sera were collected. JEV-EDIII– and ZIKV-EDIII–reactive IgG levels were measured by indirect ELISA. Data are presented as the mean ± SEM. **, P < 0.01 by two-tailed Mann–Whitney U test.
Intriguingly, we did not find the same results when we examined the effects of maternally acquired anti-ZIKV Abs on the severity of JEV infection in newborn mice. Thus, although sera from 1-d-old naive pups born to ZIKV-immune mothers contained low-to-moderate titers of ZIKV/JEV-EDIII cross-reactive Abs (Fig. S4, A and B), they did not mediate ADE of JEV infection in neonates (Fig. S4, C and D). These data indicate that maternally acquired anti-JEV Abs, but not anti-ZIKV Abs, mediate ADE of heterologous virus infection in mouse pups. This suggests that ADE occurs in a context-dependent manner and varies based on the heterologous virus infection and the maternal vs. nonmaternal origin of the preexisting Abs.
Figure S4.
Maternally acquired anti-ZIKV Abs have no effect on the survival of JEV-infected pups born to ZIKV-immune mothers. 6-wk-old female C57BL/6 mice were i.p. injected with 1 mg anti-Ifnar1 mAb (MAR1-5A3) 1 d before r.o. injection with ZIKV (102 FFU). 4 wk later, naive or ZIKV-immune females were mated with 10-wk-old naive male mice. (A and B) 1-d-old C57BL/6 pups were sacrificed, and sera were prepared. ZIKV-EDIII and JEV-EDIII–reactive IgG were measured by indirect ELISA. Mouse anti-His mAb was used as positive control. Data are presented as the mean ± SEM. (C and D) 1-d-old C57BL/6 pups were injected s.c. with JEV (12 PFU). Mouse weights and survival were recorded daily for 14 d. Data are presented as the mean ± SD and are pooled from four experiments totaling 23 infected mice born to 4 naive mothers and 25 infected mice born to 4 ZIKV-immune mothers. *, P < 0.05; ****, P < 0.0001 by two-tailed Mann–Whitney U test.
CD8+ T cells from JEV- or ZIKV-primed mice cross-react with immunogenic ZIKV or JEV epitopes in vitro
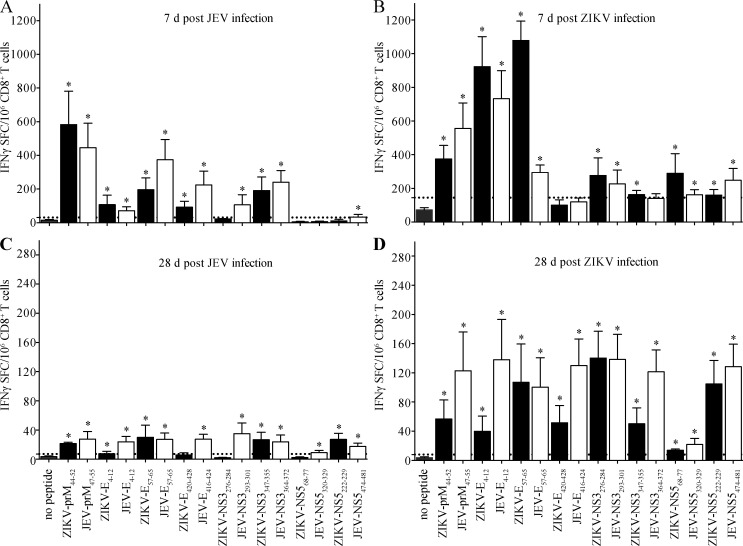
Our previous work suggested that the balance between preexisting Ab and T cell immunity to DENV, particularly CD8+ T cell immunity, determines the outcome of subsequent infections with heterotypic DENV serotype or ZIKV (Regla-Nava et al., 2018; Wen et al., 2017a; Zellweger et al., 2013, 2014, 2015). Moreover, we previously identified numerous H-2b-restricted CD8+ T cell epitopes in the ZIKV proteome (Elong Ngono et al., 2017) and demonstrated that at least five of these ZIKV-derived epitopes elicit cross-reactive CD8+ T cell responses to DENV (Wen et al., 2017a). Therefore, we next assessed whether JEV or ZIKV immunization may elicit cross-reactive CD8+ T cells, as observed for the Ab response. To this end, we tested the in vitro response to eight immunodominant H-2b-restricted ZIKV epitopes (prM44-52, E4-12, E57-65, E420-428, NS3276-284, NS3347-355, NS568-77, and NS5222-229) and the corresponding JEV variants (prM47-55, E4-12, E57-65, E416-424, NS3293-301, NS3364-372, NS5320-329, and NS5474-481; Table 1) using an IFN-γ ELISpot assay (Wen et al., 2017b). CD8+ T cells were purified from the spleens of 6-wk-old WT mice infected with ZIKV (103 FFU) or JEV (103 PFU) 7 or 28 d earlier, and then incubated with antigen-presenting cells prepulsed with peptides in vitro. After 24-h incubation, IFNγ–secreting CD8+ T cells were enumerated. CD8+ T cells from JEV-primed mice (7-d infection) mounted significant responses to seven of the eight JEV peptides tested (the exception being NS5320-329) and to five of the corresponding ZIKV peptides (except NS3276-284, NS568-77, and NS5222-229; Fig. 6, A and B). The number of CD8+ T cells responding to the ZIKV and JEV peptide analogues was similar for most peptides (Fig. 6 A). Conversely, CD8+ T cells from ZIKV-primed mice were reactive against seven of the eight ZIKV peptides (except E420-428) and six of the corresponding JEV peptides (except E416-424 and NS3364-372; Fig. 6 B). Although the magnitude of the CD8+ T cell responses to analogous ZIKV and JEV peptides was more variable in mice primed with ZIKV compared with JEV, these data demonstrate that many of the immunodominant epitopes in ZIKV and JEV elicit cross-reactive CD8+ T cells. Of note, the frequencies of peptide-specific CD8+ T cells decreased between 7 and 28 d after infection (Fig. 6, A–D), indicating that 28 d was sufficient time for the virus-elicited CD8+ T cell response to contract and develop memory.
Table 1. ZIKV-derived H-2b-restricted epitopes and JEV variants.
| Peptidesa | Sequencesb | Homologyc |
|---|---|---|
| ZIKV-prM44-52 | ATMSYECPM | 56% |
| JEV-prM47-55 | DTITYECPK | |
| ZIKV-E4-12 | IGVSNRDFV | 56% |
| JEV-E4-12 | LGMGNRDFI | |
| ZIKV-E57-65 | RSYCYEASI | 78% |
| JEV-E57-65 | RSYCYHASV | |
| ZIKV-E420-428 | RMAVLGDTA | 78% |
| JEV-E416-424 | RLAALGDTA | |
| ZIKV-NS3276-284 | SSIAARGYI | 89% |
| JEV-NS3293-301 | ASIAARGYI | |
| ZIKV-NS3347-355 | PSVRNGNEI | 67% |
| JEV-NS3364-372 | ASVKMGNEI | |
| ZIKV-NS568-77 | SSLVNGVVRL | 90% |
| JEV-NS5320-329 | SSLVNGVVKL | |
| ZIKV-NS5222-229 | RAIWYMWL | 88% |
| JEV-NS5474-481 | RAIWFMWL |
Peptide position is determined according to the amino acid sequence of ZIKV strain and JEV strain.
The shared amino acid residue is underlined.
The percentage of shared amino acid between ZIKV epitope and JEV variant.
Figure 6.
Identification of cross-reactive responses to JEV or ZIKV epitopes by CD8+ T cells from primed mice. 6-wk-old female C57BL/6 mice were i.p. injected with 1 mg anti-Ifnar1 mAb (MAR1-5A3) 1 d before r.o. injection with (A and C) JEV (103 PFU) or (B and D) ZIKV (103 FFU). 7 or 28 d later, splenocyte-derived CD8+ T cells were isolated and stimulated in vitro with the indicated CD8+ T cell epitopes from ZIKV or the corresponding JEV variants. The frequencies of IFN-γ–producing CD8+ T cells (SFCs) were detected using IFN-γ ELISpot assays. Data are presented as the mean ± SEM and are pooled from two independent experiments, each with three or four mice per group. Dotted lines represent the cutoff value. Asterisk indicates a positive response, as defined in the Materials and methods.
