Abstract
Sleep-disordered breathing symptoms may recur in some children after successful adenoidectomy. A potential etiology that warrants consideration is torus tubarius hypertrophy (TTH) as well as residual or recurrent adenoid hypertrophy. Here, we report our experience and the treatment outcomes with microscopic coblator-assisted partial resection of TTH.
Seven children who had undergone coblator-assisted partial resection of TTH under microscopy from April 2000 through January 2017 were retrospectively reviewed. The patient age at the time of initial adenotonsillectomy and the interval between the first operation and partial resection of TTH were identified. Lateral cephalometry and scores on the Korean version of the obstructive sleep apnea-18 (KOSA-18) questionnaire were reviewed.
The median age at the time of the first operation was 3.0 years and the average time interval between the first operation and subsequent tubal tonsillectomy was 44.0 months. The average width between the torus tubarius was 2.1 mm preoperatively. Symptoms of sleep-disordered breathing were relieved in all patients after operation. Preoperative and postoperative KOSA-18 scores were 73.5 and 35.5, respectively (P = .024). On polysomnography, the preoperative and postoperative apnea-hypopnea index scores were 22.9 and 4.7, respectively (P = .068). The patients were followed up for an average of 1.3 years. One patient developed a recurrence of symptoms and underwent a revision operation. Complications such as bleeding and nasopharyngeal stenosis were not observed.
Otorhinolaryngologists should keep TTH in mind as one of the differential diagnoses for recurrent upper airway obstruction symptoms after adenoidectomy. Microscopic coblator-assisted partial resection of TTH is likely to be safe and effective.
Keywords: adenoidectomy, coblation, sleep-disordered breathing, snoring, torus tubarius
1. Introduction
Adenoidectomy is one of the most common operations performed in children with sleep-disordered breathing.[1,2] Snoring, sleep apnea, nasal obstruction, or mouth breathing may recur in some children even after successful adenoidectomy. In a recent meta-analysis, the revision rate was 1.9% for pediatric adenoidectomy.[3] These children should be investigated for residual or recurrent adenoid hypertrophy and sinonasal or other diseases like gastroesophageal reflux disease should also be ruled out.[4–6]
In addition, a potential etiology that warrants consideration is the torus tubarius hypertrophy (TTH). The torus tubarius is the projecting posterior lip of the pharyngeal opening of the Eustachian tube, which forms the lateral border of nasopharynx.[7] Yanagisawa et al first reported the TTH in a patient with chronic rhinosinusitis and nasal polyposis in 1999.[8] Such TTH was reported to be able to result in impaired aeration of the middle ear space and, in some cases, serous otitis media.[8] TTH is unfamiliar to and not well understood by otorhinolaryngologists and its diagnosis and treatment remain unclear. In this article, we tried to introduce our experience of microscopic coblator-assisted partial resection of TTH and its outcomes.
2. Materials and methods
We retrospectively reviewed the medical records of 7 consecutive children who underwent coblator-assisted partial resection of TTH under microscopy at our institution from April 2000 through January 2017. All the patients had undergone adenoidectomy or adenotonsillectomy but revisited our clinic because of reappearance of sleep-disordered breathing symptoms. We identified the age of each patient at the time of the initial adenoidectomy or adenotonsillectomy and the interval between the first operation and partial resection of TTH. Lateral cephalometric radiography was performed in all patients and the Korean version of the obstructive sleep apnea-18 (KOSA-18) questionnaire[9] was completed before and 1 month after surgery. Computed tomography was performed preoperatively in 1 patient. Polysomnography was performed in 4 children twice, that is, preoperatively and 3 months postoperatively. This study was approved by the Institutional Review Board of Seoul National University Bundang Hospital (IRB No. B-1708/419-101). The need for informed consent was waived in view of the retrospective nature of the research and the anonymity of the data.
2.1. Surgical technique
Coblator-assisted partial resection of TTH was performed using a Coblator EVAC 70 (Arthrocare ENT, Sunnyvale, CA) under a microscope. Under general anesthesia, a mouth retractor was placed and 2 rubber catheters were introduced through the nasal cavities to retract the soft palate. A laryngeal mirror was used to visualize the nasopharynx. The nasopharyngeal area including the torus tubarius was amplified with a surgical microscope. The presence of the adenoids was first examined and the hypertrophied torus tubarius were identified. The smallest distance between the medial sides of the right and left torus tubarius was measured. The coblator was set at an ablation power of 9 and a coagulation power of 3. Coblator-assisted ablation was started from the medial surfaces. The mucosa around the orifice of the Eustachian tube was meticulously preserved to prevent its obstruction. The distance between the right and left torus tubarius was enlarged to be greater than 8 mm.
