Abstract
Intraperitoneal (IP) chemotherapy is believed to prolong the survival of patients with advanced ovarian cancer after primary debulking surgery. However, there is little knowledge about IP chemotherapy in the setting of neoadjuvant chemotherapy, and there are contradictory conclusions about adjuvant IP chemotherapy. Here, we evaluated the feasibility of neoadjuvant and adjuvant IP chemotherapy in patients with advanced epithelial ovarian cancer (AEOC).
We retrospectively reviewed the data of 114 patients with AEOC who received neoadjuvant chemotherapy followed by laparoscopic conservative interval debulking surgery (NACT + LIDS) in our institution from January 1, 2009 to December 31, 2017.
The median overall survival (OS) was 56 months and the median disease-free interval (DFI) was 14 months for the entire study population. Neoadjuvant IP chemotherapy cycles were crucial for the treatment of no gross residual (R0) disease (hazard ratio [HR] = 0.446, 95% confidence interval [CI] = 0.245–0.811), which was independently associated with OS of the entire study population (HR = 9.589, 95% CI = 3.911–23.507). In addition, residual disease and body mass index (BMI) were the prognostic factors for DFI (HR = 6.022, 95% CI = 3.632–9.986; HR = 1.085, 95% CI = 1.012–1.163). However, adjuvant IP cycles along with BMI were the determining factors for DFI in the R0 group (HR = 0.703, 95% CI = 0.525–0.941; HR = 1.130, 95% CI = 1.025–1.247), and were associated with OS in the R0 group (HR = 0.488, 95% CI = 0.289–0.824). The OS and DFI Kaplan-Meier curves stratified by adjuvant IP chemothearpy cycles within the R0 group were statistically significant (P = .024 and P = .033, respectively).
Our results showed improvement in patients with AEOC in terms of survival, thus suggesting the feasibility of neoadjuvant and adjuvant IP chemotherapy.
Keywords: adjuvant chemotherapy, advanced epithelial ovarian cancer, interval debulking, intraperitoneal chemotherapy, neoadjuvant chemotherapy
1. Introduction
Ovarian cancer is one of the most lethal and common gynecological cancers according to global cancer data. Most ovarian cancer patients are diagnosed at advanced stages. Complex factors result in a terrible 5-year survival rate <50%, and epithelial ovarian cancer accounts for almost 90% of the subtypes.[1,2] Neoadjuvant chemotherapy followed by interval debulking surgery (NACT + IDS), which is still under debate, was introduced for AEOC patients who could not achieve optimal primary debulking surgery (PDS) safely.[3,4] However, the exact and appropriate NACT and adjuvant chemotherapy cycles are not decided, with the advised cycles resulting from a few prospective studies,[5–8] and the medication methods by intraperitoneal or intravenous administration are still being explored. Intraperitoneal (IP) chemotherapy has gained more attention worldwide as promising results have been achieved in some large prospective studies.[9–11] A retrospective study based on these data concluded that the advantages of IP chemotherapy extended over 10 years and more IP chemotherapy cycles may lead to better survival results, but the catheter-related complications and increased toxicity dissuaded wide acceptance of IP chemotherapy.[12] However, few research studies have examined the potential benefits of neoadjuvant IP chemotherapy.[13,14] Limited research demonstrating the survival outcomes of advanced ovarian cancer patients who received IDS and adjuvant IP chemotherapy has been inconsistent.[15–20]
The main aim of this study was to evaluate the feasibility of neoadjuvant and adjuvant IP chemotherapy in AEOC patients after laparoscopic conservative interval debulking surgery.
2. Methods
2.1. Study population
This retrospective study was approved by the Institutional Review Board (IRB) of the First Affiliated Hospital of Third Military Medical University (Army Military Medical University). Informed consent was waived by the IRB. This study enrolled patients with advanced (FIGO2008 stage III to IV) epithelial ovarian cancer who received NACT + LIDS at our institution from January 2009 to December 2017. All women eligible for this study were diagnosed by biopsy or cytologic examination based on histological proofs. Data collection included demographic information and intact preoperative examinations. Histological subtype, grade, FIGO stage, and medical surgery records were also extracted from the database.
2.2. Treatment
The patients were elevated by our experts to receive treatments. The American Society of Anesthesiologists (ASA) score of these patients was no worse than grade II. Once the histological proof was obtained, the NACT started. These patients in our center received at least 2 cycles of NACT followed by adjuvant chemotherapy, and the total number was decided by our experts, but no <6. Patients received IV paclitaxel and carboplatin/cisplatin or IV docetaxel and cisplatin every 3 weeks. The IP regimen was modified based on GOG-172 (Gynecologic Oncology Group): IV paclitaxel (135 mg/m2) or docetaxel (75 mg/m2) on day 1, and IP cisplatin (75 mg/m2) or carboplatin area under the curve 5 to 6 on day 2.[9,21,22] However, we did not include IP paclitaxel on day 8 because most patients living in remote areas lacked immediate medical support in case of severe complications and it was inconvenient for them to return to the hospital. The possibly inefficient absorption of paclitaxel from the peritoneal cavity was also disadvantageous to its use.[23]
All patients showed stable, partial, or complete response to NACT. Regarding the reluctance and more complications for patients after radical surgery, which would reduce the number of adjuvant IP cycles, we chose the patients after LIDS.[23] LIDS usually includes total hysterectomy, bilateral salpingo-oophorectomy, peritoneal biopsies or excisions, infracolic omentectomy, appendectomy, selected pelvic lymphadenectomy, and/or para-aortic lymphadenectomy. They were followed regularly after the designed remedies, and routine examinations consisted of serum biomarkers, electrocardiogram, ultrasonography, and computed tomography, if necessary. A total of 114 people qualified for analysis in the database. All the planned treatments were completed. None were lost to follow-up.