JEV-elicited CD8+ T cells protect against ADE of infection with ZIKV
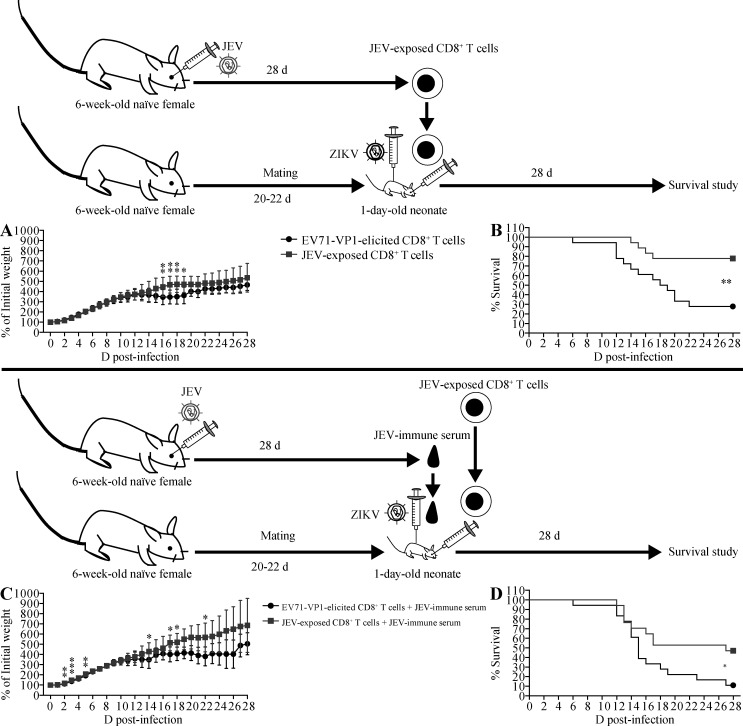
Recent studies from our group and others have demonstrated that preexisting anti-DENV CD8+ T cells contribute to cross-protection against subsequent infection with ZIKV or heterotypic DENV (Elong Ngono et al., 2016; Regla-Nava et al., 2018; Wen et al., 2017a, b; Zellweger et al., 2015), and similarly, ZIKV-elicited CD8+ T cells can mediate cross-protection against DENV infection (Huang et al., 2017). We therefore determined whether JEV-elicited CD8+ T cells protect against ZIKV infection. CD8+ T cells were purified from the spleens of 6-wk-old WT mice 28 d after infection with JEV and transferred to 1-d-old mice, which were infected 2 h later with a lethal dose of ZIKV. In sharp contrast to the effects of JEV-immune Abs, JEV-elicited CD8+ T cells significantly increased the survival of ZIKV-infected mice compared with CD8+ T cells from control enterovirus 71 VP1 protein (EV71-VP1)–immunized mice (78% vs. 28% survival; Fig. 7, A and B). Moreover, we found that CD8+ T cells purified from ZIKV-infected mice and transferred to 1-d-old mice could also significantly protect against a subsequent lethal infection with JEV, compared with EV71-VP1–elicited CD8+ T cells (33% vs. 89% death; Fig. 8, A and B). These data indicate that cross-reactive CD8+ T cells elicited by either JEV or ZIKV could protect against infection with the heterologous virus.
Figure 7.
Transfer of JEV-elicited CD8+ T cells increases the survival of ZIKV-infected mice in the absence or presence of JEV-immune serum. (A and B) 1-d-old naive C57BL/6 mice were r.o. injected with 2 × 106 CD8+ T cells from EV71-VP1–immunized mice (n = 18) or JEV-exposed mice injected r.o. with JEV (102 PFU) 28 d earlier (n = 18). 2 h later, the neonates were injected s.c. with ZIKV (102 FFU). Weights and survival were recorded daily for 28 d. (C and D) Mice were treated as described for A and B except that immediately following infusion of neonatal mice with CD8+ T cells isolated from EV71-VP1–immunized mice (n = 18) or JEV-exposed mice (n = 17), recipient mice were s.c. injected with 3 µl of JEV-immune mouse sera and then s.c. inoculated with ZIKV (102 FFU) 6 h after the infusion of T cells and sera. Data are presented as the mean ± SD and are pooled from two experiments, each with nine mice/group (A and B) or from two experiments, each with 8 or 9 mice per group (C and D). *, P < 0.05; **, P < 0.01; ***, P < 0.001 by two-tailed Mann–Whitney U test (A and C) or log-rank test (B and D).
Figure 8.
Transfer of ZIKV-elicited CD8+ T cells increases the survival of JEV-infected mice. (A and B) 1-d-old naive C57BL/6 mice were r.o. injected with 2 × 106 CD8+ T cells from EV71-VP1–immune mice that had been s.c. immunized with EV71-VP1 (50 µg/mouse/each; twice, 2 wk apart) 28 d earlier (n = 9) or ZIKV-exposed mice injected r.o. with ZIKV (102 PFU) 28 d earlier (n = 9). 2 h later, the neonates were injected s.c. with JEV (12 PFU). Mouse weights (A) and survival (B) were recorded daily for 14 d. Data are presented as the mean ± SD and are pooled from two experiments, each with four or five mice/group. *, P < 0.05; ***, P < 0.001 by two-tailed Mann–Whitney U test (A) or log-rank test (B).
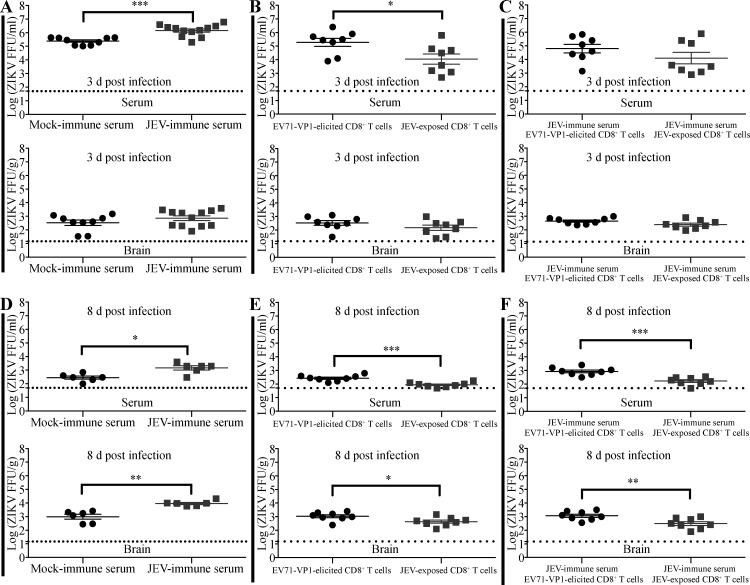
We next investigated whether JEV-elicited CD8+ T cells could rescue or attenuate the lethal effects of JEV-immune Abs on ZIKV infection. We cotransferred serum and CD8+ T cells from EV71-VP1–immune or JEV-immune mice at the same time and infected the mice with ZIKV 6 h later. Remarkably, JEV-elicited CD8+ T cells significantly protected the mice against ADE of infection mediated by JEV-immune Abs (47% vs. 11% survival; Fig. 7, C and D). Thus, JEV-elicited cross-reactive CD8+ T cells not only are protective in their own right but also abrogate ADE of ZIKV infection by JEV-immune Abs. To confirm these results, we examined the viral burden in serum and brain samples of these mice. As expected, ZIKV titers were significantly higher in the sera of mice receiving JEV-immune serum compared with naive serum, on both day 3 (Fig. 9 A) and day 8 (Fig. 9 D) after infection, consistent with ADE. In contrast, the titers were significantly lower in mice receiving JEV-elicited CD8+ T cells compared with EV71-VP1–elicited cells (Fig. 9, B and E). Notably, mice receiving both JEV-immune serum and JEV-elicited CD8+ T cells had significantly lower serum ZIKV levels than mice receiving JEV-immune serum and EV71-VP1–elicited CD8+ T cells, although this difference was statistically significant only at day 8 after infection (Fig. 9, C and F). A comparable analysis of ZIKV titers in brain tissue mirrored the results of the serum analyses on day 8 after infection (Fig. 9, D–F). Thus, ZIKV titers in serum and brain correlated with the survival outcomes of ZIKV-infected mice in that transfer of JEV-immune serum increased ZIKV titers and promoted mortality, whereas transfer of JEV-elicited CD8+ T cells had the opposite effects.
Figure 9.
