Supplemental Digital Content is available in the text
Keywords: DMARDs, hypertension, rheumatoid arthritis
Abstract
There has been some debate between biologic disease modifying anti-rheumatic drugs (bDMARDs) treatment and hypertension (HTN) in rheumatoid arthritis (RA). The aim of this study was to determine the effect of bDMARDs on the development of HTN in patients with RA.
A total of 996 patients eligible for analysis were recruited from the Korean College of Rheumatology Biologics & Targeted Therapy (KOBIO) registry from 2012 to 2018. The bDMARDs were tumor necrosis factor (TNF) inhibitors, abatacept, and tocilizumab. The cDMARDs included methotrexate, hydroxychloroquine, and leflunomide. The incidence rate and 95% confidence interval of HTN were estimated using the Kaplan–Meier method. Hazard ratio (HR) of risk factors associated with hypertension was assessed by cox proportional hazard model analysis.
Among the 996 patients, 62 patients (6.2%) were newly diagnosed with HTN. There were differences in incidence rate of HTN among conventional DMARDs (cDMARDs), TNF inhibitors, tocilizumab, and abatacept during the follow-up period (P = .015). Kaplan–Meier analysis showed that there was a significant difference in incident HTN only between cDMARDs and tocilizumab (P = .001). Systolic blood pressure and positive rheumatoid factor were associated with development of HTN (HR = 1.049, P = .016 and HR = 1.386, P = .010, respectively). Cox proportional hazard model analysis showed no difference in the development of HTN between bDMARDs and cDMARDs in RA.
This study showed that bDMARDs treatment might not increase risk of incident HTN in patients with RA, compared to cDMARDs.
1. Introduction
Rheumatoid arthritis (RA) has been established to cause increased cardiovascular morbidity and mortality.[1,2] Substantial research has yielded a growing amount of evidence in support of an association between RA and cardiovascular diseases (CVD). As with the general population, CVD in patients with RA is associated with traditional risk factors such as smoking, dyslipidemia, obesity, hypertension (HTN), and diabetes mellitus.[3,4] In addition, diverse disease-related mechanisms related to RA, including increased oxidative stress, aberrant immune response, and endothelial dysfunction, account for development of CVD.[5] Recently, evidence has indicated that RA treatment such as non-steroidal anti-inflammatory drugs (NSAIDs) and corticosteroids might contribute to the risk of CVD.[6]
The prevalence of HTN, also an important modifiable risk factor for development of CVD, seems to be high in patients with RA.[7,8] Growing evidence suggests that RA treatment may be responsible for development of HTN in such patients. In addition to NSAIDs and corticosteroids, some conventional disease modifying anti-rheumatic drugs (cDMARDs) such as leflunomide and cyclosporine may result in increased blood pressure in RA.[5,6] Over the past several decades, biologic DMARDs (bDMARDs) have revolutionized the control of disease activity in the treatment of RA. Given that considerable progress has been made in the development of effective drugs to modulate the inflammatory response and prevent joint damages in the territories of RA, bDMARDs therapy might have significant potential to modify the risk of HTN in RA. Recently, a meta-analysis of randomized controlled trials demonstrated that tumor necrosis factor (TNF) inhibitors could potentially increase the risk of HTN in RA.[9] On the other hand, there is not enough data available to determine whether other bDMARDs including tocilizumab and abatacept are associated with HTN. In this study, we identified the risk factors for development of HTN in RA patients using a patient registry for bDMARDs and cDMARDs and also assessed whether bDMARDs treatment promotes development of HTN in patients with RA.
2. Subjects and methods
2.1. Study population
The data for this study originated from the Korean College of Rheumatology Biologics & Targeted Therapy (KOBIO) registry, a prospective nationwide biologic therapy registry for RA from 2012 to 2018. The KOBIO registry for RA has a mean follow-up period of approximately 54.2 months. This study enrolled patients with RA who met the 1987 American College of Rheumatology revised classification criteria for RA diagnosis.[10]
The KOBIO registry consisted of a “bDMARDs group” and a “cDMARDs group” to evaluate differences in efficacy and safety between the 2 different therapeutic modalities. The “bDMARDs group” comprised patients who were treated with bDMARDs alone or together with cDMARDs, whereas the “cDMARDs group,” recruited as the disease control, consisted of those who had received cDMARDs but had never been exposed to any kinds of bDMARDs. The bDMARDs were TNF inhibitors (infliximab, infliximab biosimilars, etanercept, etanercept biosimilars, adalimumab, and golimumab), abatacept, and tocilizumab. The cDMARDs were methotrexate, hydroxychloroquine, and leflunomide. Corticosteroid use was assessed and presented as mean dosage per day. All study participants provided written informed consent for enrollment in the KOBIO registry. The Institutional Review Board of Daegu Catholic University Medical Center approved the study (CR-19-096).
A total of 2422 patients were initially recruited in this study, including 1738 patients treated with bDMARDs and 684 patients with cDMARDs (Fig. 1). Among patients treated with bDMARDs, those with preexisting HTN at the time of enrollment (n = 482), lost to follow-up (n = 352), or who either switched to different a bDMARD or stopped bDMARDs altogether (n = 289) were excluded during the follow-up period. Among patients treated with only cDMARDs, those with preexisting HTN (n = 199) or lost to follow-up (n = 104) were also excluded. Finally, a total of 996 patients were analyzed in this study, including 615 patients in the bDMARDs group and 381 patients in the cDMARDs group.