2.3. Definition
Overall survival (OS) in this study was defined as the period from the date of diagnosis to the day of death from any cause. The disease-free interval (DFI) was calculated from the last treatment to the first recurrence or death from any cause. Response to NACT was classified according to the Gynecological Cancer Intergroup (GCIG) criteria.[24] Our measurement of residual disease was relatively strict and conservative compared with others. No gross residual (R0) disease was defined as all diseases that were cytoreduced by electronic devices. If these diseases were not resected using an en bloc approach, leaving residual disease (RD) ≤1 cm, we considered it as optimal (R1).
2.4. Statistical analysis
Logistic regression was used to explore R0-related factors. The Cox proportional hazards model was used to evaluate the prognostic factors that affected OS and DFI. OS and DFI were calculated using the Kaplan–Meier method. The log-rank test was used to investigate the variances in Kaplan–Meier curves among the different adjuvant IP chemotherapy cycles in the R0 group. P < .05 was considered significant. Statistical analysis was performed using IBM SPSS version 20 (SPSS Inc, Chicago, IL).
3. Results
3.1. Baseline characteristics
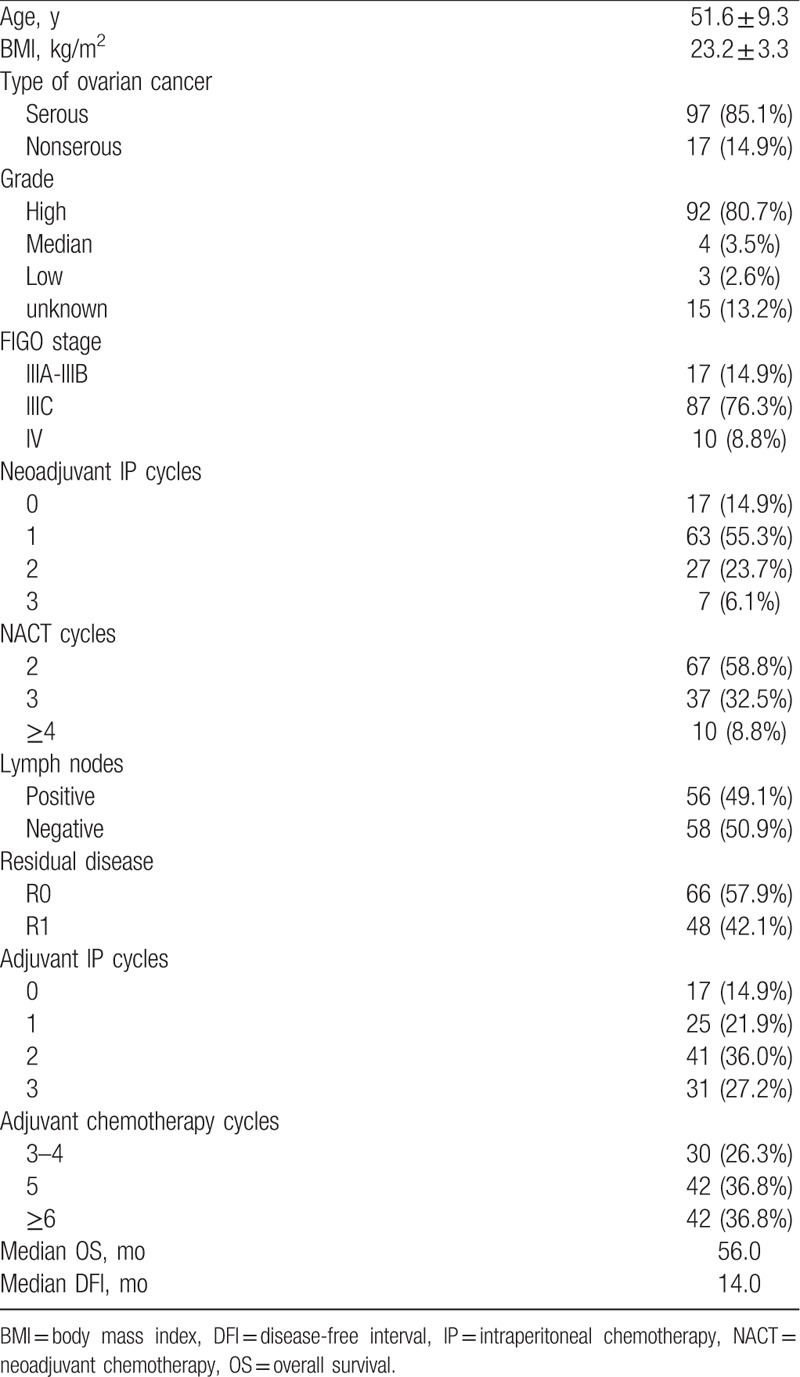
A total of 114 patients with AEOC met the inclusion criteria, with an average age of 51.6 ± 9.3 years. Most of them were serous subtype, high grade, and FIGO stage IIIC (85.1%, 80.7%, and 76.3%): nearly half had lymph node metastasis (49.1%), 17 patients did not receive neoadjuvant IP chemotherapy, 17 patients did not receive adjuvant IP chemotherapy, and 3 patients did not receive any IP chemotherapy. The median OS and DFI were 56 and 14 months, respectively. Details are shown in Table 1.