Transfer of JEV-elicited CD8+ T cells modulates anti-JEV Ab-mediated ADE of ZIKV infection. (A and D) 1-d-old C57BL/6 naive mice were s.c. injected with 3 µl of mock-immune or JEV-immune mouse serum. 6 h later, mice were injected s.c. with ZIKV (102 FFU). (B and E) 1-d-old C57BL/6 naive mice were r.o. injected with 2 × 106 CD8+ T cells from EV71-VP1–immune or JEV-exposed mice and injected s.c. with ZIKV (102 FFU) 2 h later. (C and F) Mice were treated as described for B and E except that the CD8+ T cells were from EV71-VP1–immune or JEV-exposed mice injected s.c. with 3 µl of JEV-immune mouse sera before s.c. injection of ZIKV (102 FFU). ZIKV viral loads in serum and brain were measured using the FFA at 3 or 8 d after infection. Data are presented as the mean ± SEM and are pooled from two experiments, each with three to six mice/group. *, P < 0.05; **, P < 0.01; ***, P < 0.001 by two-tailed Mann–Whitney U test.
Taken together, these data indicate that the quality of the humoral vs. cellular immunity to JEV dictates the outcome of a subsequent infection with ZIKV.
Discussion
A convergence of factors has revitalized interest in developing new flaviviral vaccines; they include the emergence of ZIKV in DENV-endemic regions; the ease of global travel, which increases not only the spread of disease but also the likelihood that individuals may be exposed to multiple flaviviruses; and the finding that flaviviral proteins share significant amino acid homology. These observations have stimulated the exploration of humoral and cellular immunity to primary flaviviral infections and their beneficial and detrimental effects on subsequent infections with heterologous flaviviruses. In particular, the spread of ZIKV into JEV-endemic countries underscores the need to understand how interplay between humoral and cellular immunity to JEV modulates the severity of disease caused by ZIKV infection and vice versa. In this study, our major goals were to examine whether JEV-elicited Abs enhance ZIKV infection via ADE, and, if so, to determine whether anti-JEV CD8+ T cell immunity transfer could abrogate JEV Ab-mediated ADE of ZIKV infection. Indeed, the results of this study have uncovered critical roles for JEV-elicited cross-reactive CD8+ T cells in protecting against ADE of infection with ZIKV. Our investigation of the reverse infection scenario revealed that ZIKV-elicited Abs and CD8+ T cells affected the outcome of JEV infection by mediating ADE and contributing to protection, respectively. These data have important implications for the design of safe and effective anti-JEV/ZIKV vaccines.
Many adult mouse models are now available for investigating ZIKV infection and pathogenesis, including mice deficient in IFN-α/β receptor 1 and/or IFN-γ receptor 1 (Ifnar1−/−, Ifngr1−/−Ifnar1−/−), IFN regulatory factors (Irf3−/−Irf5−/− Irf7−/−), and IFN signaling proteins (Stat2−/−); as well as WT SJL mice and anti-Ifnar1 mAb-treated WT C57BL/6 mice (Li et al., 2016b, 2018). Two recent studies demonstrated that ZIKV can replicate in the brain and spinal cord and cause death in 1- to 3-d-old neonatal C57BL/6 mice in a viral dose- and host age-dependent manner (Li et al., 2018; Manangeeswaran et al., 2016). In the present study, we used a model in which 1-d-old C57BL/6 mice are infected with 102 FFU of ZIKV, a dose that causes ∼40% of animals to die within 28 d. The neonatal WT mouse model was selected for this study because ADE of ZIKV pathogenesis cannot be modeled in adult WT mice, which rapidly clear ZIKV infection, likely due to heightened Ifnar1 signaling in adult compared with neonatal WT mice. Additionally, the interplay between Ab and CD8+ T cell responses to ZIKV may be more faithfully recapitulated in mice with an intact antiviral IFN response because the Ifnar1 deficiency could limit the adaptive immune response (Kolumam et al., 2005; Wang et al., 2012). We also strived to ensure that our findings were as relevant as possible to humans by using both mouse and human JEV-immune sera. However, in some experiments involving the transfer of human JEV-naive serum, 60–80% (instead of the expected 40%) of animals succumbed to ZIKV infection, suggesting that this mouse model may be better suited for studies with mouse than human sera.
We found that mice infected with live JEV produced Abs that strongly cross-reacted with purified ZIKV-EDIII protein but had modest ZIKV-neutralizing activity. Cross-recognition of JEV-EDIII by ZIKV-induced Abs was also detected. Transfer of JEV-immune serum protected mice against lethal infection with JEV but enhanced disease severity and promoted mortality following ZIKV infection, and vice versa with ZIKV-immune sera and JEV infection. These data are in line with recent studies showing ADE of DENV and YFV infections by anti-JEV Abs (Anderson et al., 2011; Chan et al., 2016; Saito et al., 2015, 2016) and ADE of DENV infection by anti-ZIKV Abs (Fowler et al., 2018; George et al., 2017). We also observed that maternally acquired anti-JEV Abs accelerated ZIKV infection and increased the mortality of 1-d-old mice after ZIKV challenge. These results are in accordance with studies showing that anti-DENV Abs and anti-WNV Abs enhance ZIKV infection in cells and exacerbate ZIKV pathogenesis in mouse models (Bardina et al., 2017; Brown et al., 2019; Rathore et al., 2019). Thus, preexisting humoral immunity to JEV or ZIKV may increase the risk of developing severe disease upon infection with ZIKV or JEV, respectively. At present, the evidence to support ADE of ZIKV or JEV in humans is weak, although ZIKV-immune human sera have been shown to mediate ADE of ZIKV infection in Ifnar1−/− mice (Shim et al., 2019), and maternal anti-ZIKV Abs are associated with ADE of ZIKV infection and fetal microcephaly in humans (Robbiani et al., 2019). Future epidemiological studies may provide evidence to support ADE of both viruses.
In contrast to our finding that anti-JEV Abs enhanced ZIKV pathogenesis, a recent report showed that passive transfer of anti-JEV Abs from mice vaccinated with the live-attenuated JEV vaccine SA14-14-2 had no effect in adult Ifnar1−/− mice infected with ZIKV (Zhang et al., 2020). This discrepancy between studies is likely due to differences in the quality of anti-JEV Abs used for passive transfer, namely, sera from JEV SA14-14-2–vaccinated mice in the study by Zhang et al. (2020) vs. Abs from JEV-vaccinated humans or JEV-immune mice in our study. We also confirmed the results of our passive transfer experiments using a model in which maternal anti-JEV Abs were transferred to mouse pups before infection with ZIKV. However, based on our finding that passive transfer of nonmaternal anti-ZIKV Abs mediated ADE in mice with JEV infection, whereas maternal Abs had no effect, we hypothesize that the influence of preexisting humoral immunity may be highly context-dependent and subject to modulation by multiple factors, including Ab quality, mouse strain, and virus. In fact, two studies have found that Abs have no effect on infection with heterologous flavivirus. One study showed that sera from individuals inoculated with tick-borne encephalitis virus vaccine did not affect the mortality of ZIKV-infected Stat2−/− mice (Duehr et al., 2018), and the second study found that sera from macaques inoculated with a YFV vaccine did not enhance ZIKV infection in vitro (Bardina et al., 2017). Phylogenetic analysis of the E protein amino acid sequences shows that JEV, WNV, and DENV are more closely related to ZIKV than are YFV and tick-borne encephalitis virus (Duehr et al., 2018); therefore, the phylogenetic relationship between flaviviruses may determine how the Ab response to one flavivirus affects the pathogenesis of a secondary heterologous flavivirus infection. It seems likely that the capacity to elicit ADE is attributable to the close phylogenetic distance between flaviviruses.
We observed that JEV-elicited CD8+ T cells protected against lethal ZIKV infection, and vice versa. This is consistent with the results of studies examining the protective effects of CD8+ T cells cross-reactive with various flaviviruses. In several mouse models, DENV-reactive CD8+ T cells have been found to cross-protect against heterotypic DENV infection (Elong Ngono et al., 2016; Zellweger et al., 2015), reduce tissue viral loads and mortality of ZIKV-infected mice (Wen et al., 2017a, b), and protect fetuses upon ZIKV infection of pregnant mice (Regla-Nava et al., 2018). ZIKV-elicited CD8+ T cells were also reported to protect against DENV infection (Huang et al., 2017), and JEV SA14-14-2–primed CD8+ T cells were recently shown to protect against ZIKV infection (Zhang et al., 2020). Importantly, we demonstrated that JEV-elicited CD8+ T cells protected against ZIKV infection even in the presence of pathogenic levels of JEV-immune Abs. These findings are in line with studies demonstrating that DENV-elicited CD8+ T cells protect against ADE induced by an inactivated DENV vaccine in adult mice (Zellweger et al., 2014) and against ADE induced by maternal Abs in infant mice (Lam et al., 2017). Taken together, these observations suggest that interplay between the Ab and CD8+ T cell responses to primary flavivirus infection dictates the outcome of a secondary heterologous flavivirus infection, and they additionally emphasize the key role of cross-reactive CD8+ T cell responses in mediating protective immunity in this setting.