2.2. Statistical analysis
The statistical analysis was performed using SPSS version 18.0 for Windows (SPSS Inc., Chicago, IL). Wilcoxon signed rank tests were performed to identify preoperative and postoperative differences in the KOSA-18 score and apnea-hypopnea index. A P-value < .05 was considered statistically significant.
3. Results
The 7 children were all boys with a median age of 5.0 years (first quartile 3.0, third quartile 12.0). The median age at the time of the first operation was 3.0 years (first quartile 2.0, third quartile 3.0) and the average time interval between the first operation and subsequent partial resection of TTH in our clinic was 44.0 (range 11–100) months (Table 1). All patients had a history of recurrent middle ear effusion. The nasopharyngeal airway space was confirmed to be narrowed in all patients on simple lateral cephalometry. Computed tomography showed bilateral enlargement of the torus tubarius area (Fig. 1). Intraoperatively, residual or recurrent adenoid tissues were minimal. The average preoperative width between the right and left torus tubarius was 2.1 (range, 1–3) mm. The width between the right torus tubarius and the left torus tubarius was enlarged to 8 mm (Fig. 2).
Table 1.
Case summary of microscopic coblator-assisted partial resection of hypertrophic torus tubarius.

Figure 1.

Axial computed tomographic image of a patient with bilateral torus tubarius hypertrophy (white arrow).
Figure 2.
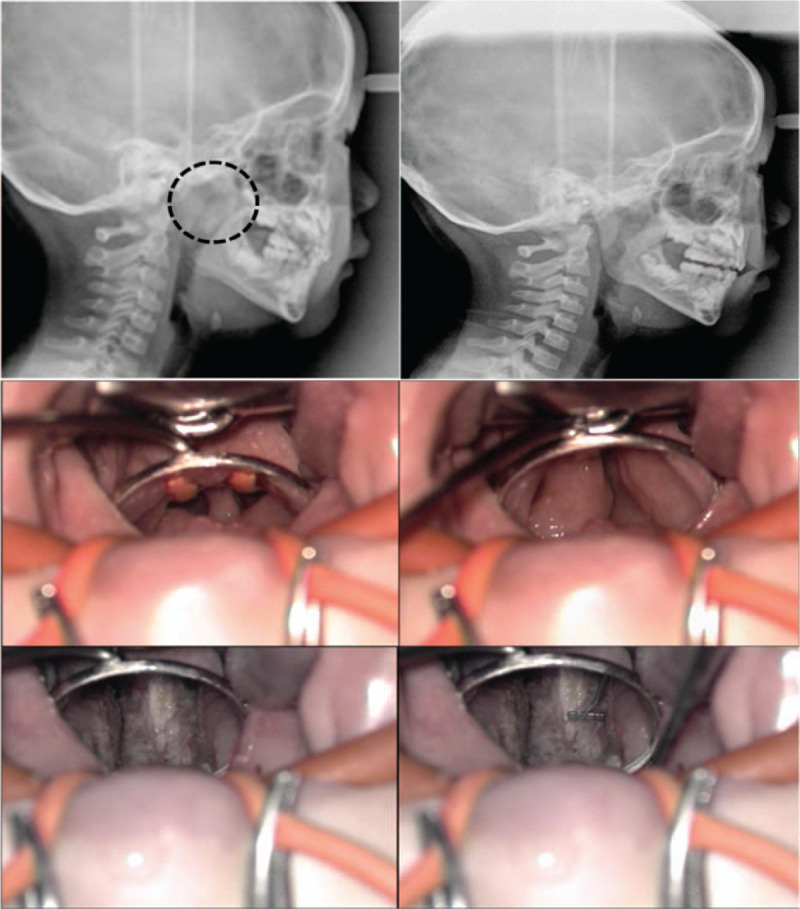
Preoperative and postoperative cephalometry (top). Intraoperatively findings shows narrowing of the airway space between hypertrophic torus tubarius (2 mm in width) (middle). Postoperatively, nasopharyngeal width becomes 8 mm with a 4-mm measure (bottom).
Symptoms of sleep-disordered breathing were relieved in all patients. The average preoperative and postoperative KOSA-18 scores were 73.5 and 35.5, respectively (P = .024). In the 4 patients who underwent polysomnography, the preoperative and postoperative apnea-hypopnea index scores were 22.9 and 4.7, respectively (P = .068).
