Abstract
Antiplatelet agents have been administered to patients with acute ischemic stroke after endovascular therapy. This study was designed to provide initial data to compare thromboelastography (TEG) with the conventional coagulation test (CCT) to analyze the coagulation function of antiplatelet drugs in such patients.
The present retrospective cohort study included 240 patients who received endovascular therapy from September 2012 to December 2017. The baseline and clinical characteristics of these patients were collected with respect to TEG (parameters: R, K, maximal amplitude (MA), and α angle) and CCT (parameters: PT, activated partial thromboplastin time (APTT), fibrinogen (FIB), international normalized ratio (INR), and platelet count (PLT)) on day 5 after aspirin and clopidogrel post-endovascular interventions. The correlation and agreement of these 2 detecting methods were analyzed. Additionally, the area under the receiver operating characteristic curve (AUROC) was used to analyze the effectiveness of these 2 methods in detecting unfavorable clinical outcomes, including symptomatic intracranial hemorrhage and early neurological deterioration.
The 3 pairs of parameters (R and APTT, K and APTT, and α angle and FIB) were in agreement for identifying hypercoagulability, while R and APTT, K and APTT, K and PLT, and α angle and PLT were in agreement for identifying hypocoagulability. The AUROC of parameter R for detecting symptomatic intracranial hemorrhage was 0.817, while that of parameter FIB for predicting early neurological deterioration was 0.887.
Parameter FIB derived from CCT might be advantageous for evaluating early neurological deterioration, while parameter R detected by TEG might be superior for evaluating symptomatic intracranial hemorrhage.
Keywords: acute ischemic stroke, antiplatelet, conventional coagulation test, endovascular treatment, thromboelastography
1. Introduction
Dual antiplatelet therapy with aspirin and clopidogrel is recommended for the clinical management of patients with acute ischemic stroke after endovascular treatment.[1] However, there are differences among individuals in terms of platelet reactivity. Patients with low drug reactivity can experience ischemic events due to their hypercoagulable state. In contrast, those with high drug reactivity may experience bleeding events. Therefore, monitoring coagulation function aids in the decision for individual treatment in patients at high risk of complications, for example in those with stent thrombosis history, suspected drug resistance, and high risk of bleeding.
The conventional coagulation test (CCT) is a common technique for monitoring blood coagulation during clinical practice. However, this test cannot confirm the active phase of the coagulation system and the tendency to form thrombi after surgery.[2,3] Moreover, the traditional diagnostic methods are weak in dynamically assessing the whole-blood strength. Thromboelastography (TEG) assesses the key information from the initiation of the blood clot to the fibrinolysis phase throughout the coagulation pathway.[3,4] Currently, few studies have compared TEG and CCT in patients after acute ischemic stroke who received endovascular treatment. Therefore, the present study was designed to analyze the correlation and agreement between these 2 methods by monitoring the coagulation function and the factors that predict unfavorable clinical outcomes after therapy with dual antiplatelet drugs to guide clinical management of such patients.
2. Patients and methods
2.1. Patients and study design
The present study was approved by the Ethics Committee on Scientific Research of the Seventh Medical Center of P.L.A. Army General Hospital, and informed consent was obtained from all patients. A total of 240 patients with acute ischemic stroke who received endovascular treatment in our Neurosurgical Department were enrolled between September 2012 and December 2017. The inclusion criteria for endovascular treatment were as previously described[5]:
-
i)
Age between 18 and 80 years.
-
ii)
Acute ischemic stroke diagnosed using brain computed tomography (CT), diffuse weighted magnetic resonance imaging (MRI), and/or MR angiography (MRA).
-
iii)
Unimproved clinical symptoms (National Institutes of Health Stroke Scale (NIHSS) score improvement <4) within 1 hour after strict intravenous thrombolysis.
-
iv)
Time window since stroke onset beyond 4.5 hours but less than 8 hours for the anterior circulation or less than 24 hours for the posterior circulation or contraindication of intravenous thrombolysis within the 4.5 hours time window for intravenous thrombolysis.
-
v)
Balloon stents for treatment and antiplatelet agents using arterial thrombectomy.