The majority of JEV-exposed individuals develop long-lasting immunity that may wane in later life (Solomon and Winter, 2004). In this context, our results suggest that JEV infection or vaccination may have a deleterious effect on subsequent heterologous viral infections. Moreover, an inadequate CD8+ T cell response may contribute to ADE and the development of severe dengue, which has been observed in individuals vaccinated for JEV (Anderson et al., 2011) or infected with heterologous DENV serotypes (Hadinegoro et al., 2015). Given that ZIKV may be spreading and JEV and DENV are already endemic in large areas of the globe, the development of JEV vaccines engineered to induce robust CD8+ T cell and Ab responses is likely to be critical for maximal efficacy and safety.
At present, three types of JEV vaccines (based on inactivated mouse brain–derived JEV, live-attenuated SA 14–14-2 strain, and inactivated Vero cell–derived JEV) are used in national immunization programs in 11 of the 24 countries currently considered at high risk for JEV endemicity. The inactivated mouse brain–derived JEV vaccine has been available for more than 50 yr and is used in six countries or territories. The live-attenuated SA14-14-2 vaccine became available in 1988 and is now used in five countries (mainland China, Cambodia, India, Nepal, and Sri Lanka). The inactivated Vero cell–derived JEV vaccine is mainly used in mainland China and Japan (Centers for Disease Control and Prevention (CDC), 2013; Gao et al., 2014). Millions of people currently carry JEV-immune Abs and CD8+ T cells resulting from either inoculation with JEV vaccines or natural infection with JEV. Thus, there is a pressing need for human cohort studies in JEV-endemic areas to clarify how the interplay between cellular and humoral immunity to JEV influences ZIKV/DENV disease severity, and vice versa.
In summary, we demonstrate here that JEV- or ZIKV-elicited human and mouse sera drive ADE of infection with the heterologous virus, and conversely, CD8+ T cells elicited by one virus mediate cross-protection against infection with the heterologous virus. Strikingly, JEV-elicited CD8+ T cells are able to attenuate the pathogenic effects of JEV-immune Abs in exacerbating ZIKV infection. These results underscore the importance of a balanced humoral and cellular response to the primary infection, which has crucial implications for vaccine design. Our finding that maternally acquired anti-JEV Abs can enhance the pathogenicity of ZIKV suggests that the developing fetuses and neonates of women with natural or vaccine-acquired JEV immunity may be at even higher risk of developing disease upon ZIKV infection. This knowledge should be taken into account in the design of vaccines to avoid exacerbation of disease. Encounters with multiple flaviviruses in a lifetime seem increasingly likely in many regions of the world, and our study provides new models of Ab-enhanced ZIKV and JEV pathogenesis in WT mice and sets a framework for investigating how the interplay between humoral and cellular immunity to one flavivirus modifies the development of immunity and clinical outcome to another flavivirus.
Materials and methods
Peptide synthesis
Eight previously identified H-2b-restricted CD8+ T cell epitopes in ZIKV (ZIKV-prM44-52, ZIKV-E4-12, ZIKV-E57-65, ZIKV-E420-428, ZIKV-NS3276-284, ZIKV-NS3347-355, ZIKV-NS568-77, and ZIKV-NS5222-229; Elong Ngono et al., 2017) and their variants in JEV (JEV-prM47-55, JEV-E4-12, JEV-E57-65, JEV-E416-424, JEV-NS3293-301, JEV-NS3364-372, JEV-NS5320-329, and JEV-NS5474-481) were synthesized by ChinaPeptides with a purity >95% (Table 1). All peptides were dissolved in dimethyl sulfoxide at 20 mg/ml.
Cell lines, viruses, and reagents
Vero, U937, and BHK-21 cells were purchased from the American Type Culture Collection and were maintained in RPMI-1640 (Vero and U937 cells) or MEM-α (BHK-21 cells) medium (Gibco). JEV strain ZJ14-52 (GenBank accession no. MK558811) was originally isolated from the serum of a patient with Japanese encephalitis in July 2014. ZIKV strain Zhejiang04 (GenBank accession no. KX117076.1) was originally isolated from the serum of a ZIKV-infected patient who lived in Wenzhou, China, and traveled to the Republic of Suriname in February 2016 during the ZIKV South American epidemic. ZIKV and JEV viral stocks were produced by propagating in Vero cells for two passages followed by titering in a BHK-21 cell–based focus-forming assay (FFA) in the case of ZIKV (Elong Ngono et al., 2017) or plaque-forming assay (PFA) in the case of JEV. Complete Freund’s adjuvant, incomplete Freund’s adjuvant, carboxymethyl cellulose (CMC), and PMA were purchased from Sigma-Aldrich. Dextran sulfate and LPS were from Merck. Abs and mAbs were as follows: Ifnar1-blocking Ab MAR1-5A3 (BP0241, BioXCell), mouse anti-ZIKV E protein mAb (ARG66286, clone SQab1749, Arigo Biolaboratories), mouse anti-JEV E protein mAb (clone C926M, Creative Diagnostics), mouse anti-hexahistidine (His) mAb (ab001, MultiSciences Biotech), and HRP-conjugated goat anti-mouse IgG and anti-human IgG (SA00012-1 and SA00001-17, respectively, Proteintech). Magnetic beads for positive selection (480076) and negative selection (480035) of mouse CD8+ T cell were purchased from BioLegend. Mouse IFN-γ ELISpot kits were from DAKEWE. Protein-G affinity column and nickel-nitrilotriacetic acid column were from Phygene. TrueBlue and 3,3′,5,5′-tetramethylbenzidine (TMB) peroxidase substrates were from KPL and Sigma-Aldrich, respectively.
Human sera and mice
This study was approved by the ethical committee of Zhejiang Provincial Center for Disease Control and Prevention, and written informed consent was obtained from all subjects. Blood was collected from healthy JEV-vaccinated donors (7-, 8-, 9-, 10-, 11-, and 20-yr-old individuals who had been vaccinated with live-attenuated JEV vaccine SA14-14-2 twice; once each at 8 mo and 2 yr of age) and JEV-naive donors (7-, 8-, 9-, 10-, 11-, and 20-yr-old individuals who had not been vaccinated against or infected by JEV or exposed to DENV or ZIKV; 6-mo-old infants born to JEV-naive mothers [never vaccinated or infected with JEV]) at the Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University. Serum samples were prepared and inactivated by heating for 30 min at 56°C before use in experiments. IgG was purified from 6-mo-old JEV-naive and 8-yr-old JEV-vaccinated donors using protein-G affinity columns according to the manufacturer’s instructions.
6-wk-old male and female WT C57BL/6 mice were purchased from the Animal Model Research Center at Nanjing University, China, and were maintained and bred in the Animal Center of Zhejiang Provincial Center for Disease Control and Prevention, China. The experiments were performed in strict accordance with the animal welfare act and public health service policy on humane care and use of laboratory animals and were approved by the ethical committee of Zhejiang Provincial Center for Disease Control and Prevention (no. LCKY2019-03). Sample sizes were estimated based on previous similar studies. Animal experiments were not randomized or blinded.
FFA and PFA
For ZIKV titering, the BHK-21 cell–based FFA was performed as described previously (Wen et al., 2017a). In brief, the ZIKV viral stock was serially diluted 10-fold, added to BHK-21 cells, and incubated for 2 h. The supernatant was then discarded, the cells were overlaid with 1% CMC MEM-α medium, and the plates were incubated for 4–5 d. The cells were then fixed with 4% paraformaldehyde, permeabilized with 1% Triton X-100, blocked with 5% FBS/PBS, and incubated with 1 µg/ml mouse anti-ZIKV E protein mAb (ARG66286) for 1 h at 25°C. After washing, the cells were incubated with HRP-conjugated goat anti-mouse IgG mAb at 1:1,000 for 1 h at 25°C. Finally, the cells were incubated with TrueBlue substrate, and the foci were counted manually. ZIKV viral titers are expressed as FFU per milliliter.