Figure 1.
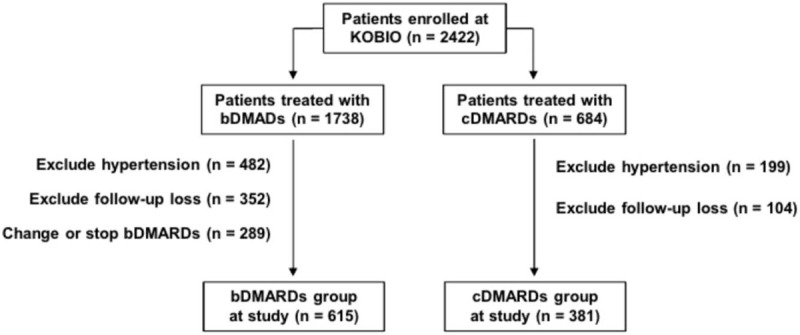
Schematic presentation for study population. bDMARDs = biologic disease modifying anti-rheumatic drugs, cDMARDs = conventional disease modifying anti-rheumatic drugs. bDMARDs group includes TNF inhibitors, abatacept, and tocilizumab.
2.2. Collection of clinical information
The baseline characteristics of patients were sex, age (years, <60 and ≧60), systolic blood pressure (SBP, mmHg), diastolic blood pressure (DBP, mmHg), smoking status (ex-smokers, current smokers, and never smokers), and body mass index (BMI, kg/m2). BMI was classified into 3 groups, normal (BMI < 23.0), overweight (23.0 ≤ BMI < 25.0), and obese (BMI ≤ 25). Comorbidities that are recognized as traditional risk factors for HTN, such as dyslipidemia and diabetes mellitus, were identified. Blood urea nitrogen (BUN, mg/dL) and creatinine (mg/dL) were also assessed.
The following RA-related disease activity indexes were assessed at both baseline and follow-up: erythrocyte sedimentation rate (ESR, mm/hour), C-reactive protein (CRP, mg/dL), swollen joint count (SJC), tender joint count (TJC), patient global assessment (PTGA), physician global assessment (PHGA), disease activity score 28 (DAS28)-ESR, DAS28-CRP, simplified disease activity index (SDAI), clinical disease activity index (CDAI), and routine assessment of patient index data 3 (RAPID3). In addition, ESR and CRP were dichotomically classified into normal and abnormal. Positivity for rheumatoid factor (RF, positive and negative) and anti-cyclic citrullinated peptide antibody (anti-CCP antibody, positive, and negative) was also determined.
2.3. Definition of HTN
A standard mercury sphygmomanometer with an inflatable cuff was used to measure blood pressure from the right arm. After sitting for at least 5 minutes, measurements of 2 consecutive blood pressures were carried out and recorded. We used the average values of the 2 blood pressures. In this study, HTN was defined as an SBP greater than 140 mm Hg and/or DBP greater than 90 mm Hg according to guidelines proposed by the British HTN Society.[11] Any RA patients receiving new antihypertensive drugs such as β-blockers, calcium channel blockers, angiotensin converting enzyme inhibitors, or angiotensin II receptor blockers during the follow-up period, were defined as having HTN.
2.4. Statistical analysis
Values are presented as mean (standard deviation, SD) for quantitative variables or as frequency (%) for qualitative variables. A comparison of baseline characteristics between the bDMARDs and cDMARDs groups and a comparison of variables according to HTN were analyzed using the two-sample t test for quantitative variables and the chi-square test for qualitative variables. The differences in SBP and DBP among cDMARDs, TNF inhibitors, abatacept, and tocilizumab were analyzed by one-way analysis of variance (ANOVA) method.
To obtain difference values from the baseline visit to the last follow-up visit for changes in the disease activity indexes ESR, CRP, SJC, TJC, PTGA, PHGA, DAS28-ESR, DAS28-CRP, SDAI, CDAI, and RAPID3, the following formula was used: [difference value = values at follow-up − values at baseline]. In addition, difference values for changes in SBP, DBP, and disease activity indexes among cDMARDs, TNF inhibitors, tocilizumab, and abatacept were calculated from the baseline visit to the last follow-up visit.
The Kaplan–Meier method was used to calculate HTN incidence rate by DMARD group (cDMARDs, TNF inhibitors, abatacept, and tocilizumab). The 95% confidence intervals (CI) of HTN incidence rate by each DMARD group were also presented. Among cDMARDs, TNF inhibitors, abatacept, and tocilizumab, the log-rank test was used to compare overall and pair-wise HTN incidence rates, and post hoc analysis was applied to identify the differences in SBP, DBP, and disease activity indexes.
In the multivariate analysis for determination of risk factors for HTN, the candidate variables were the variables were statistically significant at the univariate analysis and traditional risk factors such as BMI, dyslipidemia, and diabetes mellitus in Model 1. Additional candidate variables such as ESR, CRP, RF, anti-CCP antibody, corticosteroid, methotrexate, leflunomide, or bDMARDs were assessed in the analysis of Model 2 and Model 3. The Cox's proportional hazards model was used, and the hazard ratio (HR) and 95% CI for HR were calculated. All statistical analyses were performed using the IBM SPSS software package for Windows (version 19.0, Chicago, IL). All tests were 2-sided, and a P-value less than .05 was considered to indicate statistical significance.