The patients were followed up postoperatively for an average of 1.3 years (range, 8 months to 2 years). One patient (2) developed a recurrence of symptoms at 3 months postoperatively, and underwent revision operation. No patients had middle ear effusion after partial resection of TTH, and complications such as bleeding and nasopharyngeal stenosis were not observed during the follow-up period.
4. Discussion
To the best of our knowledge, we are the first to report TTH as a cause of recurrent symptoms after adenoidectomy. There have been some reports on hypertrophy of the tubal tonsil.[10,11] One study reported that 50% of cases of otitis media with effusion after adenoidectomy were associated with tubal tonsil hypertrophy.[10] In another study, 21% of 72 patients with symptoms of adenoid regrowth were found to have tubal tonsil hypertrophy.[11] The opinion of the authors is that these reports of tubal tonsil hypertrophy might also be TTH. In previous reports, examinations were performed with the aid of a mirror or endoscope. However, on microscopic examination, unlike the tubal tonsil, which has the shape of a lymphoid follicle, TTH has a hard symmetric appearance. Pathologic confirmation was not possible in our cases because tissue could not be retrieved after coblation.
The pathogenesis of TTH is unknown. Given that all the patients in our study had previously undergone adenoidectomy, removal of the adenoids might have placed a greater burden on the remaining tissues in the nasopharynx, leading to their progressive and compensatory hypertrophy.[5] Lymphoid tissues actively grow during the first several years of life, so the younger age of the child at the time of the initial adenoidectomy (less than 4 years) has been suggested to be a significant risk factor for adenoid regrowth.[12–14] Like adenoid regrowth, TTH would be more likely to occur in children who undergo their initial adenoidectomy at a younger age. Six children in our series underwent their initial adenoidectomy at 3 years of age or younger. The prospective large population study is warranted to validate our retrospective findings and to demonstrate the pathogenesis and risk factors of TTH.
In the present series, microscopic coblator-assisted partial resection of TTH was successful. There were no complications related to partial resection of TTH, such as nasopharyngeal, choanal stenosis, or Eustachian tube scarring with subsequent middle ear problems. Many techniques for surgical removal of the adenoids have been proposed to reduce morbidity and surgical risk (eg, curettage, suction cautery, microdebrider, or coblation).[15–17] Among those techniques, authors have been using coblation adenoidectomy. The overall advantages of coblation adenoidectomy are the decrease in intra- and postoperative bleeding, better safety, precision of adenoid removal and less injury to adjacent tissues.[18,19] It has been reported that the use of coblation particularly efficacious in removing adenoid tissue localized in the lateral walls of the nasopharynx surrounding the eustachian tube orifices.[15] In addition, the shallow depth of penetration of energy and low tissue temperatures at the coblated interface means that there is very little surrounding tissue damage with preservation of tissue architecture.[20,21] This makes it ideal for working near the delicate eustachian tube cushions.[18] For same reason, coblator-assisted technique seems better in partial resection of TTH. The chief surgical pitfall to avoid during partial resection of TTH may be trauma to the opening of the Eustachian tube, same as during the adenoidectomy. The coblator system makes it possible to perform irrigation, coagulation, and ablation at the same time, so the operative field can be maintained bloodless and surgery can be performed meticulously around the Eustachian tube with more precise visualization.[22] In addition, we used a microscope with laryngeal mirror instead of an endoscope, which also has several advantages. Curettage with blind manner is not recommended in recent years, direct or indirect visualization is necessary.[4] Usually, laryngeal mirror is used with naked eye or angled endoscope is used, however, the authors performed the adenoidectomy with microscope from the beginning. As early as 1993, Andrea recommended the use of a surgical microscope for better visualization, more gentle dissection, and more precise hemostasis, thereby leading to less post-operative pain during tonsillectomy.[23,24] Another advantage is that there is no distortion of vision compared to endoscope. The endoscope can allow an widened exposure; however, provide an image distortion with fish-eye view, especially in angled endoscope.[25,26] In addition, the endoscope has loss of the microscopic 3-dimensional perspective.[26] The nasopharyngeal space is not narrow, therefore it is not necessary to widen the exposure with the endoscope. With the straight-line view of the microscope without distortion, meticulous surgery can be performed with enlarged microscopic view.