For JEV titering, a BHK-21 cell–based PFA was performed. 10-fold serially diluted JEV viral stock was added to BHK-21 cells and incubated for 2 h. The supernatant was then discarded, the cells were overlaid with 1% CMC MEM-α medium, and the plates were incubated for 4–5 d. The cells were then fixed with 4% paraformaldehyde and stained with 1% crystal violet. After washing, the plaques were counted. JEV viral titers are expressed as PFU per milliliter.
For measurement of ZIKV burden in serum and tissues, mouse brains were homogenized in 0.5 ml medium using a TissueLyser 24 (Jingxin) and then centrifuged for 8 min at 400 g. Aliquots of the resulting tissue supernatant (200 µl) or mouse serum samples (10 µl) were serially diluted 10-fold and added to BHK-21 cells. The remaining steps are as described above. ZIKV viral burden is expressed as FFU per milliliter serum or per gram tissue.
Expression of JEV and ZIKV EDIII proteins
Gene sequences encoding the viral (JEV and ZIKV) EDIII proteins and EV71-VP1 protein were synthesized by Genewiz and cloned into plasmid pET21a downstream of a 6× His-tag. The plasmids were transfected into Escherichia coli BL21, expression was induced, and the recombinant proteins were purified using a nickel-nitrilotriacetic acid column according to the manufacturer’s instructions.
Viral infection of adult mice
Three protocols were employed. In the first, 6-wk-old male WT mice were i.p. injected with 1 mg of the anti-Ifnar1 Ab MAR1-5A3 1 d before retro-orbital (r.o.) injection with 103 PFU of JEV or 103 FFU of ZIKV in 200 µl 10% FBS/PBS. 7 or 28 d later, naive and virus-infected mice were sacrificed, and the spleens were collected. CD8+ T cells were isolated from splenocytes by positive selection with magnetic beads and used for the IFN-γ ELISpot assays.
In the second protocol, 6-wk-old female WT mice were injected i.p. with 1 mg of the anti-Ifnar1 Ab MAR1-5A3 1 d before r.o. injection of 103 PFU of JEV or 103 FFU of ZIKV in 200 µl 10% FBS/PBS. Mock-immune mice were injected r.o. with 200 µl 10% FBS/PBS. As additional controls, EV71-VP1 was emulsified in complete Freund’s adjuvant and injected s.c. into the backs of 6-wk-old female mice (50 µg/mouse). After 2 wk, mice were boosted with the same amount of EV71-VP1 in incomplete Freund’s adjuvant. 28 d later, the mock-immune mice, EV71-VP1-immune mice, JEV-immune mice, and ZIKV-immune mice were sacrificed, blood samples were collected and processed, and sera were used for ELISA, microneutralization assays, in vitro ADE assays, and passive transfer experiments. IgG was purified from mock-immune and JEV-immune sera using protein-G affinity columns and used for passive transfer experiments. CD8+ T cells were purified from splenocytes by negative selection with magnetic beads and used for adoptive transfer experiments.
In the third protocol, 6-wk-old female WT mice were injected i.p. with 1 mg of the anti-Ifnar1 Ab MAR1-5A3 1 d before r.o. injection of 102 PFU of JEV or 102 FFU of ZIKV in 200 µl 10% FBS/PBS. 28 d later, the naive, JEV-immune, and ZIKV-immune mice were mated with 10-wk-old naive male mice. 1-d-old pups born to naive, JEV-immune, or ZIKV-immune mice were sacrificed, blood was collected and prepared, and sera were used for ELISA.
Anti-EDIII IgG capture ELISAs
Anti-JEV-EDIII– and anti-ZIKV-EDIII–reactive IgG in human and mouse sera were detected by indirect capture ELISAs. In brief, 96-well plates were coated with 1 µg/ml recombinant JEV-EDIII or ZIKV-EDIII proteins overnight at 4°C, and the wells were then blocked with 10% FBS/PBS for 1 h at 25°C. Human sera and mouse sera (mock-, JEV-, and ZIKV-immune) were serially diluted threefold starting from 1:9 dilutions. Sera from 1-d-old pups born to naive, JEV-immune, or ZIKV-immune mice were serially diluted threefold starting with 1:15 dilutions. Threefold serially diluted mouse anti-His mAb (starting with 6 µg/ml) was used as a positive control. Diluted serum samples (100 µl) were added to the wells of JEV-EDIII or ZIKV-EDIII protein-coated ELISA plates and incubated for 1.5 h at 25°C. After washing the wells, bound IgG was detected by incubation with HRP-conjugated goat anti-human or mouse IgG mAb (1:5,000) for 1 h at 25°C. The plates were then developed with TMB for 10 min at 25°C, and the absorbance at 450 nm was measured. Endpoint titers were defined as the reciprocal of the serum dilution that gave twice the average OD value obtained with naive serum.
Microneutralization assay
ZIKV- and JEV-neutralizing activities of human and mouse sera were determined using a microneutralization assay as described previously (Abbink et al., 2016) with slight modifications. In brief, 50 µl of twofold serially diluted (from 1:4 to 1:256) serum samples was incubated with 50 µl of ZIKV (102 FFU) or JEV (102 PFU) at 37°C for 1 h. The mixture was then added to Vero cell monolayers that had been cultured overnight in 96-well plates, and the cells were incubated for 1 h. Samples containing virus but not serum were included as controls. The viral supernatants were removed, and the cells were overlaid with 1% CMC medium and incubated for 2 d at 37°C. Cells were then fixed with 4% paraformaldehyde/PBS, permeabilized with 1% Triton X-100, and incubated with mouse anti-ZIKV E protein mAb (1 µg/ml) or mouse anti-JEV E protein mAb (1 µg/ml) followed by HRP-conjugated goat anti-mouse IgG (1:5,000). Color development was achieved by the addition of TMB substrate, and the absorbance at 450 nm (OD450) was recorded. Percent neutralization of infection was calculated as (OD450 in control wells – OD450 in serum-containing wells)/OD450 in control wells. The 50% neutralizing titer was calculated as the reciprocal of the average serum dilution yielding 50% inhibition of the OD450 compared with naive serum values.
In vitro ADE assay
To assess ADE in vitro, we developed an assay similar in principle to the previously described microneutralization assay (Abbink et al., 2016). Human and mouse sera were serially diluted twofold from 1:4 to 1:256 in PBS and added to 96-well plates at 50 µl/well. Aliquots of ZIKV (104 FFU/50 µl/well) were added, and the plates were incubated for 1 h at 37°C. The well contents were transferred to wells containing 104 U937 cells that had been pretreated with 0.5 µg/ml PMA for 2 d, and the plates were incubated for 2 h at 37°C. Viral supernatants were removed, and the cells were overlaid with 1% CMC medium and incubated for 2 d at 37°C. Cells treated in the same manner but without serum served as controls. After incubation, the U937 cells were fixed with 4% paraformaldehyde/PBS, permeabilized with 1% Triton X-100, and incubated with mouse anti-ZIKV E protein mAb (1 µg/ml) followed by HRP-conjugated goat anti-mouse IgG (1:1,000). Color development was achieved by addition of TMB, and the absorbance at 450 nm was measured. ADE of infection was expressed as the fold increase compared with control wells: (OD450 in serum-containing wells – OD450 in control wells)/OD450 in control wells.
IFN-γ ELISpot assay
IFN-γ–producing CD8+ T cells were enumerated using an IFN-γ ELISpot assay as previously described (Wen et al., 2017b). In brief, CD8+ T cells were isolated from splenocytes from JEV- or ZIKV-infected mice by positive selection using magnetic beads. LPS blasts (antigen-presenting cells) were prepared by stimulation of naive C57BL/6 splenocytes with LPS and dextran sulfate as described previously (Wen et al., 2017b). CD8+ T cells (2 × 105), LPS blasts (105), and 10 µg of JEV or ZIKV peptides were added to each well of ELISpot plates precoated with anti–IFN-γ mAb (CT655-10, U-CyTech Biosciences). After 24 h incubation, the wells were incubated sequentially with biotinylated anti-mouse IFN-γ mAb (CT655-10, U-CyTech Biosciences), HRP-conjugated streptavidin, and 3-amino-9-ethylcarbazole color development solution. The results are expressed as the number of peptide-reactive spot-forming cells (SFCs) per 106 CD8+ T cells. The presence of >20 SFCs/well with a stimulation index of >2 (index = ratio of test SFCs to control SFCs) was considered a positive response.