Table 1.
Baseline characteristics of the study population (n = 114).
3.2. Predictors of R0 surgery
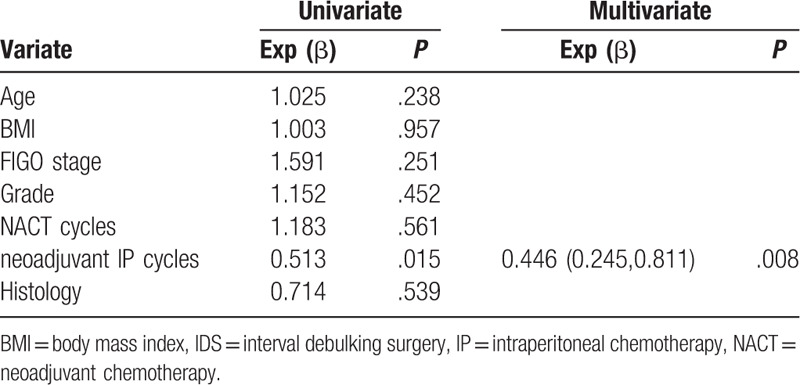
We included clinical and therapeutic factors that were reportedly related to optimal surgery in the logistics regression. Univariate and multivariate analyses showed that only neoadjuvant IP cycles were R0-related factors (hazard ratio [HR] = 0.446, 95% confidence interval [CI] = 0.245–0.811, P = .008). Details are shown in Table 2.
Table 2.
Univariate and Multivariate logistics regression of R0 IDS-related factors.
3.3. Association between IP cycles and survival results
Univariate analysis identified age and residual disease as OS-related factors (Table 3). Multivariate analysis of all study patients demonstrated that only residual disease was associated with OS (HR = 9.589, 95% CI = 3.911–23.507), as shown in Table 3. The univariate Cox proportional hazards model associated lymph node, residual disease, and BMI with DFI (Table 4), but multivariate analysis revealed that residual disease and BMI were associated with DFI (HR = 6.022, 95% CI = 3.632–9.986; HR = 1.085,95% CI = 1.012–1.163), as shown in Table 4. Multivariate Cox regression analysis within the R0 group showed that adjuvant IP cycles and BMI were related to DFI, and only adjuvant IP cycles were associated with OS (HR = 0.703, 95% CI = 0.525–0.941; HR = 1.130, 95% CI = 1.025–1.247; HR = 0.488, 95% CI = 0.289–0.824, respectively), as shown in Table 5. The Kaplan–Meier curves of OS and DFI divided by adjuvant IP chemotherapy cycles showed significant differences in the R0 group (P = .024 and P = .033, respectively) (Figs. 1 and 2).
Table 3.
Univariate and Multivariate COX regression of OS in the whole study patients.
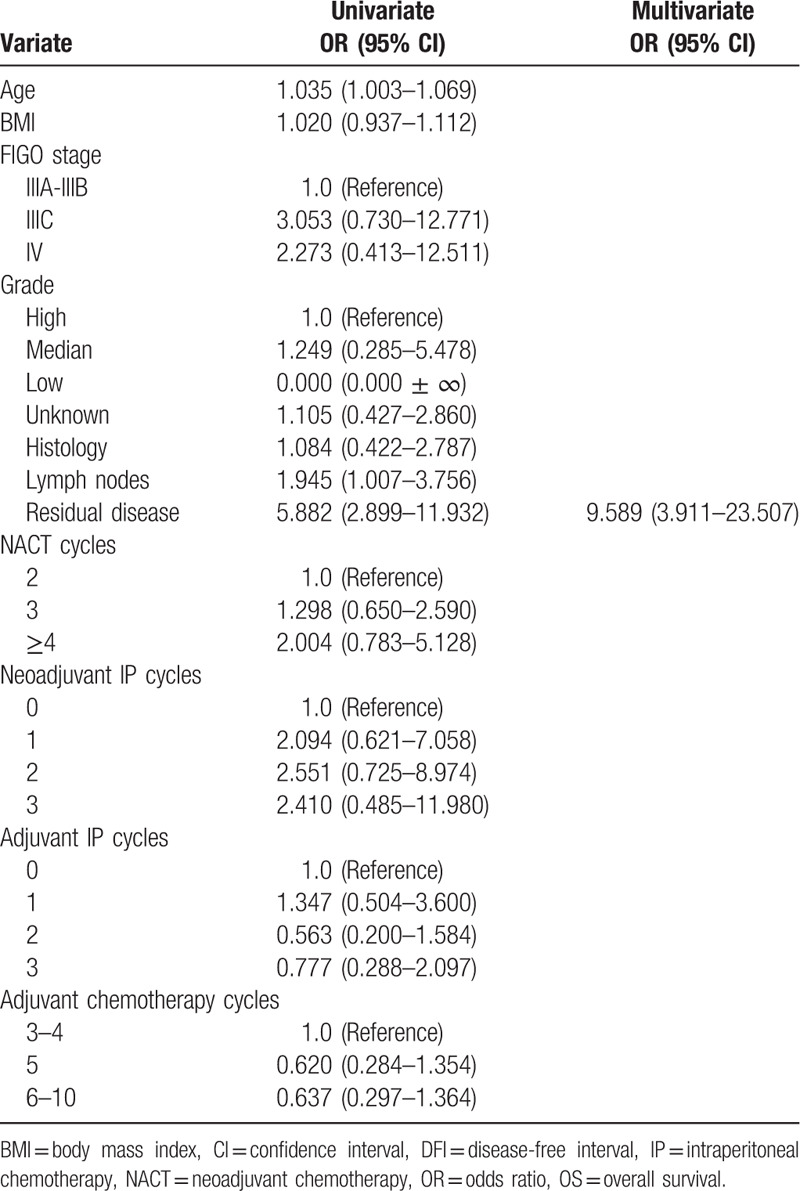
Table 4.