2.2. Etiology of ischemic stroke
The Trial of ORG 10172 in Acute Stroke Treatment (TOAST) classification was used to classify the etiology of ischemic stroke into different subtypes.[6,7] According to this classification, large artery atherosclerosis (LAA) in the clinical and brain imaging findings of either severe stenosis (≥50%) or occlusion of a major brain artery or branch cortical artery. This occurrence might be attributed to atherosclerosis without potential sources of cardiogenic embolism. Cardioembolism needs to fit the evidence of cardiac disease that has potential for embolism; however, there was no evidence of 50% stenosis in intracranial or extracranial large arteries. Stroke of other determined cause (SOD) included vasospasm associated with subarachnoid hemorrhage, a broad array of hereditary arteriopathies with diverse pathophysiologies (such as moyamoya disease and fibromuscular dysplasia), primary or parainfectious cerebral vasculitis, and coagulopathies (including those associated with malignancy, genetic disorders, and medical therapy). Stroke of undetermined cause (SUD) included multiple causes, no identified cause, or incomplete investigation.
2.3. Evaluation and treatment method
Neurological function was assessed using the NIHSS score after admission and therapy on days 1 and 5. Endovascular treatments, including intra-arterial thrombolysis (IAT), mechanical clot disruption using the clot retrieval device (Solitaire FR, Penumbra system), angioplasty with balloon angioplasty (Aviator, Gateway balloon), stent implantation (Acculink, Precise, Wingspan, Enterprise), and/or the combination of these approaches. The choice of endovascular treatment was decided by the senior physicians in our hospital.
2.4. Data acquisition
Data were acquired during the hospitalization of the patients. Patients were orally administered 100 mg of aspirin and 75 mg of clopidogrel daily since 24 hours post-treatment. Peripheral venous blood was collected by venipuncture on day 5. However, the initial 2 to 4 mL of blood was discarded to avoid spontaneous platelet interference. Follow-up data at 3 months post-treatment were additionally collected but were not analyzed for the purposes of the present study.
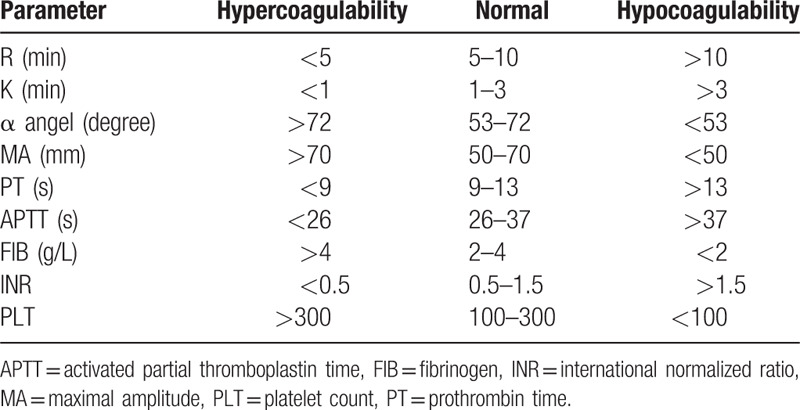
TEG parameters, including R (min), K (min), α angle (°), and maximal amplitude (MA, mm) were immediately detected by a computerized TEG coagulation analyzer (Hemonetics Co., Niles, IL). CCT indicators, including prothrombin time (PT, s), activated partial thromboplastin time (APTT, s), fibrinogen (FIB, g/L), international normalized ratio (INR), and platelets count (PLT) were identified using an automatic coagulation analyzer (Instrumentation Laboratory, Bedford, MA). All the parameters were recorded within 2 hours and divided into 3 types, that is, hypercoagulability, normal, and hypocoagulability based on the reference value provided by the manufacturer (Table 1).
Table 1.
Reference values of each parameter.
2.5. Classification of outcomes
The unfavorable clinical outcomes, classified as symptomatic intracranial hemorrhage (SIH), according to ECASS II,[8] and early neurological deterioration (END), were referred to as follows:
-
(1)
increased NIHSS score ≥4 points on day 5 compared to that on day 1 post-treatment except in cases of patients,
-
(2)
who died, or
- (3)
The outcome was assessed by 2 authors, Z.J. He and S. Ma, who were blinded to the treatment the patients received.
2.6. Statistical analysis
Continuous variables are expressed as mean ± standard deviation 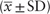 , while categorical variables are expressed as number (percentage). Nonparametric Spearman's rank correlation (r) was used to evaluate the correlations between TEG and CCT parameters, and the agreement between these 2 monitoring methods was decided by the kappa value. In addition, receiver operating characteristics (ROC) curves were analyzed and the area under the ROC curve (AUROC) was used to compare the effectiveness of TEG and CCT for detecting unfavorable outcomes (SIH and END). A 2-sided P value < .05 was considered to be significant. Statistical analysis was performed using the SPSS Statistics 20 (IBM Company, Chicago).