Viral challenge of mice transferred with serum and/or CD8+ T cells
For the serum transfer experiments, two protocols were used. In the first protocol, 1-d-old C56BL/6 mice were injected s.c. with 3 µl, 15 µl, or 75 µl of pooled human sera (from JEV-naive or 8-yr-old JEV-vaccinated donors) or mouse sera (mock- or JEV-immune); or with 75 µg IgG purified from human JEV-naive or JEV-immune sera (8-yr-old JEV-vaccinated donors); or with 12 µg IgG purified from mouse mock-immune or JEV-immune sera. Transfer of 75 µl of sera was accomplished with two injections of 37.5 µl each, administered 3 h apart. 6 h later, mice were injected s.c. with 12 PFU of JEV or 102 FFU of ZIKV in 30 µl 10% FBS/PBS. In the second protocol, 1-d-old C56BL/6 mice were injected s.c. with 0.05 µl or 10 µl of pooled sera from mock- or ZIKV-immune mice. 6 h later, mice were injected s.c. with 12 PFU of JEV or 102 FFU of ZIKV in 30 µl 10% FBS/PBS.
For the cell transfer experiments, three protocols were used. CD8+ T cells were isolated from the spleens of EV71-VP1-, JEV-, or ZIKV-immune C57BL/6 mice, as described above. For the first protocol, aliquots of 2 × 106 CD8+ T cells in 10 µl of 10% FBS/PBS were injected r.o. into 1-d-old naive mice, and 2 h later, the mice were injected s.c. with 102 FFU of ZIKV in 30 µl 10% FBS/PBS. For the second protocol, 1-d-old naive mice were injected r.o. with 2 × 106 CD8+ T cells in 10 µl of 10% FBS/PBS and s.c. with 3 µl mouse JEV-immune serum. 6 h later, the mice were injected s.c. with 102 FFU of ZIKV in 30 µl of 10% FBS/PBS. For the third protocol, 1-d-old naive mice were injected r.o. with 2 × 106 CD8+ T cells in 10 µl of 10% FBS/PBS and injected s.c. with 12 PFU of JEV in 30 µl 10% FBS/PBS 6 h later. For all experiments, mouse weights and deaths were recorded daily for up to 28 d. In selected experiments, ZIKV viral burden in serum and brain was measured on days 3 and 8 after ZIKV challenge.
ZIKV/JEV challenge of pups born to JEV/ZIKV-immune mice
Three protocols were used. In the first, 1-d-old pups born to naive and JEV-immune mice were injected s.c. with 12 PFU of JEV or 102 FFU of ZIKV in 30 µl of 10% FBS/PBS. Mouse weights and deaths were recorded daily for up to 28 d. In the second protocol, 1-d-old pups born to naive and JEV-immune mice were injected s.c. with 102 FFU of ZIKV in 30 µl of 10% FBS/PBS. 3 d after infection, the mice were sacrificed, and blood and brain were collected for measurement of viral burden. In the third protocol, 1-d-old pups born to naive and ZIKV-immune mice were injected s.c. with 12 PFU of JEV in 30 µl of 10% FBS/PBS. Mouse weights and deaths were recorded daily for up to 14 d.
Statistics
All data were analyzed using Prism 6 software (GraphPad Software). Data are expressed as the means ± standard errors. Grubbs’s test was performed to detect outliers. Two-group comparison was performed using the two-tailed Mann–Whitney U test, and survival data were analyzed using a log-rank test. P < 0.05 was considered statistically significant.
Data availability
Data supporting the findings of this study are available from the corresponding authors upon request.
Online supplemental material
Fig. S1 shows the neutralizing and enhancing activity of mouse and human JEV-immune serum against ZIKV and JEV infection in vitro, which is related to Fig. 3 and Fig. 4. Fig. S2 shows the effect of mouse and human JEV-immune serum-derived IgG on the outcome of ZIKV-infected mice, which is related to Fig. 3 and Fig. 4. Fig. S3 shows the level of ZIKV-reactive IgG in the serum of mice receiving different volume of mouse JEV-immune serum, which is related to Fig. 5. Fig. S4 shows the effect of maternally acquired anti-ZIKV Ab on the outcome of JEV-infected mice, which is related to Fig. 5.
Acknowledgments
J. Wen was supported by the K.C. Wong Magna Fund of Ningbo University and grants from the Zhejiang Provincial Natural Science Foundation (LY17C010004) and the National Natural Science Foundation of China (31870159). S. Shresta was supported by institutional funds from the La Jolla Institute for Immunology.
Author contributions: J. Wen and S. Shresta designed the study. D. Chen, Z. Duan, W. Zhou, W. Zou, S. Jin, D. Li, X. Chen, Y. Zhou, L. Yang, and Y. Zhang performed the experiments. D. Chen, Y. Zhang, J. Wen, and S. Shresta interpreted the data. J. Wen and S. Shresta wrote the manuscript. S. Shresta edited the manuscript.
References
- Abbink P., Larocca R.A., De La Barrera R.A., Bricault C.A., Moseley E.T., Boyd M., Kirilova M., Li Z., Ng’ang’a D., Nanayakkara O., et al. 2016. Protective efficacy of multiple vaccine platforms against Zika virus challenge in rhesus monkeys. Science. 353:1129–1132. 10.1126/science.aah6157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson K.B., Gibbons R.V., Thomas S.J., Rothman A.L., Nisalak A., Berkelman R.L., Libraty D.H., and Endy T.P.. 2011. Preexisting Japanese encephalitis virus neutralizing antibodies and increased symptomatic dengue illness in a school-based cohort in Thailand. PLoS Negl. Trop. Dis. 5 e1311 10.1371/journal.pntd.0001311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki K., Yoshimatsu K., Lee B.H., Okumura M., Kariwa H., Takashima I., and Arikawa J.. 2004. Age-dependent hantavirus-specific CD8(+) T-cell responses in mice infected with Hantaan virus. Arch. Virol. 149:1373–1382. 10.1007/s00705-003-0285-4 [DOI] [PubMed] [Google Scholar]
- Balsitis S.J., Williams K.L., Lachica R., Flores D., Kyle J.L., Mehlhop E., Johnson S., Diamond M.S., Beatty P.R., and Harris E.. 2010. Lethal antibody enhancement of dengue disease in mice is prevented by Fc modification. PLoS Pathog. 6 e1000790 10.1371/journal.ppat.1000790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardina S.V., Bunduc P., Tripathi S., Duehr J., Frere J.J., Brown J.A., Nachbagauer R., Foster G.A., Krysztof D., Tortorella D., et al. 2017. Enhancement of Zika virus pathogenesis by preexisting antiflavivirus immunity. Science. 356:175–180. 10.1126/science.aal4365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J.A., Singh G., Acklin J.A., Lee S., Duehr J.E., Chokola A.N., Frere J.J., Hoffman K.W., Foster G.A., Krysztof D., et al. 2019. Dengue Virus Immunity Increases Zika Virus-Induced Damage during Pregnancy. Immunity. 50:751–762.e5. 10.1016/j.immuni.2019.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell G.L., Hills S.L., Fischer M., Jacobson J.A., Hoke C.H., Hombach J.M., Marfin A.A., Solomon T., Tsai T.F., Tsu V.D., et al. 2011. Estimated global incidence of Japanese encephalitis: a systematic review. Bull. World Health Organ. 89:766–774: 774A–774E. 10.2471/BLT.10.085233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) 2013. Japanese encephalitis surveillance and immunization--Asia and the Western Pacific, 2012. MMWR Morb. Mortal. Wkly. Rep. 62:658–662. [PMC free article] [PubMed] [Google Scholar]