3. Results
3.1. Baseline characteristics of enrolled patients
A total of 996 RA patients (n = 615 for the bDMARDs group and n = 381 for the cDMARDs group) were enrolled in this study. Baseline characteristics according to group, bDMARDs or cDMARDs, are described in Table 1. Frequencies of sex, age (<60 or ≧60), BMI (normal, overweight, and obese), smoking status, dyslipidemia, diabetes mellitus, anti-CCP antibody positivity, and hydroxychloroquine use were similar between the bDMARDs and cDMARDs groups (P > .05 for all). Patients treated with bDMARDs were significantly younger than those treated with cDMARDs (P = .006). There were no differences in SBP and DBP, BMI (kg/m2), and BUN between the 2 groups.
Table 1.
Baseline characteristics of enrolled patients.
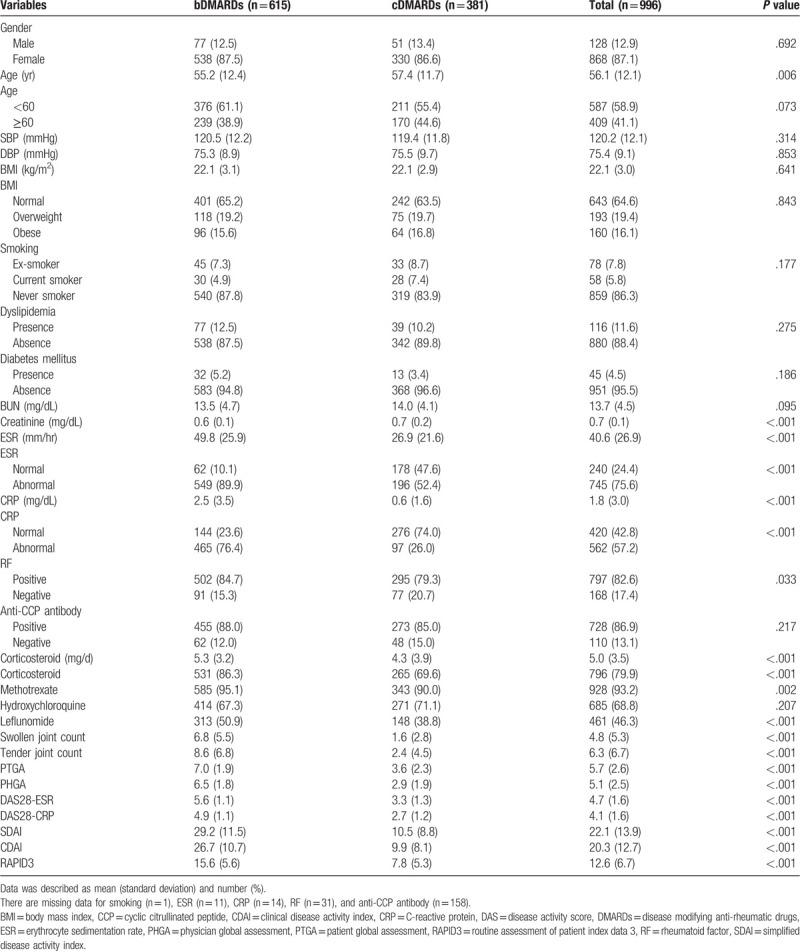
Patients in the bDMARDs group showed lower creatinine and higher ESR, CRP, and corticosteroid dosage than those in the cDMARDs group (P < .001 for all). The number of bDMARDs-treated patients with abnormal ESR, abnormal CRP, RF positivity, corticosteroid use, methotrexate use, and leflunomide use was higher than that of cDMARDs patients (P < .001, P < .001, P = .033, P < .001, P = .002, and P < .001, respectively). There were significant differences in disease activity indexes of SJC, TJC, PTGA, PHGA, DAS28-ESR, DAS28-CRP, SDAI, CDAI, and RAPID3 between the bDMARDs and cDMARDs groups (P < .001 for all).