, while categorical variables are expressed as number (percentage). Nonparametric Spearman's rank correlation (r) was used to evaluate the correlations between TEG and CCT parameters, and the agreement between these 2 monitoring methods was decided by the kappa value. In addition, receiver operating characteristics (ROC) curves were analyzed and the area under the ROC curve (AUROC) was used to compare the effectiveness of TEG and CCT for detecting unfavorable outcomes (SIH and END). A 2-sided P value < .05 was considered to be significant. Statistical analysis was performed using the SPSS Statistics 20 (IBM Company, Chicago).
3. Results
3.1. Patient characteristics
The average age of the 240 patients enrolled in the study was 63.01 ± 12.82 years. One hundred seventy-one (71.25%) patients were male. According to the TOAST classification, 89 (37.08%) had LAA, 77 (32.08%) had cardioembolism (CE), 40 (16.67%) had SOD, and 34 (14.17%) had SUD. IAT was applied in 106 (44.17%), mechanical clot disruption was used in 117 (48.75%), and angioplasty was conducted in 115 (47.92%) patients. The platelet receptor inhibition in response to arachidonic acid (AA) and adenosine diphosphate (ADP) was 74.92 ± 34.69% and 50.29 ± 25.33%, respectively. Table 2 shows the results of clinical and laboratory characteristics.
Table 2.
Demographic and clinical characteristics of the enrolled patients (n = 240).
3.2. Correlations between TEG and CCT parameters
The correlations between the TEG and CCT parameters are demonstrated in Table 3 and Fig. 1a–g. Positive correlations were found between R and APTT (r = 0.330, P < .001), K and APTT (r = 0.228, P = .001), alpha angle and FIB (r = 0.292, P < .001), and PLT (r = 0.384, P < .001). Negative correlations were detected between K and FIB (r = −0.281, P < .001), PLT (r = −0.376, P < .001), and alpha angle and APTT (r = 0.231, P = .001).