Univariate and Multivariate COX regression of DFI in the whole study patients.
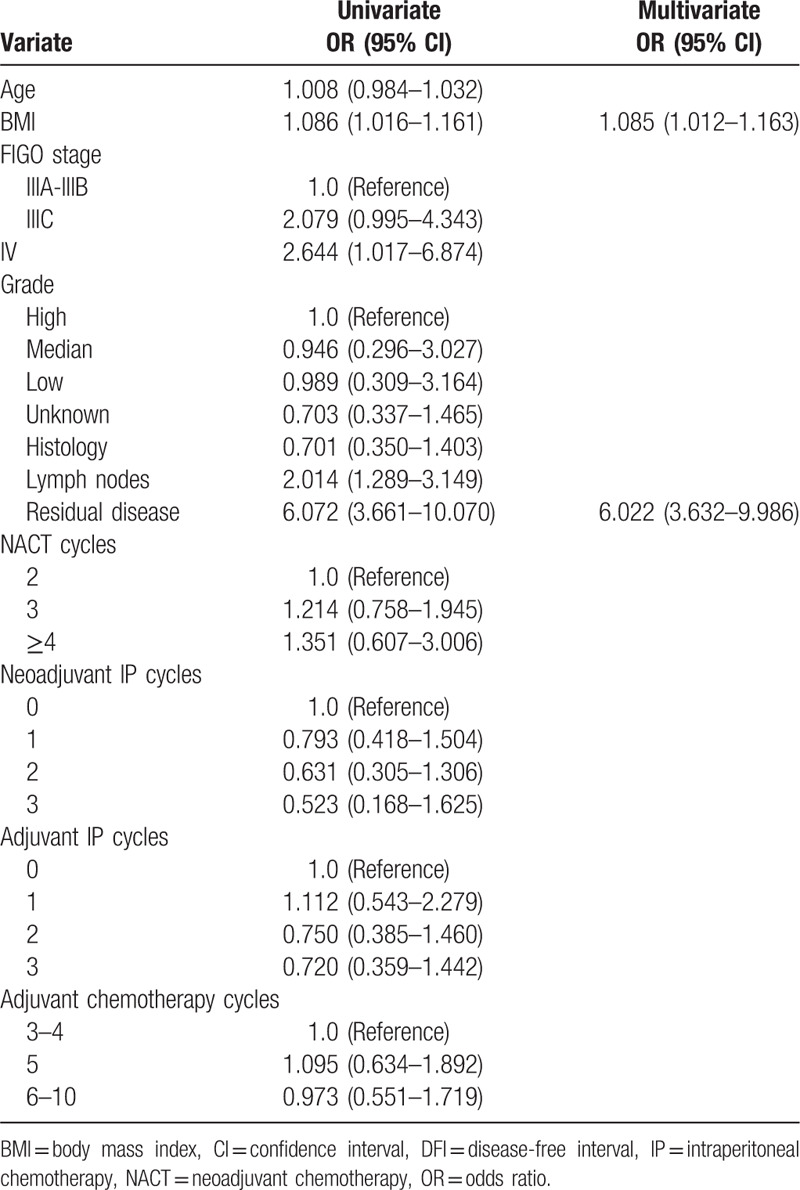
Table 5.
Multivariate analysis of OS and DFI within R0 group.
Figure 1.