There have been no studies on how much the nasopharyngeal width should be enlarged. Only 1 study has investigated the nasopharyngeal width in pediatric patients.[27] They reported that the average nasopharyngeal width was 11.9 mm (range, 7.0–18.0 mm), and the increase in width was only 0.26 mm per year, from 3 years to 11 years.[27] The authors thought age differences were almost negligible, and 8 mm width may be enough for improving symptoms compared to the preoperative 2.1 mm width. Our experience suggests that widening of the nasopharyngeal airway space up to 8 mm would be adequate and enough for improvement of symptoms without causing any injury to the orifice of the Eustachian tube.
The preoperative diagnosis of TTH seems to be difficult. Although cephalometry clearly shows a narrowing of the nasopharyngeal airway space, it is difficult to differentiate between adenoid regrowth and TTH. A CT scan can be effective for differentiation but cannot be recommended because of the radiation hazard in children. Nasopharyngoscopy would be helpful if the child is cooperative. Yanagisawa et al. recommended that the transnasal endoscopic nasopharyngoscopy is a useful tool to document changes of the torus tubarius.[8] They also recommended that differentiating the 2 types of tissue is facilitated by transnasal suctioning; the adenoid tissue is mobile and easily manipulated by suctioning, whereas the torus tubarius is not.[8]
Our study has some limitations. Its retrospective nature with small samples as a case series is first limitation. Additional limitations of this study are there was no information on body mass index or adenoid size at initial operation, and that affects adenoid regrowth such as acid reflux or allergic components in each patients.[5,6] However, this is the first report of surgical outcomes of partial resection of TTH, therefore our study may be appreciable. The prospective, longer-follow up, and large population study is required in the future.
5. Conclusion
Although patients with TTH are not encountered frequently, otorhinolaryngologists should keep TTH in mind as one of the differential diagnoses in children with recurrent symptoms of upper airway obstruction after adenoidectomy. As shown in the present study, microscopic coblator-assisted partial resection of TTH is likely to be safe and effective.
Author contributions
Conceptualization: Jeong-Whun Kim.
Data curation: Chae Seo Rhee, Hahn Jin Jung.
Formal analysis: Hahn Jin Jung.
Investigation: Hahn Jin Jung.
Software: Hahn Jin Jung.
Supervision: Jeong-Whun Kim.
Validation: Jeong-Whun Kim.
Writing – original draft: Hahn Jin Jung.
Writing – review and editing: Hahn Jin Jung.
Hahn Jin Jung orcid: 0000-0002-6015-7048.
Footnotes
Abbreviations: KOSA-18 = Korean version of the obstructive sleep apnea-18, TTH = torus tubarius hypertrophy.
How to cite this article: Kim JW, Rhee CS, Jung HJ. Partial resection of hypertrophic torus tubarius for recurred snoring: Case series. Medicine. 2020;99:10(e19329).
The authors have no conflicts of interest to disclose.
References
- [1].Emerick KS, Cunningham MJ. Tubal tonsil hypertrophy: a cause of recurrent symptoms after adenoidectomy. Arch Otolaryngol Head Neck Surg 2006;132:153–6. [DOI] [PubMed] [Google Scholar]
- [2].Passàli D, De Benedetto M, Lauriello M, et al. Influence of Waldeyer's ring hypertrophy on snoring and sleep apnea. Adv Otorhinolaryngol 2011;72:132–5. [DOI] [PubMed] [Google Scholar]
- [3].Lee CH, Hsu WC, Ko JY, et al. Revision adenoidectomy in children: a meta-analysis. Rhinology 2019;57:411–9. [DOI] [PubMed] [Google Scholar]
- [4].Elhassan HA, Bozkurt G, Emre IE. Revision adenoidectomy in children: residual vs regrowth? Eur Arch Otorhinolaryngol 2018;275:1035. [DOI] [PubMed] [Google Scholar]
- [5].Dearking AC, Lahr BD, Kuchena A, et al. Factors associated with revision adenoidectomy. Otolaryngol Head Neck Surg 2012;146:984–90. [DOI] [PubMed] [Google Scholar]