Table 3.
Correlations between TEG and CCT parameters.
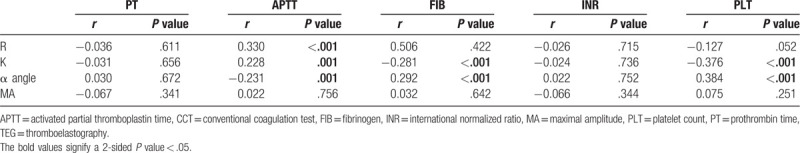
Figure 1.
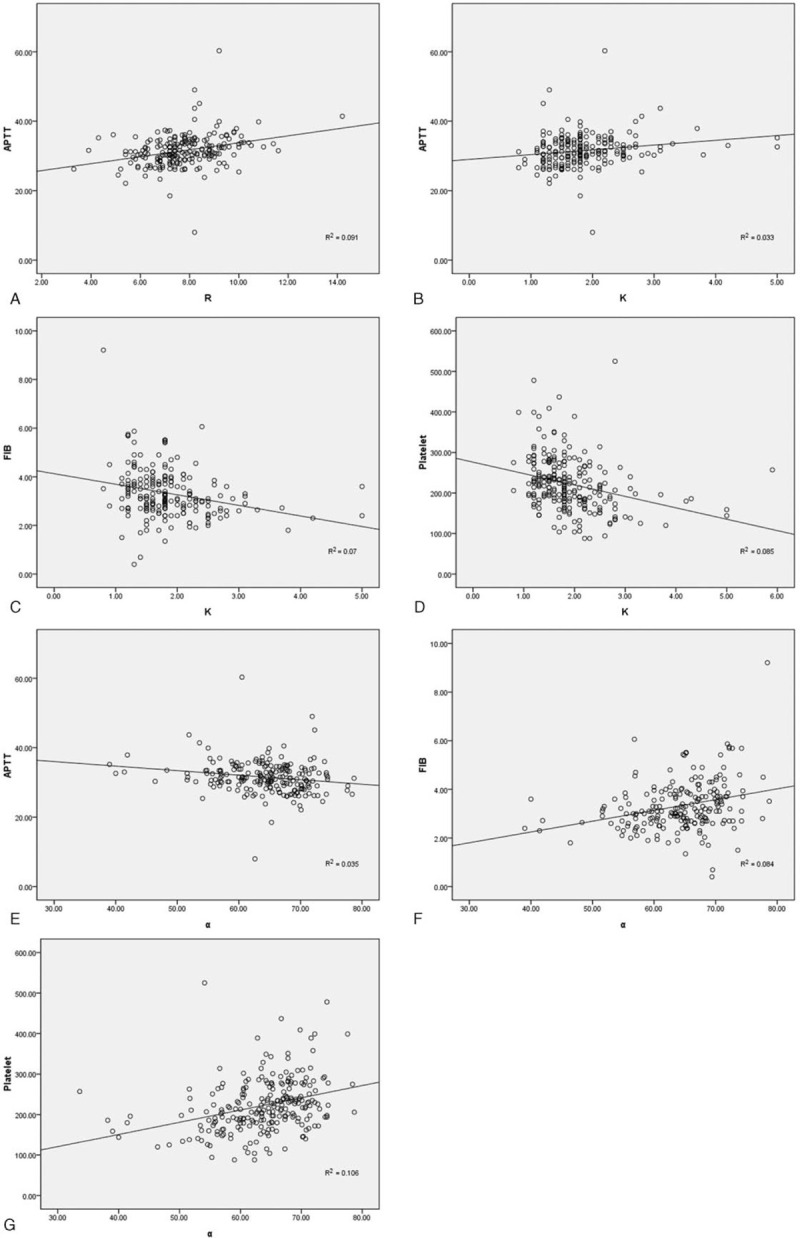
Correlations between TEG and CCT parameters. (a) R and APTT, (b) K and APTT, (c) K and FIB, (d) K and PLT, (e) alpha angle and APTT, (f) alpha angle and FIB, and (g) alpha angle and PLT. APTT = activated partial thromboplastin time, CCT = conventional coagulation test, FIB = fibrinogen, PLT = platelets, TEG = thromboelastography.
3.3. Agreement between TEG and CCT parameters
A weak agreement was detected between TEG and CCT among parameters R and APTT (κ = 0.266, P < .001), K and APTT (κ = 0.134, P = .024), and alpha angle and FIB (κ = 0.162, P = .007) in identifying hypercoagulability. Moreover, a weak agreement was established among parameters R and APTT (κ = 0.211, P = .001), K and APTT (κ = 0.198, P = .002), K and PLT (κ = 0.388, P < .001), and alpha angle and PLT (κ = 0.099, P = .041) in identifying hypocoagulability (Tables 4 and 5).
Table 4.
Agreement between TEG and CCT in identifying hypercoagulability and hypocoagulability.
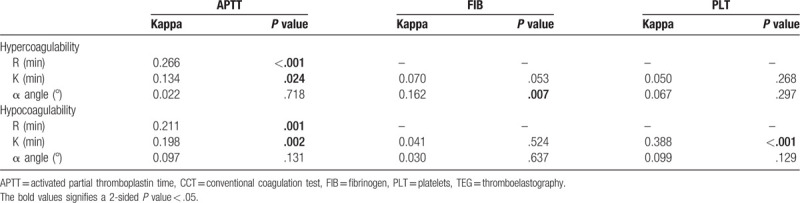
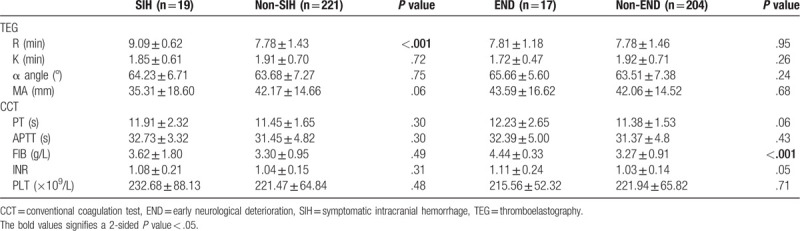
Table 5.
Parameters of TEG and CCT for indicating SIH and END.
3.4. Prediction of unfavorable outcomes
SIH and END were found in 19 (7.92%) and 17 (7.69%) patients, respectively. The AUROC was 0.817 (95% confidence interval (CI): 0.746–0.887) for parameter R to indicate SIH, while it was 0.887 (95% CI: 0.840–0.935) for FIB to predict END (Fig. 2a–d).
Figure 2.