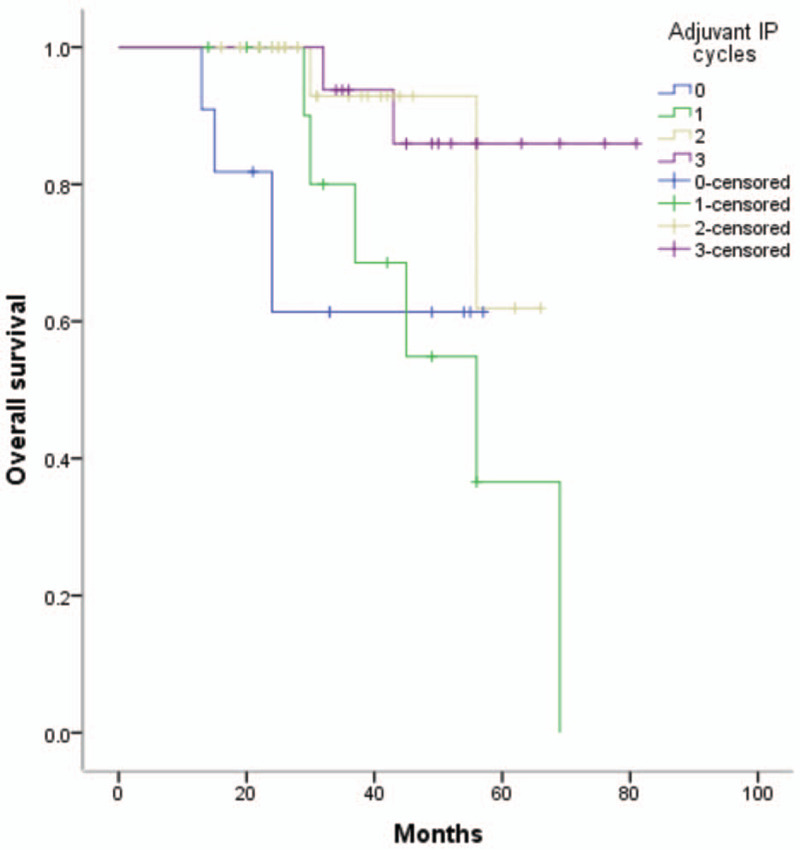
Kaplan-Meier curves of OS among 0-3 adjuvant IP cycles, P = .024. IP = intraperitoneal chemotherapy, OS = overall survival.
Figure 2.
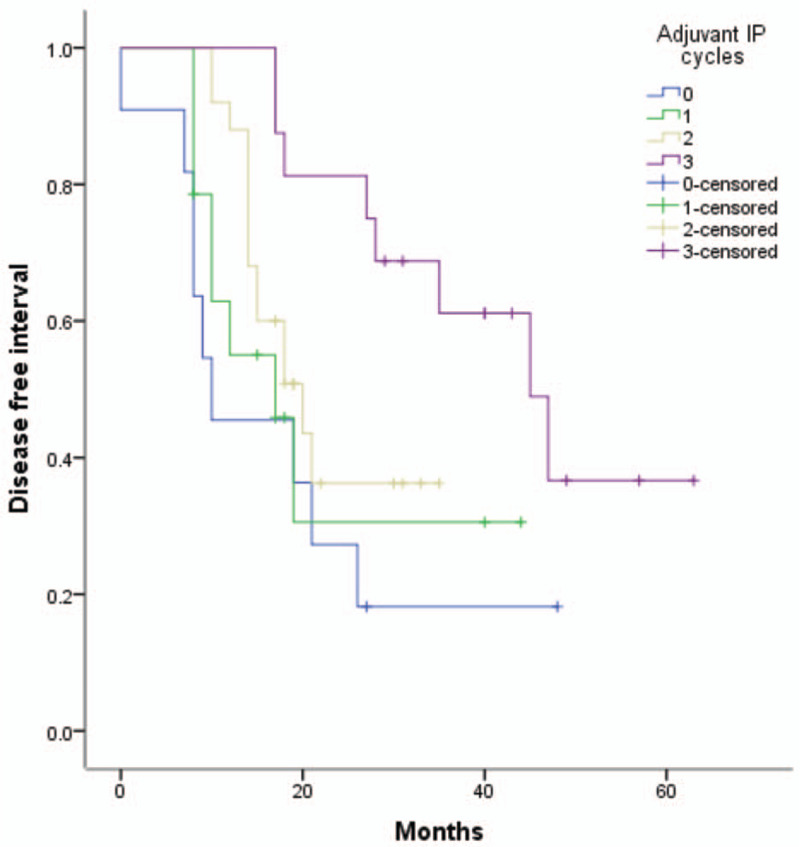
Kaplan-Meier curves of DFI among 0-3 adjuvant IP cycles, P = .033. DFI = disease-free interval, IP = intraperitoneal chemotherapy.