3.2. Comparison of variables for HTN according to variables at baseline
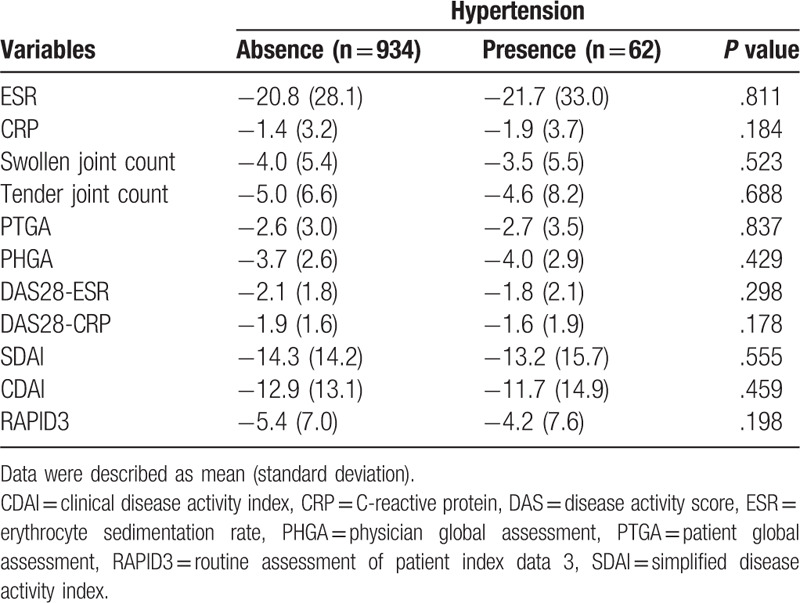
We also compared baseline variables between patients with HTN and patients without HTN at the end of follow-up (Table 2). Older age in HTN patients was noted compared to non-HTN patients (P < .001), with more prevalence of patients 60 years and older (P = .005). There was no difference of BMI (normal, overweight, and obese) between HTN group and non-HTN group. The number of patients with dyslipidemia and diabetes mellitus were similar between HTN patients and non-HTN patients (P = .051 and P = .074, respectively). Frequencies for other variables of sex, smoking status, abnormal ESR, abnormal CRP, RF positivity, anti-CCP antibody positivity, corticosteroid use, methotrexate use, hydroxychloroquine use, and leflunomide use were not significantly different between the 2 groups. There were no differences in BMI (kg/m2), creatinine, ESR, CRP, and mean corticosteroid dosage between 2 groups (P > .05 for all). All disease activity indexes such as SJC, TJC, PTGA, PHGA, DAS28-ESR, DAS28-CRP, SDAI, CDAI, and RADIP3 were similar between the 2 groups (P > .05 for all). In addition, we did not find significant differences in changes for any of the disease activity indexes between patients with and without HTN over time (P > .05 for all) (Table 3).
Table 2.
Comparison of variable according to variables at baseline.
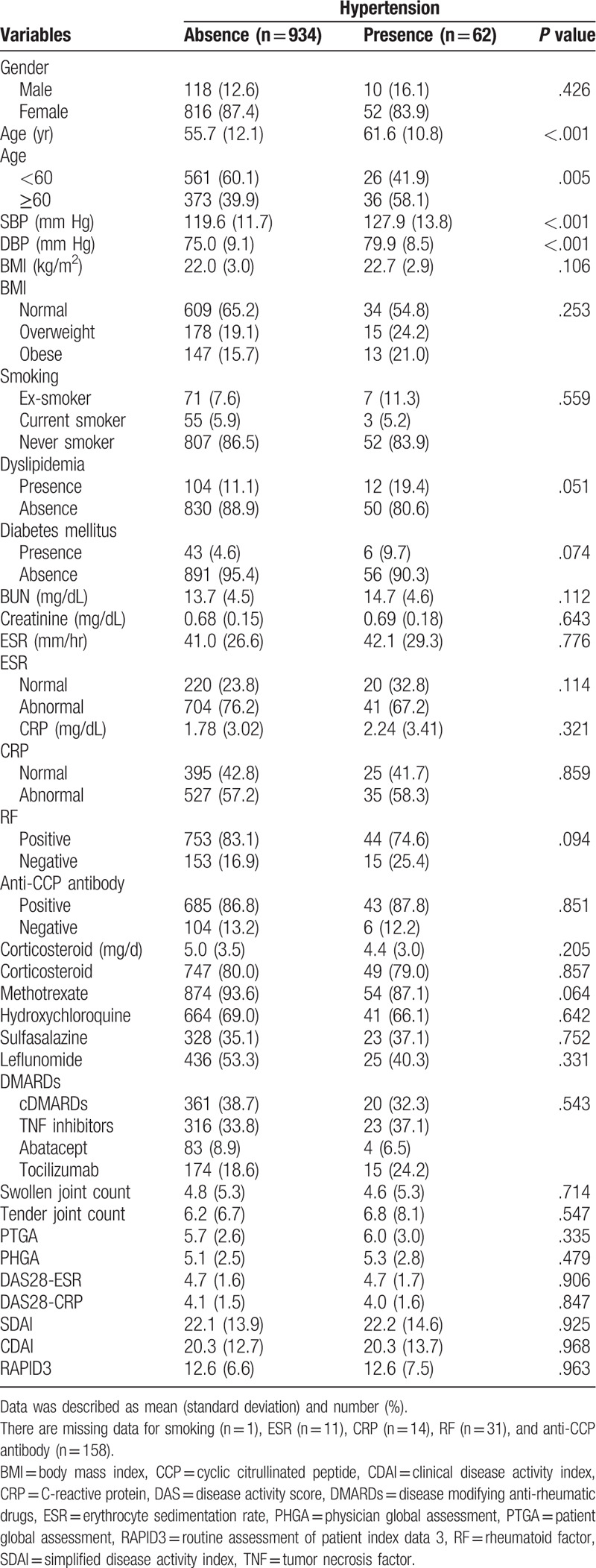
Table 3.
Comparison for changes of disease activity indexes according to hypertension.