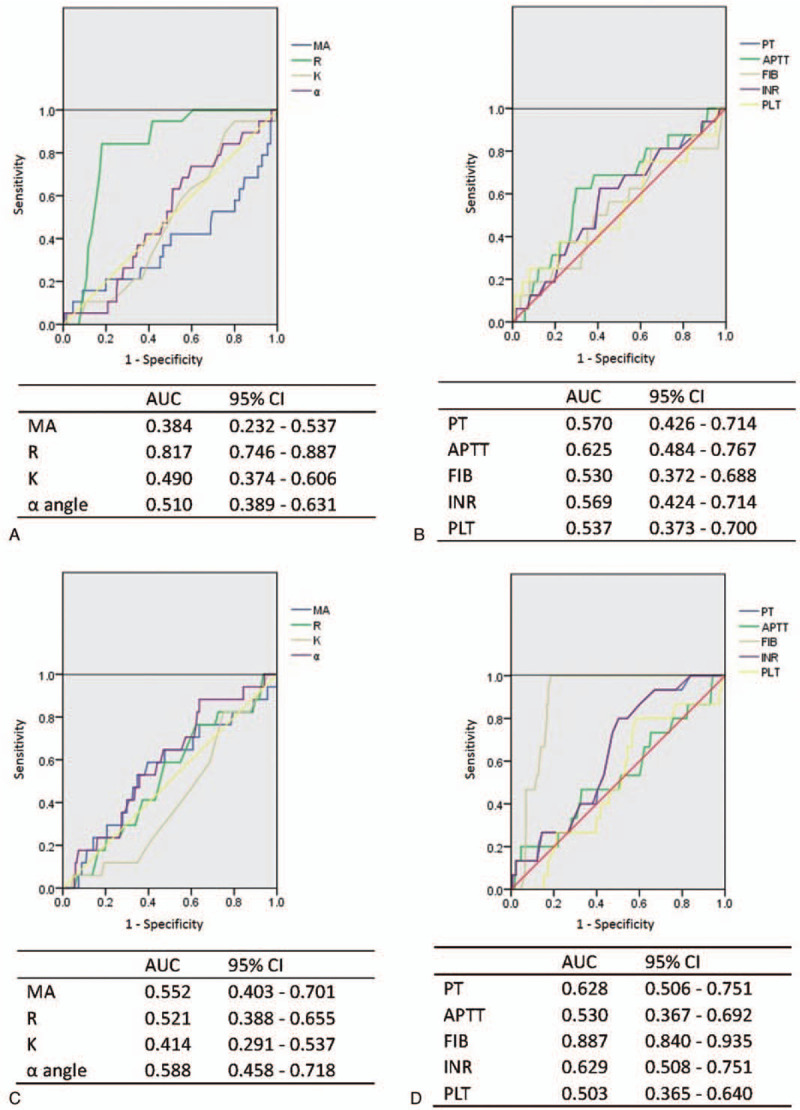
Receiver operating characteristic (ROC) curves of TEG and CCT parameters for detecting unfavorable outcomes (SIH and END). (a) ROC curves of TEG to detect SIH. (b) ROC curves of CCT to detect SIH. (c) ROC curves of TEG to detect END. (d) ROC curves of CCT to detect END. The area under the curve (AUROC) and 95% confidence interval (CI) are listed below each ROC curve. APTT = activated partial thromboplastin time, CCT = conventional coagulation test, FIB = fibrinogen, INR = international normalized ratio, MA = maximal amplitude, PLT = platelets, PT = prothrombin time, TEG = thromboelastography.
4. Discussion
The current study compared the effectiveness of TEG and CCT in analyzing the coagulation function of antiplatelet drugs in patients with acute ischemic stroke who underwent endovascular treatment. We found that parameter FIB derived from CCT might be advantageous in evaluating END, while parameter R detected by TEG might be superior in evaluating SIH. TEG has been in use since 1948, however, only recently its practical application has been feasible after the advent of user-friendly computerized analysis.[11] It has the advantage of offering a comprehensive view of the entire coagulation process, including the dynamic balance of coagulation and fibrinolysis, as well as the dynamic clotting process from the coagulation-triggered cascade reaction to clotting.[12,13] Hitherto, this approach has been applied to the diagnosis of coagulopathy, hemorrhage monitoring, and evaluation of hemostasis.[12,13] In addition, its application has been extended to the tumor field to predict postoperative thrombosis complications. Interestingly, the results of hypercoagulability assessment were inconsistent in a prospective case–control study.[14,15]
Age is usually considered as a significant role for the coagulation test results. In the existing publications, variation of the age-dependent coagulation parameters has been reported in infancy, adolescence and younger adults, mainly during the first year of life.[16–18] However, no study has been reported regarding older individuals. One study suggested that the TEG parameters in participants aged over 50 years were more hypercoagulable than their younger counterparts (less than 50 years) in normal population. Nevertheless, no further stratification for age over 50 years was conducted without any explanation.[18] In another study, different subgroups were divided based on age, but the individuals over 60 years were in 1 group without further exploration.[19] All the patients in the study were between 50 and 70 years old. Since this is a small age group, although the coagulation parameters fluctuated with increase in every 10 years, no statistical significance of the fluctuation was shown. Therefore, we did not stratify by age in the current study, which might be the reason why the 2 studies mentioned above also did not conduct further stratification by age in individuals over 50 and 60 years old.