4. Discussion
The idea of locoregional chemotherapy for ovarian cancer patients was first introduced in 1978, and further research followed.[25] The most famous 3 prospective studies accomplished by the GOG laid solid foundations for the wide use of IP chemotherapy.[9–11] The National Cancer Institute issued an alert recommending IP chemotherapy,[26] but the underuse of IP chemotherapy was obvious due to intolerable toxicity. Although the IP chemotherapy regimen and dose in these 3 studies were different, the long-term survival results were still statistically significant. For example, GOG-104 did not use paclitaxel and included patients with residual disease ≤2 cm in the largest diameter. However, GOG-252 failed to demonstrate progression-free survival benefit because of the differences of the use of bevacizumab in all arms.[27] Sequent studies adopted various modifications of the IP chemotherapy regimen based on GOG-172 to subside toxicity, but these did not affect the survival results.[28–30]
IP chemotherapy has been approved for its obvious advantages after optimal PDS,[9–11] but the survival results have been inconsistent for patients after IDS,[14–20] and the appropriate adjuvant chemotherapy cycles for patients with advanced ovarian cancer patients are still undecided. Moreover, no consensus has been reached about how many cycles should be given definitely during NACT. Many studies have suggested that too many NACT cycles would affect the survival and increase the risk of platinum-resistant recurrence.[31–33] Moreover, few articles have reported the utility of neoadjuvant IP chemotherapy.[13,14]
Here, we analyzed the data of patients going through NACT + LIDS. Our results showed that neoadjuvant and adjuvant IP chemotherapy were supportive; they are the predictive factors that affected the surgery and survival outcomes. Our logistic analysis showed that neoadjuvant IP cycles were more important than NACT cycles related to R0. Previous studies have revealed that patients treated with NACT acquired a higher rate of optimal surgery compared with PDS, but this could not translate into better survival results,[4] NACT probably left platinum-resistant colonies of cancer stem cells,[34] which are difficult to remove absolutely.[35] IP chemotherapy in the setting of neoadjuvant chemotherapy enhanced the R0 accomplishments but failed to improve survival results in our study. However, adjuvant IP chemotherapy was identified as a meaningful factor for OS and DFI. Previous studies found that IP chemotherapy could increase several-fold drug concentration in the abdominal cavity, and the slow absorption of the drug due to the peritoneum barrier could induce remarkable penetration into the tumor cells and reduce the tumor load apparently.[12] However, this benefit of IP chemotherapy may fade as permeating into gross residual is difficult,[36,37] yet other articles have contemplated that multiple cycles of IP could enhance the penetrating effects by high concentration and long exposure.[12,38] In addition, the temperature of our solution (approximately 40–42°C) was higher than that in previous studies, which could produce a better effect than normothermic IP according to the theory of hyperthermia.[39] Possibly, the merits of neoadjuvant IP chemotherapy were weakened by the increasing number of NACT cycles and large bulky disease; therefore, this form of chemotherapy could not demonstrate its advantages on survival.
Some studies were against the positive functions of adjuvant IP chemotherapy.[15–19] Our multivariate Cox regression analysis concerning adjuvant IP cycles among the whole study population was consistent with previous results, but adjuvant IP cycles were the most important factor for OS and DFI within the R0 group. Although Kaplan–Meier curves of OS and DFI did show the significant differences and a promising tendency, our results did not stand by the suggestion that R0 patients would benefit from more adjuvant IP cycles, which needed ongoing research for verification. One retrospective study pointed out that the definition of “optimal” should be R0 rather than R1 for ovarian cancer patients after IDS[4]; our results support this idea. Those mentioned above may partially explain why adjuvants, but not neoadjuvant IP chemotherapy, behave as an essential element to improve survival results.
Our results demonstrated the importance and feasibility of neoadjuvant and adjuvant IP chemotherapy to improve the survival of patients with AEOC, but since this was a small, retrospective study with a very specific subset of women and only 1 single center experience, bias was unavoidable. Patients with decreasing tumor loads, for whom optimal surgery by laparoscopy was easier to achieve, were enrolled in the study. Second, we should pay attention to the changes in chemotherapeutic drugs between cisplatin and carboplatin; some patients firmly refused the proposal because of cisplatin-related toxicity. On the contrary, lower toxic events and equal survival results after IP carboplatin chemotherapy were reported.[22] Third, we have to modify the recommended regimens for patients and remove IP paclitaxel on day 8 because many patients who lived far away from hospitals could not obtain essential medical care to attenuate side effects. This compromise may affect the survival results. Although direct comparisons with previous studies were impossible, OS and DFI in our study were similar to previous survival results, suggesting a practicable modification.[16,19,20] Otherwise, the lack of comparison with patients who underwent neoadjuvant and adjuvant IP chemotherapy necessitated laparotomy, and the specific samples in our study meant that the conclusions could not be generalized, but still signified the potential value of neoadjuvant and adjuvant IP chemotherapy in AEOC patients.
5. Conclusions
Our results showed improvement in the survival rate of patients with AEOC who received neoadjuvant and adjuvant IP chemotherapy, especially for those without residual disease. Further studies with a larger sample are needed to validate this finding before generalization.
Acknowledgments
The authors acknowledge all the help provided by everyone.
Author contributions
All the authors contributed equally to the paper.
Formal analysis: Wenting Wang.
Funding acquisition: Wenting Wang.
Investigation: Wenting Wang.
Footnotes
Abbreviations: AEOC = advanced epithelial ovarian cancer, ASA = American Society of Anesthesiologists, AUC = area under the curve, DFI = disease-free interval, GCIG = Gynecological Cancer Intergroup, GOG = Gynecologic Oncology Group, IDS = interval debulking surgery, IP = intraperitoneal chemotherapy, LIDS = laparoscopic conservative interval debulking surgery, NACT = neoadjuvant chemotherapy, OS = overall survival, RD = residual disease.
How to cite this article: Liu Y, Cao L, Chen W, Wang J, Wang W, Liang Z. Feasibility of neoadjuvant and adjuvant intraperitoneal chemotherapy in patients with advanced epithelial ovarian cancer: A single-center experience. Medicine. 2020;99:36(e22100).
All authors contributed equally to this article.