The lack of differences among patients treated with cDMARDs, TNF inhibitors, abatacept, or tocilizumab in changes of SBP and DBP during the follow-up period (P = .419 and P = .434, respectively) (S1 Fig.). However, differences in disease activity indexes between cDMARDs and TNF inhibitors, tocilizumab, or abatacept were significant (S1 Table).
3.3. Assessment of incident HTN according to DMARDs
The frequency of incident HTN was not different among patients treated with cDMARDs, TNF inhibitors, abatacept, or tocilizumab (P = .543) (Table 2). There was no difference of incident HTN between patients with cDMARDs and bDMARDs (P = .316). The incidence rate of HTN was 4.6% of cDMARDs, 6.9% of TNF inhibitors, 1.4% of abatacept, and 7.2% of tocilizumab (S2 Fig.). The incidence rates of HTN with 95% CI for cDMARDs, TNF inhibitors, abatacept, and tocilizumab over time were analyzed using Kaplan–Meier curves (4.613%, 95% CI 4.022–5.204 for cDMARDs; 6.878%, 95% CI 6.164–7.591 for TNF inhibitors; 1.389%, 95% CI 0.139–2.639 for abatacept; and 7.177%, 95% CI 6.022–8.332 for tocilizumab). The incidence rates were significantly different among all DMARDs (log-rank test, P = .015), as shown in Figure 2. However, only the difference of incidence rate between cDMARDs and tocilizumab was significant (P = .001).
Figure 2.
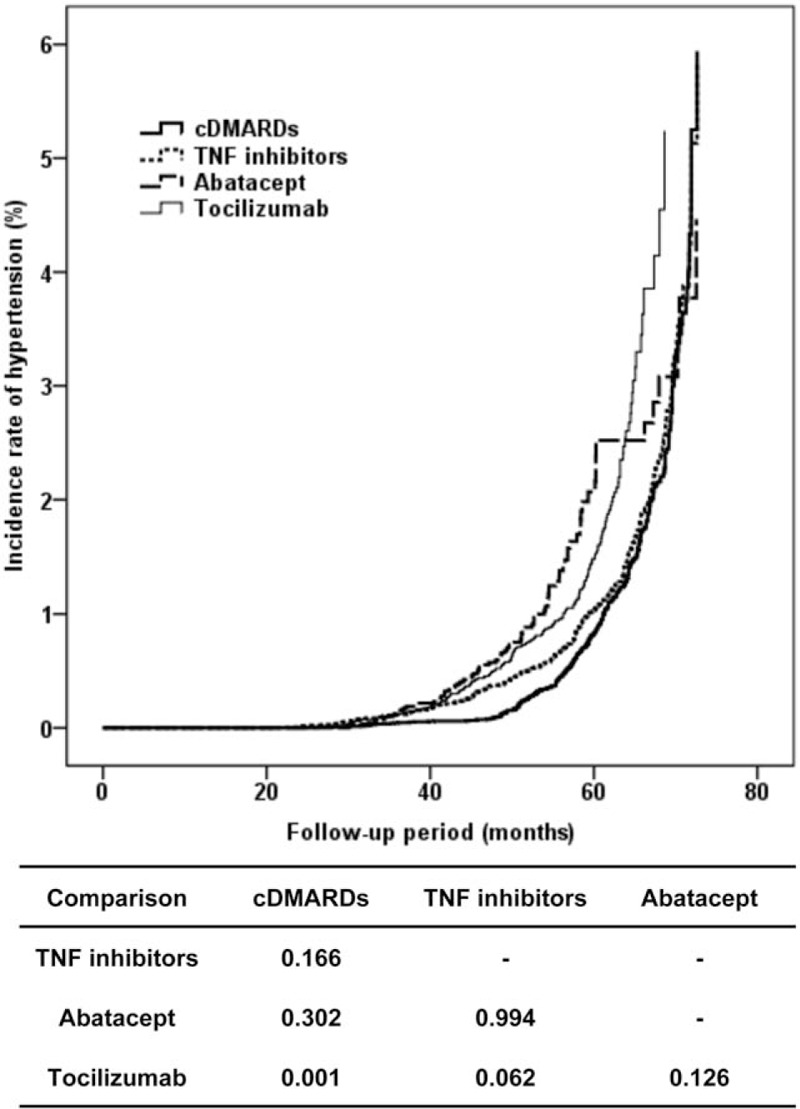
Kaplan–Meier curves for incidence rate of hypertension according to cDMARDs, TNF inhibitors, abatacept, and tocilizumab over time. Line patterns of each prescription for abatacept (thick solid line), tocilizumab (thin solid line), TNF inhibitors (thin dashed line), and cDMARD (thick dashed line) were presented. Beneath the figure, P values in the table are presented by pair-wise comparison analysis between 2 DMARDs. cDMARDs = conventional disease modifying anti-rheumatic drugs, TNF = tumor necrosis factor.
3.4. Determination of risk factors related with development of HTN
Risk factors for development of HTN in the study population were identified by Cox's proportional hazards model after adjusting for clinical variables and treatment modalities (Table 4). Multivariate analysis (Model 1) adjusting for age, sex, BMI, dyslipidemia, diabetes mellitus, and systolic/diastolic blood pressure showed that only systolic blood pressure at baseline predicted increased risk of development of HTN (HR = 1.050, 95% CI 1.019–1.083, P = .002).
Table 4.