- Chan K.R., Wang X., Saron W.A.A., Gan E.S., Tan H.C., Mok D.Z.L., Zhang S.L., Lee Y.H., Liang C., Wijaya L., et al. 2016. Cross-reactive antibodies enhance live attenuated virus infection for increased immunogenicity. Nat. Microbiol. 1:16164 10.1038/nmicrobiol.2016.164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejnirattisai W., Jumnainsong A., Onsirisakul N., Fitton P., Vasanawathana S., Limpitikul W., Puttikhunt C., Edwards C., Duangchinda T., Supasa S., et al. 2010. Cross-reacting antibodies enhance dengue virus infection in humans. Science. 328:745–748. 10.1126/science.1185181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejnirattisai W., Supasa P., Wongwiwat W., Rouvinski A., Barba-Spaeth G., Duangchinda T., Sakuntabhai A., Cao-Lormeau V.M., Malasit P., Rey F.A., et al. 2016. Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with zika virus. Nat. Immunol. 17:1102–1108. 10.1038/ni.3515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duehr J., Lee S., Singh G., Foster G.A., Krysztof D., Stramer S.L., Bermúdez González M.C., Menichetti E., Geretschläger R., Gabriel C., et al. 2018. Tick-Borne Encephalitis Virus Vaccine-Induced Human Antibodies Mediate Negligible Enhancement of Zika Virus Infection InVitro and in a Mouse Model. MSphere. 3 e00011-18 10.1128/mSphereDirect.00011-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elong Ngono A., and Shresta S.. 2019. Cross-Reactive T Cell Immunity to Dengue and Zika Viruses: New Insights Into Vaccine Development. Front. Immunol. 10:1316 10.3389/fimmu.2019.01316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elong Ngono A., Chen H.W., Tang W.W., Joo Y., King K., Weiskopf D., Sidney J., Sette A., and Shresta S.. 2016. Protective Role of Cross-Reactive CD8 T Cells Against Dengue Virus Infection. EBioMedicine. 13:284–293. 10.1016/j.ebiom.2016.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elong Ngono A., Vizcarra E.A., Tang W.W., Sheets N., Joo Y., Kim K., Gorman M.J., Diamond M.S., and Shresta S.. 2017. Mapping and Role of the CD8+ T Cell Response During Primary Zika Virus Infection in Mice. Cell Host Microbe. 21:35–46. 10.1016/j.chom.2016.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler A.M., Tang W.W., Young M.P., Mamidi A., Viramontes K.M., McCauley M.D., Carlin A.F., Schooley R.T., Swanstrom J., Baric R.S., et al. 2018. Maternally Acquired Zika Antibodies Enhance Dengue Disease Severity in Mice. Cell Host Microbe. 24:743–750.e5. 10.1016/j.chom.2018.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X., Li X., Li M., Fu S., Wang H., Lu Z., Cao Y., He Y., Zhu W., Zhang T., et al. 2014. Vaccine strategies for the control and prevention of Japanese encephalitis in Mainland China, 1951-2011. PLoS Negl. Trop. Dis. 8 e3015 10.1371/journal.pntd.0003015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George J., Valiant W.G., Mattapallil M.J., Walker M., Huang Y.S., Vanlandingham D.L., Misamore J., Greenhouse J., Weiss D.E., Verthelyi D., et al. 2017. Prior Exposure to Zika Virus Significantly Enhances Peak Dengue-2 Viremia in Rhesus Macaques. Sci. Rep. 7:10498 10.1038/s41598-017-10901-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A., Gresh L., Ojeda S., Katzelnick L.C., Sanchez N., Mercado J.C., Chowell G., Lopez B., Elizondo D., Coloma J., et al. 2019. Prior dengue virus infection and risk of Zika: A pediatric cohort in Nicaragua. PLoS Med. 16 e1002726 10.1371/journal.pmed.1002726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadinegoro S.R., Arredondo-García J.L., Capeding M.R., Deseda C., Chotpitayasunondh T., Dietze R., Muhammad Ismail H.I., Reynales H., Limkittikul K., Rivera-Medina D.M., et al. ; CYD-TDV Dengue Vaccine Working Group . 2015. Efficacy and Long-Term Safety of a Dengue Vaccine in Regions of Endemic Disease. N. Engl. J. Med. 373:1195–1206. 10.1056/NEJMoa1506223 [DOI] [PubMed] [Google Scholar]
- Huang H., Li S., Zhang Y., Han X., Jia B., Liu H., Liu D., Tan S., Wang Q., Bi Y., et al. 2017. CD8+ T Cell Immune Response in Immunocompetent Mice during Zika Virus Infection. J. Virol. 91 e00900-17 10.1128/JVI.00900-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzelnick L.C., Gresh L., Halloran M.E., Mercado J.C., Kuan G., Gordon A., Balmaseda A., and Harris E.. 2017. Antibody-dependent enhancement of severe dengue disease in humans. Science. 358:929–932. 10.1126/science.aan6836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaiboullina S., Uppal T., Martynova E., Rizvanov A., Baranwal M., and Verma S.C.. 2018. History of ZIKV Infections in India and Management of Disease Outbreaks. Front. Microbiol. 9:2126 10.3389/fmicb.2018.02126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolumam G.A., Thomas S., Thompson L.J., Sprent J., and Murali-Krishna K.. 2005. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J. Exp. Med. 202:637–650. 10.1084/jem.20050821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutsuna S., Kato Y., Takasaki T., Moi M., Kotaki A., Uemura H., Matono T., Fujiya Y., Mawatari M., Takeshita N., et al. 2014. Two cases of Zika fever imported from French Polynesia to Japan, December 2013 to January 2014 [corrected]. Euro Surveill. 19:20683 10.2807/1560-7917.ES2014.19.4.20683 [DOI] [PubMed] [Google Scholar]
- Lam J.H., Chua Y.L., Lee P.X., Martínez Gómez J.M., Ooi E.E., and Alonso S.. 2017. Dengue vaccine-induced CD8+ T cell immunity confers protection in the context of enhancing, interfering maternal antibodies. JCI Insight. 2 e94500 10.1172/jci.insight.94500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Xu D., Ye Q., Hong S., Jiang Y., Liu X., Zhang N., Shi L., Qin C.F., and Xu Z.. 2016a Zika Virus Disrupts Neural Progenitor Development and Leads to Microcephaly in Mice. Cell Stem Cell. 19:120–126. 10.1016/j.stem.2016.04.017 [DOI] [PubMed] [Google Scholar]
- Li H., Saucedo-Cuevas L., Shresta S., and Gleeson J.G.. 2016b The Neurobiology of Zika Virus. Neuron. 92:949–958. 10.1016/j.neuron.2016.11.031 [DOI] [PubMed] [Google Scholar]
- Li J., Gao N., Fan D., Chen H., Sheng Z., Fu S., Liang G., and An J.. 2016c Cross-protection induced by Japanese encephalitis vaccines against different genotypes of Dengue viruses in mice. Sci. Rep. 6:19953 10.1038/srep19953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Armstrong N., Zhao H., Hou W., Liu J., Chen C., Wan J., Wang W., Zhong C., Liu C., et al. 2018. Zika Virus Fatally Infects Wild Type Neonatal Mice and Replicates in Central Nervous System. Viruses. 10:49 10.3390/v10010049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luca V.C., AbiMansour J., Nelson C.A., and Fremont D.H.. 2012. Crystal structure of the Japanese encephalitis virus envelope protein. J. Virol. 86:2337–2346. 10.1128/JVI.06072-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manangeeswaran M., Ireland D.D., and Verthelyi D.. 2016. Zika (PRVABC59) Infection Is Associated with T cell Infiltration and Neurodegeneration in CNS of Immunocompetent Neonatal C57Bl/6 Mice. PLoS Pathog. 12 e1006004 10.1371/journal.ppat.1006004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlakar J., Korva M., Tul N., Popović M., Poljšak-Prijatelj M., Mraz J., Kolenc M., Resman Rus K., Vesnaver Vipotnik T., Fabjan Vodušek V., et al. 2016. Zika Virus Associated with Microcephaly. N. Engl. J. Med. 374:951–958. 10.1056/NEJMoa1600651 [DOI] [PubMed] [Google Scholar]
- Monsalve D.M., Pacheco Y., Acosta-Ampudia Y., Rodríguez Y., Ramírez-Santana C., and Anaya J.M.. 2017. Zika virus and autoimmunity. One-step forward. Autoimmun. Rev. 16:1237–1245. 10.1016/j.autrev.2017.10.008 [DOI] [PubMed] [Google Scholar]
- Ngono A.E., and Shresta S.. 2018. Immune Response to Dengue and Zika. Annu. Rev. Immunol. 36:279–308. 10.1146/annurev-immunol-042617-053142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedroso C., Fischer C., Feldmann M., Sarno M., Luz E., Moreira-Soto A., Cabral R., Netto E.M., Brites C., Kümmerer B.M., et al. 2019. Cross-Protection of Dengue Virus Infection against Congenital Zika Syndrome, Northeastern Brazil. Emerg. Infect. Dis. 25:1485–1493. 10.3201/eid2508.190113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quyen N.T.H., Kien D.T.H., Rabaa M., Tuan N.M., Vi T.T., Van Tan L., Hung N.T., Tuan H.M., Van Tram T., Le Da Ha N., et al. 2017. Chikungunya and Zika Virus Cases Detected against a Backdrop of Endemic Dengue Transmission in Vietnam. Am. J. Trop. Med. Hyg. 97:146–150. 10.4269/ajtmh.16-0979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathore A.P.S., Saron W.A.A., Lim T., Jahan N., and St John A.L.. 2019. Maternal immunity and antibodies to dengue virus promote infection and Zika virus-induced microcephaly in fetuses. Sci. Adv. 5:v3208 10.1126/sciadv.aav3208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regla-Nava J.A., Elong Ngono A., Viramontes K.M., Huynh A.T., Wang Y.T., Nguyen A.T., Salgado R., Mamidi A., Kim K., Diamond M.S., et al. 2018. Cross-reactive Dengue virus-specific CD8+ T cells protect against Zika virus during pregnancy. Nat. Commun. 9:3042 10.1038/s41467-018-05458-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remot A., Descamps D., Jouneau L., Laubreton D., Dubuquoy C., Bouet S., Lecardonnel J., Rebours E., Petit-Camurdan A., and Riffault S.. 2016. Flt3 ligand improves the innate response to respiratory syncytial virus and limits lung disease upon RSV reexposure in neonate mice. Eur. J. Immunol. 46:874–884. 10.1002/eji.201545929 [DOI] [PubMed] [Google Scholar]