4.1. Correlations between TEG and CCT
Parameter R refers to the coagulation reaction time, namely thromboplastin generation time, mainly reflecting the function of coagulation factors. K is the agglutination time of the blood cells and is associated with the function of thrombin. Moreover, APTT reflects the level of coagulation factors XII, XI, IX, and VIII. In the current study, the weak correlation among these factors may be interpreted as TEG analyzing the whole-blood coagulation function, while CCT detecting a subset of the coagulation factors in separated plasma. Furthermore, the alpha angle was found to be positively correlated with FIB and PLT. The value of the alpha angle represented a high blood-clot formation rate and a rapid fibrous-protein formation rate. The positive correlations among the alpha angle, FIB, and PLT revealed an interaction between fibrinogen and platelets at the beginning of blood-clot formation. In a prospective study of 100 pre-eclampsia/eclampsia cases, Ahmad and Kohli reported that PT, APTT, and thrombin time were directly proportional to R and K and inversely proportional to the alpha angle.[20] In addition, a negative correlation was established among K and FIB, PLT, alpha angle, and APTT. In a study of 40 consecutive patients with fractures, Liu et al[13] reported positive correlations between FIB and MA and alpha angle, as well as between PLT and MA and APTT and further confirmed the negative correlation between K and FIB.
4.2. Hypercoagulability prediction
Elliott et al[11] investigated hypercoagulability in patients with acute ischemic stroke that was detected based on TEG monitoring; short R, high alpha angle, and short K indicated rapid clotting. In the current study, we used the reference value (Table 1) to define the hypercoagulability and non-hypercoagulability of each parameter. Further analysis indicated a weak agreement between TEG and CCT with respect to parameters R and APTT, K and APTT, and alpha angle and FIB in identifying hypercoagulability. Similarly, CCT detects the cascade of the coagulation reaction in the plasma, while TEG measures the various components of hemostasis as they interact with one another in vivo, which includes the characteristics of not only thrombin and coagulation but also of the blood clots.[21]
Further exploration showed that after excluding the SIH cases, the AUROC of FIB was 0.887 for END. Wang et al[3] reported a study of 80 patients with prostate cancer, wherein TEG and CCT were applied 24 hours before surgery. The results showed that TEG was superior in evaluating hypercoagulation. In addition, the maximum amplitude of adenosine diphosphate-induced platelet-fibrin clots (MAADP), one of the TEG parameters, was designated as an indicator of ischemic events or an independent factor predicting the risk of infarction recurrences in other studies.[22–24]
4.3. Hypocoagulability prediction
A previous study suggested that compared to no medication, dual antiplatelet therapy was associated with prolonged mean R, decreased MA, and alpha angle, which is a decrease in both the rate and strength of clot formation along with high risk of bleeding.[25] The current results demonstrated that in identifying hypocoagulability, a weak correlation was detected between the TEG and CCT parameters (R and APTT, K and APTT, K and PLT, and alpha angle and PLT). Ahmad et al[20] reported that there was agreement between the TEG and CCT parameters in detecting hypocoagulation.
Furthermore, after analyzing the data of 19 patients with SIH, the AUROC of R (detected by TEG) was 0.817, designating it as a reliable parameter to predict SIH. Previously, Wang et al[3] reported that the sensitivity and specificity of TEG for predicting bleeding were higher than those of CCT. Li et al[26] also showed that MAADP measured by TEG is valuable for the prediction of major bleeding in patients with acute coronary syndrome treated with in ticagrelor. In addition, other studies have demonstrated that TEG could effectively monitor hypocoagulability and predict bleeding tendency.[27,28]
4.4. Limitations
One limitation of the present study is the single-center and retrospective analysis, which might not adequately represent the majority of the population and control for potential bias. Additionally, the components, size, and position of the coagulation, for example the percentage of PLT, might be associated with the results of thrombolysis therapy. Herein, we only detected the main factors in one stage of this disease. Thus, a prospective trial, including comprehensive analysis, that is, of the composition and subtype of clotting, as well as varying durations, is essential to obtain accurate results.