Cox proportional hazard model of risk factors of development of hypertension in RA according to baseline variables.
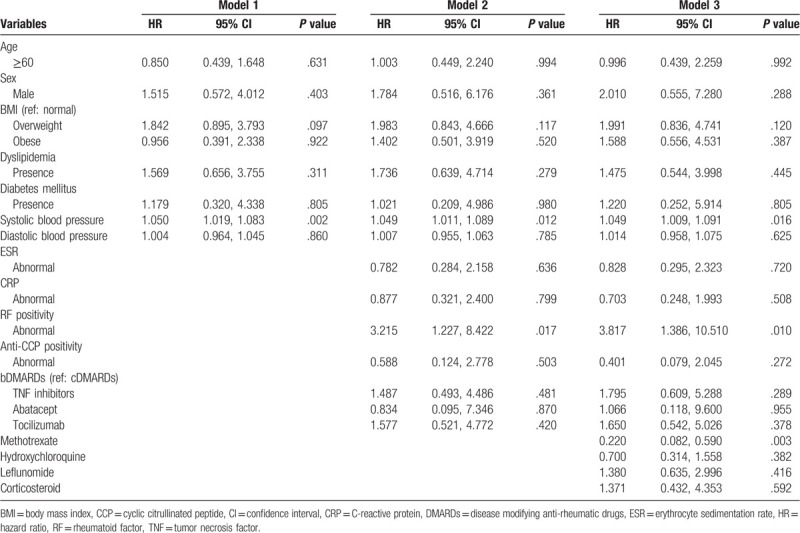
Following analysis adjusting for addition of ESR, CRP, RF, anti-CCP antibody, and bDMARDs use (Model 2), increased risk of HTN was found in patients with systolic blood pressure and RF positivity (HR = 1.049, 95% CI 1.011–1.089, P = .012 and HR = 3.215, 95% CI 1.227–8.422, P = .017, respectively). Interestingly, increased risk of HTN was not noted in patients treated with TNF inhibitors, abatacept, or tocilizumab compared to those treated with cDMARDs (P > .05 for all).
In addition, Model 3 analysis also showed systolic blood pressure and RF positivity as risk factors for development of HTN (HR = 1.049, 95% CI 1.009–1.091, P = .016 and HR = 3.817, 95% CI 1.386–10.510, P = .010, respectively). In contrast, methotrexate use was negatively associated with HTN (HR = 0.220, 95% CI 0.082–0.590, P = .003). Any bDMARDs use was not associated with development of HTN.
4. Discussion
The causes for increased morbidity and mortality observed in RA patients are multifactorial, including CVD, lung involvement, and infectious diseases. It is well recognized that patients with RA have a higher risk of CVD compared to the general population.[12–14] Generally, HTN is an important traditional risk factor for CVD and has also been considered a potent target for prevention of CVD, as well as for reduction of CVD-related mortality.[15] There is a growing need to assess the risk factors for HTN in patients treated with RA. Recently, bDMARDs therapy has become an important therapeutic modality for treatment of RA.[16] In this study, we assessed whether bDMARDs might have differential potentials for increased risk of HTN compared to cDMARDs. The main finding of this study was that treatment with bDMARDs, including TNF inhibitors, tocilizumab, and abatacept, did not increase the risk of incident HTN compared to treatment with cDMARDs.
It is well known that an increased level of inflammatory cytokines such as TNF-α and IL-6 is an important feature in patients with RA.[12] These cytokines were found to be closely associated with elevation of blood pressure in hypertensive patients and healthy subjects.[17,18] This evidence suggests that the anti-inflammatory effect of bDMARDs including TNF inhibitors, IL-6 receptor antagonist (tocilizumab), and possibly cytotoxic T-lymphocyte antigen 4-Ig (CTLA4-Ig, abatacept), may be an important preventive or therapeutic strategy against development of HTN. However, there has been considerable debate about the role of bDMARDs in incident HTN in patients with RA. Some observational studies have shown that TNF inhibitors have a lowering effect on blood pressure in RA.[19,20] TNF inhibitor treatment with infliximab, etanercept, and adalimumab induced vascular dilatation through significant change in microvascular endothelium-dependent function using acetylcholine compared to the baseline measurement (P = .001). Three months of anti-TNF therapy resulted in lower SBP after 3 months of anti-TNF therapy compared to nonbiologic DMARDs controls (P = .021).[19] Yoshida et al demonstrated the lowering effect of infliximab on ambulatory blood pressure through attenuation of sympathetic nerve tone but not renin angiotensin activity in RA.[20] In contrast, a cohort study using insurance claims data from the US revealed that treatment with TNF inhibitors did not reduce the risk of incident HTN compared to treatment with nonbiologic DMARDs (HR 0.85, 95% CI 0.67–1.1).[21] In contrast, a recent meta-analysis of 11 randomized controlled trials demonstrated that TNF inhibitors might be associated with increased risk of HTN in RA.[9] Specifically, statistical significance was only found between certolizumab pegol and HTN (OR 3.62, 95% CI 1.50–8.73, P = .0002) but not with etanercept, golimumab, and infliximab. Consistently, our study also found that treatment with TNF inhibitors including infliximab/infliximab similars, etanercept/etanercept similars, adalimumab, and golimumab was not related with increased incidence of HTN compared to those treated with cDMARDs.