- Robbiani D.F., Olsen P.C., Costa F., Wang Q., Oliveira T.Y., Nery N. Jr., Aromolaran A., do Rosário M.S., Sacramento G.A., Cruz J.S., et al. 2019. Risk of Zika microcephaly correlates with features of maternal antibodies. J. Exp. Med. 216:2302–2315. 10.1084/jem.20191061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Barraquer I., Costa F., Nascimento E.J.M., Nery N., Castanha P.M.S., Sacramento G.A., Cruz J., Carvalho M., De Olivera D., Hagan J.E., et al. 2019. Impact of preexisting dengue immunity on Zika virus emergence in a dengue endemic region. Science. 363:607–610. 10.1126/science.aav6618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruchusatsawat K., Wongjaroen P., Posanacharoen A., Rodriguez-Barraquer I., Sangkitporn S., Cummings D.A.T., and Salje H.. 2019. Long-term circulation of Zika virus in Thailand: an observational study. Lancet Infect. Dis. 19:439–446. 10.1016/S1473-3099(18)30718-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y., Moi M.L., Takeshita N., Lim C.K., Shiba H., Hosono K., Saijo M., Kurane I., and Takasaki T.. 2016. Japanese encephalitis vaccine-facilitated dengue virus infection-enhancement antibody in adults. BMC Infect. Dis. 16:578 10.1186/s12879-016-1873-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salje H., Cummings D.A.T., Rodriguez-Barraquer I., Katzelnick L.C., Lessler J., Klungthong C., Thaisomboonsuk B., Nisalak A., Weg A., Ellison D., et al. 2018. Reconstruction of antibody dynamics and infection histories to evaluate dengue risk. Nature. 557:719–723. 10.1038/s41586-018-0157-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato R., Hamada N., Kashiwagi T., Imamura Y., Hara K., Nishimura M., Kamimura T., Takasaki T., Watanabe H., and Koga T.. 2015. Dengue Hemorrhagic Fever in a Japanese Traveler with Pre-existing Japanese Encephalitis Virus Antibody. Trop. Med. Health. 43:85–88. 10.2149/tmh.2014-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim B.S., Kwon Y.C., Ricciardi M.J., Stone M., Otsuka Y., Berri F., Kwal J.M., Magnani D.M., Jackson C.B., Richard A.S., et al. 2019. Zika Virus-Immune Plasmas from Symptomatic and Asymptomatic Individuals Enhance Zika Pathogenesis in Adult and Pregnant Mice. MBio. 10 e00758-19 10.1128/mBio.00758-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirohi D., Chen Z., Sun L., Klose T., Pierson T.C., Rossmann M.G., and Kuhn R.J.. 2016. The 3.8 Å resolution cryo-EM structure of Zika virus. Science. 352:467–470. 10.1126/science.aaf5316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon T., and Winter P.M.. 2004. Neurovirulence and host factors in flavivirus encephalitis--evidence from clinical epidemiology. Arch. Virol. Suppl. 18:161–170. [DOI] [PubMed] [Google Scholar]
- Tesh R.B., Travassos da Rosa A.P., Guzman H., Araujo T.P., and Xiao S.Y.. 2002. Immunization with heterologous flaviviruses protective against fatal West Nile encephalitis. Emerg. Infect. Dis. 8:245–251. 10.3201/eid0803.010238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vázquez-Calvo Á., Blázquez A.B., Escribano-Romero E., Merino-Ramos T., Saiz J.C., Martín-Acebes M.A., and Jiménez de Oya N.. 2017. Zika virus infection confers protection against West Nile virus challenge in mice. Emerg. Microbes Infect. 6 e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Swiecki M., Cella M., Alber G., Schreiber R.D., Gilfillan S., and Colonna M.. 2012. Timing and magnitude of type I interferon responses by distinct sensors impact CD8 T cell exhaustion and chronic viral infection. Cell Host Microbe. 11:631–642. 10.1016/j.chom.2012.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen J., and Shresta S.. 2019. Antigenic cross-reactivity between Zika and dengue viruses: is it time to develop a universal vaccine? Curr. Opin. Immunol. 59:1–8. 10.1016/j.coi.2019.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen J., Elong Ngono A., Regla-Nava J.A., Kim K., Gorman M.J., Diamond M.S., and Shresta S.. 2017a Dengue virus-reactive CD8+ T cells mediate cross-protection against subsequent Zika virus challenge. Nat. Commun. 8:1459 10.1038/s41467-017-01669-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen J., Tang W.W., Sheets N., Ellison J., Sette A., Kim K., and Shresta S.. 2017b Identification of Zika virus epitopes reveals immunodominant and protective roles for dengue virus cross-reactive CD8+ T cells. Nat. Microbiol. 2:17036 10.1038/nmicrobiol.2017.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization 2017. Zika situation report: Zika virus, microcephaly and Guillain-Barre syndrome. https://apps.who.int/iris/bitstream/handle/10665/254714/zikasitrep10Mar17-eng.pdf
- Wilcox D.R., Wadhwani N.R., Longnecker R., and Muller W.J.. 2015. Differential reliance on autophagy for protection from HSV encephalitis between newborns and adults. PLoS Pathog. 11 e1004580 10.1371/journal.ppat.1004580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zellweger R.M., Prestwood T.R., and Shresta S.. 2010. Enhanced infection of liver sinusoidal endothelial cells in a mouse model of antibody-induced severe dengue disease. Cell Host Microbe. 7:128–139. 10.1016/j.chom.2010.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zellweger R.M., Miller R., Eddy W.E., White L.J., Johnston R.E., and Shresta S.. 2013. Role of humoral versus cellular responses induced by a protective dengue vaccine candidate. PLoS Pathog. 9 e1003723 10.1371/journal.ppat.1003723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zellweger R.M., Eddy W.E., Tang W.W., Miller R., and Shresta S.. 2014. CD8+ T cells prevent antigen-induced antibody-dependent enhancement of dengue disease in mice. J. Immunol. 193:4117–4124. 10.4049/jimmunol.1401597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zellweger R.M., Tang W.W., Eddy W.E., King K., Sanchez M.C., and Shresta S.. 2015. CD8+ T Cells Can Mediate Short-Term Protection against Heterotypic Dengue Virus Reinfection in Mice. J. Virol. 89:6494–6505. 10.1128/JVI.00036-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Chen W., Wong G., Bi Y., Yan J., Sun Y., Chen E., Yan H., Lou X., Mao H., et al. 2016. Highly diversified Zika viruses imported to China, 2016. Protein Cell. 7:461–464. 10.1007/s13238-016-0274-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Xu Y., Zhao F., Tarbe M., Zhou S., Wang W., Zhang S., Zhang W., Xu Q., Shi L., et al. 2020. The pre-existing cellular immunity to Japanese encephalitis virus heterotypically protects mice from Zika virus infection. Sci. Bull. (Beijing). 65:402–409. 10.1016/j.scib.2019.11.006 [DOI] [PubMed] [Google Scholar]
- Zimmerman M.G., Quicke K.M., O’Neal J.T., Arora N., Machiah D., Priyamvada L., Kauffman R.C., Register E., Adekunle O., Swieboda D., et al. 2018. Cross-Reactive Dengue Virus Antibodies Augment Zika Virus Infection of Human Placental Macrophages. Cell Host Microbe. 24:731–742.e6. 10.1016/j.chom.2018.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data supporting the findings of this study are available from the corresponding authors upon request.