Funding: This study was supported by the Chongqing Science and Technology Commission (No. cstc2016shms-ztzx10001).
The authors report no conflicts of interest.
Data availability statement: Data are available from the corresponding authors upon reasonable request.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
References
- [1].Torre LA, Trabert B, DeSantis CE, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin 2018;68:284–96.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7–30.. [DOI] [PubMed] [Google Scholar]
- [3].Bristow RE, Tomacruz RS, Armstrong DK, et al. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol 2002;20:1248–59.. [DOI] [PubMed] [Google Scholar]
- [4].Bian C, Yao K, Li L, et al. Primary debulking surgery vs. neoadjuvant chemotherapy followed by interval debulking surgery for patients with advanced ovarian cancer. Arch Gynecol Obstet 2016;293:163–8.. [DOI] [PubMed] [Google Scholar]
- [5].Vergote I, Trope CG, Amant F, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med 2010;363:943–53.. [DOI] [PubMed] [Google Scholar]
- [6].Kehoe S, Hook J, Nankivell M, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet 2015;386:249–57.. [DOI] [PubMed] [Google Scholar]
- [7].Fagotti A, Ferrandina G, Vizzielli G, et al. Phase III randomised clinical trial comparing primary surgery versus neoadjuvant chemotherapy in advanced epithelial ovarian cancer with high tumour load (SCORPION trial): Final analysis of peri-operative outcome. Eur J Cancer 2016;59:22–33.. [DOI] [PubMed] [Google Scholar]
- [8].Onda T, Satoh T, Saito T, et al. Comparison of treatment invasiveness between upfront debulking surgery versus interval debulking surgery following neoadjuvant chemotherapy for stage III/IV ovarian, tubal, and peritoneal cancers in a phase III randomised trial: Japan Clinical Oncology Group Study JCOG0602. Eur J Cancer 2016;64:22–31.. [DOI] [PubMed] [Google Scholar]
- [9].Armstrong DK, Bundy B, Wenzel L, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med 2006;354:34–43.. [DOI] [PubMed] [Google Scholar]
- [10].Alberts DS, Liu PY, Hannigan EV, et al. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med 1996;335:1950–5.. [DOI] [PubMed] [Google Scholar]
- [11].Markman M, Bundy BN, Alberts DS, et al. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J Clin Oncol 2001;19:1001–7.. [DOI] [PubMed] [Google Scholar]
- [12].Tewari D, Java JJ, Salani R, et al. Long-term survival advantage and prognostic factors associated with intraperitoneal chemotherapy treatment in advanced ovarian cancer: a gynecologic oncology group study. J Clin Oncol 2015;33:1460–6.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gao T, Huang XX, Wang WY, et al. Feasibility and safety of neoadjuvant laparoscopic hyperthermic intraperitoneal chemotherapy in patients with advanced stage ovarian cancer: a single-center experience. Cancer Manag Res 2019;11:6931–40.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Shi TY, Jiang R, Yu JJ, et al. Addition of intraperitoneal cisplatin and etoposide to first-line chemotherapy for advanced ovarian cancer: a randomised, phase 2 trial. Brit J Cancer 2018;119:12–8.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Provencher DM, Gallagher CJ, Parulekar WR, et al. OV21/PETROC: a randomized Gynecologic Cancer Intergroup phase II study of intraperitoneal versus intravenous chemotherapy following neoadjuvant chemotherapy and optimal debulking surgery in epithelial ovarian cancer. Ann Oncol 2018;29:431–8.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lee J, Curtin JP, Muggia FM, et al. Timing is everything: intraperitoneal chemotherapy after primary or interval debulking surgery for advanced ovarian cancer. Cancer Chemother Pharmacol 2018;82:55–63.. [DOI] [PubMed] [Google Scholar]
- [17].Al Mutairi NJ, Le T. Does modality of adjuvant chemotherapy after interval surgical debulking matter in epithelial ovarian cancer?: An exploratory analysis. Int J Gynecol Cancer 2014;24:461–7.. [DOI] [PubMed] [Google Scholar]
- [18].Le T, Latifah H, Jolicoeur L, et al. Does intraperitoneal chemotherapy benefit optimally debulked epithelial ovarian cancer patients after neoadjuvant chemotherapy? Gynecol Oncol 2011;121:451–4.. [DOI] [PubMed] [Google Scholar]
- [19].Mueller JJ, Kelly A, Zhou Q, et al. Intraperitoneal chemotherapy after interval debulking surgery for advanced-stage ovarian cancer: feasibility and outcomes at a comprehensive cancer center. Gynecol Oncol 2016;143:496–503.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bixel K, Vetter M, Davidson B, et al. Intraperitoneal chemotherapy following neoadjuvant chemotherapy and optimal interval tumor reductive surgery for advanced ovarian cancer. Gynecol Oncol 2020;156:530–4.. [DOI] [PubMed] [Google Scholar]