Data on the development of HTN induced by tocilizumab are still insufficient. In some clinical trials, disturbance of lipid profiles, especially low-density lipoprotein cholesterol (LDL-C), was noted after treatment with tocilizumab.[22,23] A retrospective post hoc analysis of data from patients who received tocilizumab showed that baseline lipid profile was associated with increased risk of major adverse cardiovascular events (MACE).[24] These clinical results have raised concerns about the occurrence of CVD in patients treated with tocilizumab. However, there is still a debate as to whether changes in lipid parameters by tocilizumab could increase CVD events. In contrast, a prospective observational study of treatment with tocilizumab for 6 months revealed significant reduction of circulating CD4+/CD28− T cells, an important marker of accelerated atherosclerosis in RA patients with a clinical response to DAS28 < 2.6.[25] Recently, 2 different cohort studies have confirmed that the risk of CVD in tocilizumab is similar to that of other bDMARDs such as abatacept or TNF inhibitors.[26,27] In this study, we did not find any increased risk of tocilizumab in the development of HTN compared to the cDMARDs group, although Kaplan–Meier analysis showed a marked difference in HTN between tocilizumab and cDMARDs. Consistent with recent cohort studies,[26,27] the risk of incident HTN in tocilizumab was comparable to that of abatacept or TNF inhibitors, as shown in Kaplan–Meier analysis.
The clinical implication of an association between abatacept and cardiovascular outcomes has not been determined until now. A recent cohort study using claims data from Medicare and MarketScan for RA patients who initiated abatacept or TNF inhibitors revealed that the abatacept group was associated with reduced cardiovascular risk compared to the TNF inhibitors group, especially in patients with diabetes mellitus.[28] The beneficial cardiovascular mechanism of abatacept can be explained as follows. First, abatacept improves cardiovascular risk factors such as insulin sensitivity and some components of the lipid profile.[29,30] Second, CTLA-4 co-inhibitory pathways are known to be responsible for the pathogenesis of atherosclerosis through marked attenuation of accelerated atherosclerosis by abatacept treatment in hypercholesterolemic ApoE3∗Leiden mice.[31] Abatacept could more potently inhibit activation of T cells including proatherogenic T cells such as Th1 cells and CD8+ T cells through modulation of interaction between CD28 and CD80/CD86, rather than specific cytokine-targeted agents such as TNF inhibitors or IL-6R antagonists. However, abatacept treatment for 6 months worsened aortic stiffness as measured by pulse wave velocity, which might be caused by inappropriate control of systemic inflammation.[30] Until now, there has been no study of whether abatacept affects the development of HTN. We firstly observed that abatacept treatment did not increase the risk of HTN compared to cDMARDs treatment. Additionally, its effects were comparable to those of TNF inhibitors and tocilizumab, as shown in Kaplan–Meier analysis.
The factors that can explain increased risk of HTN in patients with RA remain to be fully explained. Several possible candidates are presented, including inflammation, oxidative stress, physical inactivity, and therapeutic medications.[5] Considering cDMARDs-related risk for HTN, the uses of some drugs such as methotrexate might affect the development of HTN. Our study found that methotrexate use contributed to lower risk of incident of HTN. A recent meta-analysis demonstrated that use of methotrexate in diverse rheumatic diseases was significantly associated with lower risk of all cardiovascular events including myocardial infarction (relative risk, 0.72, 95% CI 0.057–0.091, P = .007).[32] Mangoni et al identified the lowering effect on clinical and 24-hour peripheral and central blood pressure in patients with RA on MTX treatment compared to those who were not on MTX treatment,[33] which could be explained by the presumptive hypothesis that MTX restores vasodilation-related adenosine in the body. Some studies demonstrated that seropositivity for RF and/or anti-CCP antibody was associated with ischemic heart diseases and atherosclerosis.[34,35] In contrast, Montes et al revealed no association between RF and subclinical atherosclerosis in patients with RA.[36] In our study, trends in the blood pressure-lowering effect of RF positivity did showed statistical significance.
The prevalence of HTN in RA was higher than the approximate 29% in the general population.[3] Panoulas et al reported that the prevalence of HTN in RA widely varied from about 3.8% to 73% in the outcome analysis of earlier studies.[5] This difference in prevalence in RA seems to be derived from differences in study population (community or secondary care), diagnostic criteria for HTN, disease severity, therapeutic modalities, participation criteria, and study design (case–control, retrospective cohort, or cross-sectional). A recent study using the 2010–2012 Korea National Health and Nutrition Examination Survey (KNHANES) demonstrated that the prevalence of HTN in RA patients was approximately 30.3%, which was significantly higher than 16.8% in the non-RA study population.[37] Compatible with earlier studies, we found that 681 of the total of 2422 RA patients had preexisting HTN (28.1%), although this study did not evaluate a control population. A meta-analysis revealed that incidence of anti-TNF inhibitor-related HTN was 3.25% (95% CI 1.51–6.89%).[9] In this study, the overall incidence of HTN in RA patients after treatment with TNF inhibitors was estimated at 5.8%, which is consistent with results within the confidence interval of the previous meta-analysis.