5. Conclusions
The parameters derived from TEG and CCT are correlated and consistent in identifying hypercoagulability and hypocoagulability. Furthermore, CCT, especially parameter FIB, might be superior in evaluating END, while parameter R, detected by TEG, might be advantageous in evaluating SIH in patients with acute ischemic stroke.
Author contributions
Conceptualization: Chunyang Liang, Yang Yang.
Data curation: Chunyang Liang, Zijun He, Shang Ma, Xuenan Qu.
Formal analysis: Yang Yang, Xuenan Qu.
Funding acquisition: Chunyang Liang, Ruxiang Xu.
Investigation: Zijun He, Shang Ma.
Methodology: Yang Yang.
Resources: Chunyang Liang, Yongchun Luo, Chunsen Shen, Ruxiang Xu.
Writing – original draft: Chunyang Liang, Zijun He, Shang Ma.
Writing – review & editing: Yang Yang.
Yang Yang orcid: 0000-0001-6645-2454.
Footnotes
Abbreviations: AA = arachidonic acid, ADP = adenosine diphosphate, APTT = activated partial thromboplastin time, AUROC = area under the ROC curve, CCT = conventional coagulation test, CE = cardioembolism, END = early neurological deterioration, FIB = fibrinogen, IAT = intra-arterial thrombolysis, INR = international normalized ratio, LAA = large artery atherosclerosis, MA = maximal amplitude, MRA = magnetic resonance angiography, MRI = magnetic resonance imaging, NIHSS = National Institutes of Health Stroke Scale, PLT = platelet count, ROC = receiver operating characteristic, SIH = symptomatic intracranial hemorrhage, SOD = stroke of other determined cause, SUD = stroke of undetermined cause, TEG = thromboelastography, TOAST = Trial of ORG 10172 in Acute Stroke Treatment.
How to cite this article: Liang C, Yang Y, He Z, Ma S, Qu X, Luo Y, Shen C, Xu R. Comparison between thromboelastography and the conventional coagulation test in detecting effects of antiplatelet agents after endovascular treatments in acute ischemic stroke patients: A STROBE-compliant study. Medicine. 2020;99:10(e19447).
The study was supported by Science and Technology Committee of Beijing (project No.: Z171100001017165) and the Capital Medical Development Fund (2009–2050).
The authors have no conflicts of interest to disclose.
References
- [1].Davis KA, Miyares MA, Dietrich E. Dual antiplatelet therapy with clopidogrel and aspirin after ischemic stroke: a review of the evidence. Am J Health Syst Pharm 2015;72:1623–9. [DOI] [PubMed] [Google Scholar]
- [2].Baglin T. Using the laboratory to predict recurrent venous thrombosis. Int J Lab Hematol 2011;33:333–42. [DOI] [PubMed] [Google Scholar]
- [3].Wang Z, Li J, Cao Q, et al. Comparison between thromboelastography and conventional coagulation tests in surgical patients with localized prostate cancer. Clin Appl Thromb Hemost 2018;24:755–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Rosafio F, Vandelli L, Bigliardi G, et al. Usefulness of thromboelastography in the detection and management of tissue plasminogen activator-associated hyperfibrinolysis. J Stroke Cerebrovasc Dis 2017;26:e29–31. [DOI] [PubMed] [Google Scholar]
- [5].Yang Y, Liang C, Zhang Q, et al. Analysis of prognostic factors of endovascular therapy in 59 patients with acute anterior circulation stroke: a retrospective cohort study – observational. Int J Surg 2015;16(Pt A):36–41. [DOI] [PubMed] [Google Scholar]
- [6].Adams HP, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- [7].Gomes J, Wachsman AM. Types of Strokes. 2013;15–31. [Google Scholar]
- [8].Larrue V, von Kummer R, Muller A, et al. Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator: a secondary analysis of the European–Australasian Acute Stroke Study (ECASS II). Stroke 2001;32:438–41. [DOI] [PubMed] [Google Scholar]
- [9].Awadh M, MacDougall N, Santosh C, et al. Early recurrent ischemic stroke complicating intravenous thrombolysis for stroke: incidence and association with atrial fibrillation. Stroke 2010;41:1990–5. [DOI] [PubMed] [Google Scholar]
- [10].Ois A, Martinez-Rodriguez JE, Munteis E, et al. Steno-occlusive arterial disease and early neurological deterioration in acute ischemic stroke. Cerebrovasc Dis 2008;25:151–6. [DOI] [PubMed] [Google Scholar]