- [21].Becker DA, Leath CA, 3rd, Walters-Haygood CL, et al. Utilization of an alternative docetaxel-based intraperitoneal chemotherapy regimen in patients with ovarian, fallopian tube or primary peritoneal carcinoma: a continued need for ovarian cancer patients. Am J Clin Oncol 2019;42:12–6.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Eoh KJ, Lee JY, Nam EJ, et al. Long-term survival analysis of intraperitoneal versus intravenous chemotherapy for primary ovarian cancer and comparison between carboplatin- and cisplatin-based intraperitoneal chemotherapy. J Korean Med Sci 2017;32:2021–8.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Jaaback K, Johnson N, Lawrie TA. Intraperitoneal chemotherapy for the initial management of primary epithelial ovarian cancer. Cochrane Database Syst Rev 2016;CD005340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rustin GJ, Vergote I, Eisenhauer E, et al. Definitions for response and progression in ovarian cancer clinical trials incorporating RECIST 1.1 and CA 125 agreed by the Gynecological Cancer Intergroup (GCIG). Int J Gynecol Cancer 2011;21:419–23.. [DOI] [PubMed] [Google Scholar]
- [25].Dedrick RL. Theoretical and experimental bases of intraperitoneal chemotherapy. Semin Oncol 1985;12: 3 suppl 4: 1–6.. [PubMed] [Google Scholar]
- [26].Trimble EL, Christian MC. Intraperitoneal chemotherapy for women with advanced epithelial ovarian carcinoma. Gynecol Oncol 2006;100:3–4.. [DOI] [PubMed] [Google Scholar]
- [27].Walker JL, Brady MF, Wenzel L, et al. Randomized trial of intravenous versus intraperitoneal chemotherapy plus bevacizumab in advanced ovarian carcinoma: an NRG Oncology/Gynecologic Oncology Group Study. J Clin Oncol 2019;37:1380–90.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Bruixola G, Domingo S, Diaz R, et al. Feasibility and safety of a modified outpatient regimen with intravenous/intraperitoneal chemotherapy for optimally debulked stage III ovarian cancer. Int J Gynecol Cancer 2015;25:214–21.. [DOI] [PubMed] [Google Scholar]
- [29].Oaknin A, Roda D, Gonzalez-Martin A, et al. Feasibility of a modified outpatient regimen of intravenous/intraperitoneal chemotherapy in optimally debulked stage III ovarian cancer patients: a GEICO study. Int J Gynecol Cancer 2011;21:1048–55.. [DOI] [PubMed] [Google Scholar]
- [30].Blinman P, Gainford C, Donoghoe M, et al. Feasibility, acceptability and preferences for intraperitoneal chemotherapy with paclitaxel and cisplatin after optimal debulking surgery for ovarian and related cancers: an ANZGOG study. J Gynecol Oncol 2013;24:359–66.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Xu X, Deng F, Lv M, et al. The number of cycles of neoadjuvant chemotherapy is associated with prognosis of stage IIIc-IV high-grade serous ovarian cancer. Arch Gynecol Obstet 2017;295:451–8.. [DOI] [PubMed] [Google Scholar]
- [32].da Costa AA, Valadares CV, Baiocchi G, et al. Neoadjuvant chemotherapy followed by interval debulking surgery and the risk of platinum resistance in epithelial ovarian cancer. Ann Surg Oncol 2015;22: suppl 3: S971–8.. [DOI] [PubMed] [Google Scholar]
- [33].Rauh-Hain JA, Nitschmann CC, Worley MJ, Jr, et al. Platinum resistance after neoadjuvant chemotherapy compared to primary surgery in patients with advanced epithelial ovarian carcinoma. Gynecol Oncol 2013;129:63–8.. [DOI] [PubMed] [Google Scholar]
- [34].Lim MC, Song YJ, Seo SS, et al. Residual cancer stem cells after interval cytoreductive surgery following neoadjuvant chemotherapy could result in poor treatment outcomes for ovarian cancer. Onkologie 2010;33:324–30.. [DOI] [PubMed] [Google Scholar]
- [35].Ren YL, Shi TY, Jiang R, et al. Multiple cycles of neoadjuvant chemotherapy associated with poor survival in bulky stage IIIC and IV ovarian cancer. Int J Gynecol Cancer 2015;25:1398–404.. [DOI] [PubMed] [Google Scholar]
- [36].Los G, Mutsaers PH, van der Vijgh WJ, et al. Direct diffusion of cis-diamminedichloroplatinum(II) in intraperitoneal rat tumors after intraperitoneal chemotherapy: a comparison with systemic chemotherapy. Cancer Res 1989;49:3380–4.. [PubMed] [Google Scholar]
- [37].Los G, Mutsaers PH, Lenglet WJ, et al. Platinum distribution in intraperitoneal tumors after intraperitoneal cisplatin treatment. Cancer Chemother Pharmacol 1990;25:389–94.. [DOI] [PubMed] [Google Scholar]
- [38].Fujiwara K, Nagao S, Kigawa J, et al. Comparative phase II study of intraperitoneal (IP) versus intravenous (IV) carboplatin administration with IV paclitaxel in patients with bulky residual disease after primary debulking surgery for epithelial ovarian or primary peritoneal cancer: A Sankai Gynecology Study Group (SGSG) study. J Clin Oncol 2007;25(18.): [Google Scholar]
- [39].van Driel WJ, Koole SN, Sikorska K, et al. Hyperthermic intraperitoneal chemotherapy in ovarian cancer. N Engl J Med 2018;378:230–40.. [DOI] [PubMed] [Google Scholar]