- [6].Carr MM, Poje CP, Ehrig D, et al. Incidence of reflux in young children undergoing adenoidectomy. Laryngoscope 2001;111:2170–2. [DOI] [PubMed] [Google Scholar]
- [7].Merati AL, Rieder AA. Normal endoscopic anatomy of the pharynx and larynx. Am J Med 2003;115: Suppl 3A: 10S–4S. [DOI] [PubMed] [Google Scholar]
- [8].Yanagisawa E, Joe JK. Endoscopic view of the torus tubarius. Ear Nose Throat J 1999;78:404–6. [PubMed] [Google Scholar]
- [9].Franco RA, Jr, Rosenfeld RM, Rao M. Quality of life for children with obstructive sleep apnea. Otolaryngol Head Neck Surg 2000;123:9–16. [DOI] [PubMed] [Google Scholar]
- [10].Honda K, Tanke M, Kumazawa T. Otitis media with effusion and tubal tonsil. Acta Otolaryngol Suppl 1988;454:218–21. [DOI] [PubMed] [Google Scholar]
- [11].Monroy A, Behar P, Brodsky L. Revision adenoidectomy--a retrospective study. Int J Pediatr Otorhinolaryngol 2008;72:565–70. [DOI] [PubMed] [Google Scholar]
- [12].Kim SY, Lee WH, Rhee CS, et al. Regrowth of the adenoids after coblation adenoidectomy: cephalometric analysis. Laryngoscope 2013;123:2567–72. [DOI] [PubMed] [Google Scholar]
- [13].Papaioannou G, Kambas I, Tsaoussoglou M, et al. Age-dependent changes in the size of adenotonsillar tissue in childhood: implications for sleep-disordered breathing. J Pediatr 2013;162:269–74.e4. [DOI] [PubMed] [Google Scholar]
- [14].Lesinskas E, Drigotas M. The incidence of adenoidal regrowth after adenoidectomy and its effect on persistent nasal symptoms. Eur Arch Otorhinolaryngol 2009;266:469–73. [DOI] [PubMed] [Google Scholar]
- [15].Timms MS, Ghosh S, Roper A. Adenoidectomy with the coblator: a logical extension of radiofrequency tonsillectomy. J Laryngol Otol 2005;119:398–9. [DOI] [PubMed] [Google Scholar]
- [16].Krajewski M, Samoliaski B, Schmidt J. Endoscopic adenotomy--clinical assessment of value and safety--an own experience. Otolaryngol Pol 2007;61:21–4. [DOI] [PubMed] [Google Scholar]
- [17].Songu M, Altay C, Adibelli ZH, et al. Endoscopic-assisted versus curettage adenoidectomy: a prospective, randomized, double-blind study with objective outcome measures. Laryngoscope 2010;120:1895–9. [DOI] [PubMed] [Google Scholar]
- [18].Di Rienzo Businco L, Angelone AM, Mattei A, et al. Paediatric adenoidectomy: endoscopic coblation technique compared to cold curettage. Acta Otorhinolaryngol Ital 2012;32:124–9. [PMC free article] [PubMed] [Google Scholar]
- [19].Di Rienzo Businco L, Coen Tirelli G. Paediatric tonsillectomy: radiofrequency-based plasma dissection compared to cold dissection with sutures. Acta Otorhinolaryngol Ital 2008;28:67–72. [PMC free article] [PubMed] [Google Scholar]
- [20].Rotenberg B, Tan S. Endoscopic-assisted radiofrequency lingual tonsillectomy. Laryngoscope 2011;121:994–6. [DOI] [PubMed] [Google Scholar]
- [21].Plant RL. Radiofrequency treatment of tonsillar hypertrophy. Laryngoscope 2002;112:20–2. [DOI] [PubMed] [Google Scholar]
- [22].Bhandari N, Don DM, Koempel JA. The incidence of revision adenoidectomy: a comparison of four surgical techniques over a 10-year period. Ear Nose Throat J 2018;97:E5–9. [DOI] [PubMed] [Google Scholar]
- [23].Andrea M. Microsurgical bipolar cautery tonsillectomy. Laryngoscope 1993;103:1177–8. [DOI] [PubMed] [Google Scholar]
- [24].Schrötzlmair F, Geerke L, Kisser U, et al. Optical magnification devices in tonsillectomy: a prospective randomised clinical study. Eur Arch Otorhinolaryngol 2015;272:3031–7. [DOI] [PubMed] [Google Scholar]
- [25].Montibeller GR, Hendrix P, Fries FN, et al. Comparison of microscopic and endoscopic view of the internal acoustic meatus: a cadaveric study. Clin Anat 2018;31:398–403. [DOI] [PubMed] [Google Scholar]
- [26].Peris-Celda M, Da Roz L, Monroy-Sosa A, et al. Surgical anatomy of endoscope-assisted approaches to common aneurysm sites. Neurosurgery 2014;10: Suppl 1: 121–44. [DOI] [PubMed] [Google Scholar]
- [27].Lee SY, Kim JW. Nasopharyngeal width and its association with sleep-disordered breathing symptoms in children. Clin Exp Otorhinolaryngol 2019;12:399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]