- [11].Elliott A, Wetzel J, Roper T, et al. Thromboelastography in patients with acute ischemic stroke. Int J Stroke 2015;10:194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].David JS, Durand M, Levrat A, et al. Correlation between laboratory coagulation testing and thromboelastometry is modified during management of trauma patients. J Trauma Acute Care Surg 2016;81:319–27. [DOI] [PubMed] [Google Scholar]
- [13].Liu C, Guan Z, Xu Q, et al. Relation of thromboelastography parameters to conventional coagulation tests used to evaluate the hypercoagulable state of aged fracture patients. Medicine (Baltimore) 2016;95:e3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Girdauskas E, Kempfert J, Kuntze T, et al. Thromboelastometrically guided transfusion protocol during aortic surgery with circulatory arrest: a prospective, randomized trial. J Thorac Cardiovasc Surg 2010;140:1117–24. e1112. [DOI] [PubMed] [Google Scholar]
- [15].Moganasundram S, Hunt BJ, Sykes K, et al. The relationship among thromboelastography, hemostatic variables, and bleeding after cardiopulmonary bypass surgery in children. Anesth Analg 2010;110:995–1002. [DOI] [PubMed] [Google Scholar]
- [16].Weidhofer C, Meyer E, Ristl R, et al. Dynamic reference intervals for coagulation parameters from infancy to adolescence. Clin Chim Acta 2018;482:124–35. [DOI] [PubMed] [Google Scholar]
- [17].Toulon P, Berruyer M, Brionne-Francois M, et al. Age dependency for coagulation parameters in paediatric populations. Results of a multicentre study aimed at defining the age-specific reference ranges. Thromb Haemost 2016;116:9–16. [DOI] [PubMed] [Google Scholar]
- [18].Ho P, Ng C, Rigano J, et al. Significant age, race and gender differences in global coagulation assays parameters in the normal population. Thromb Res 2017;154:80–3. [DOI] [PubMed] [Google Scholar]
- [19].Wu J, Zhao HR, Zhang HY, et al. Thrombin generation increasing with age and decreasing with use of heparin indicated by calibrated automated thrombogram conducted in Chinese. Biomed Environ Sci 2014;27:378–84. [DOI] [PubMed] [Google Scholar]
- [20].Ahmad A, Kohli M, Malik A, et al. Role of thromboelastography versus coagulation screen as a safety predictor in pre-eclampsia/eclampsia patients undergoing lower-segment caesarean section in regional anaesthesia. J Obstet Gynaecol India 2016;66: Suppl 1: 340–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sharma S, Kumar S, Tewari P, et al. Utility of thromboelastography versus routine coagulation tests for assessment of hypocoagulable state in patients undergoing cardiac bypass surgery. Ann Card Anaesth 2018;21:151–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hou X, Han W, Gan Q, et al. Relationship between thromboelastography and long-term ischemic events as gauged by the response to clopidogrel in patients undergoing elective percutaneous coronary intervention. Biosci Trends 2017;11:209–13. [DOI] [PubMed] [Google Scholar]
- [23].Rao Z, Zheng H, Wang F, et al. High on-treatment platelet reactivity to adenosine diphosphate predicts ischemic events of minor stroke and transient ischemic attack. J Stroke Cerebrovasc Dis 2017;26:2074–81. [DOI] [PubMed] [Google Scholar]
- [24].Wang B, Li XQ, Ma N, et al. Association of thrombelastographic parameters with post-stenting ischemic events. J Neurointerv Surg 2017;9:192–5. [DOI] [PubMed] [Google Scholar]
- [25].McDonald MM, Almaghrabi TS, Saenz DM, et al. Dual antiplatelet therapy is associated with coagulopathy detectable by thrombelastography in acute stroke. J Intensive Care Med 2017;doi: 10.1177/0885066617729644. [DOI] [PubMed] [Google Scholar]
- [26].Li DD, Wang XY, Xi SZ, et al. Relationship between ADP-induced platelet-fibrin clot strength and anti-platelet responsiveness in ticagrelor treated ACS patients. J Geriatr Cardiol 2016;13:282–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].McDonald MM, Wetzel J, Fraser S, et al. Thrombelastography does not predict clinical response to rtPA for acute ischemic stroke. J Thromb Thrombolysis 2016;41:505–10. [DOI] [PubMed] [Google Scholar]
- [28].Ranucci M, Baryshnikova E, Ciotti E, et al. Hemodilution on cardiopulmonary bypass: thromboelastography patterns and coagulation-related outcomes. J Cardiothorac Vasc Anesth 2017;31:1588–94. [DOI] [PubMed] [Google Scholar]