This study included several limitations. First, there is a possibility that the accuracy of determining HTN could be reduced by measuring blood pressure only at the time of visit. When defining HTN, it is reasonable to measure blood pressure more frequently or use 24-hour blood pressure measurement. Second, the risk factors associated with RA were mainly considered in evaluating HTN of bDMARDs. It is necessary to consider confounding variables that are not directly related to RA.
In conclusion, the main observation of this study was that bDMARDs treatment did not increase the risk of development of HTN compared to cDMARDs treatment. In addition, risk of development of HTN was not associated with changes in disease activity indexes in RA. Finally, systolic blood pressure and RF positivity were responsible for the risk of HTN in RA. In addition, methotrexate use might reduce the risk of HTN in RA. The result of this study should be confirmed through prospective studies in a larger population.
Acknowledgments
This study was based on the Korean College of Rheumatology project KOBIO-19-01-01.
Author contributions
Data curation: Seong-Kyu Kim, Jung-Yoon Choe.
Formal analysis: Seong-Kyu Kim, Sang Gyu Kwak.
Investigation: Seong-Kyu Kim, Jung-Yoon Choe.
Supervision: Seong-Kyu Kim.
Writing – original draft: Seong-Kyu Kim, Sang Gyu Kwak, Jung-Yoon Choe
Writing – review & editing: Seong-Kyu Kim.
Seong-Kyu Kim orcid: 0000-0002-7780-0167.
Supplementary Material
Supplementary Material
Footnotes
Abbreviation: KOBIO = Korean College of Rheumatology Biologics & Targeted Therapy.
How to cite this article: Kim SK, Kwak SG, Choe JY. Association between biologic disease modifying anti-rheumatic drugs and incident hypertension in patients with rheumatoid arthritis: Results from prospective nationwide KOBIO Registry. Medicine. 2020;99:9(e19415).
This work was supported by a research grant from Daegu Catholic University Medical Center.
The authors have no conflicts of interest to disclose.
References
- [1].Wolfe F, Freundlich B, Straus WL. Increase in cardiovascular and cerebrovascular disease prevalence in rheumatoid arthritis. J Rheumatol 2003;30:36–40. [PubMed] [Google Scholar]
- [2].Solomon D, Karlson E, Rimm E, et al. Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis. Circulation 2003;107:1303–7. [DOI] [PubMed] [Google Scholar]
- [3].Jagpal A, Navarro-Millan I. Cardiovascular co-morbidity in patients with rheumatoid arthritis: a narrative review of risk factors, cardiovascular risk assessment and treatment. BMC Rheumatol 2018;2:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gonzalez A, Maradit Kremers H, Crowson CS, et al. Do cardiovascular risk factors confer the same risk for cardiovascular outcomes in rheumatoid arthritis patients as in non-rheumatoid arthritis patients? Ann Rheum Dis 2008;67:64–9. [DOI] [PubMed] [Google Scholar]
- [5].Panoulas VF, Metsios GS, Pace AV, et al. Hypertension in rheumatoid arthritis. Rheumatology (Oxford) 2008;47:1286–98. [DOI] [PubMed] [Google Scholar]
- [6].Atzeni F, Turiel M, Caporali R, et al. The effect of pharmacological therapy on the cardiovascular system of patients with systemic rheumatic diseases. Autoimmun Rev 2010;9:835–9. [DOI] [PubMed] [Google Scholar]
- [7].Panoulas VF, Douglas KM, Milionis HJ, et al. Prevalence and associations of hypertension and its control in patients with rheumatoid arthritis. Rheumatology (Oxford) 2007;46:1477–82. [DOI] [PubMed] [Google Scholar]
- [8].Chung CP, Oeser A, Solus JF, et al. Prevalence of the metabolic syndrome is increased in rheumatoid arthritis and is associated with coronary atherosclerosis. Atherosclerosis 2008;196:756–63. [DOI] [PubMed] [Google Scholar]
- [9].Zhao Q, Hong D, Zhang Y, et al. Association between anti-TNF therapy for rheumatoid arthritis and hypertension: a meta-analysis of randomized controlled trials. Medicine (Baltimore) 2015;94:e731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24. [DOI] [PubMed] [Google Scholar]
- [11].Williams B, Poulter NR, Brown MJ, et al. Guidelines for management of hypertension: report of the fourth working party of the British Hypertension Society, 2004-BHS IV. J Hum Hypertens 2004;18:139–85. [DOI] [PubMed] [Google Scholar]
- [12].Klareskog L, Catrina AI, Paget S. Rheumatoid arthritis. Lancet 2009;373:659–72. [DOI] [PubMed] [Google Scholar]
- [13].Avina-Zubieta JA, Thomas J, Sadatsafavi M, et al. Risk of incident cardiovascular events in patients with rheumatoid arthritis: a metaanalysis of observational studies. Ann Rheum Dis 2012;71:1524–9. [DOI] [PubMed] [Google Scholar]
- [14].Pujades-Rodriguez M, Duyx B, Thomas SL, et al. Rheumatoid arthritis and incidence of twelve initial presentations of cardiovascular disease: a population record-linkage cohort study in England. PLoS One 2016;11:e0151245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kjeldsen SE. Hypertension and cardiovascular risk: general aspects. Pharmacol Res 2018;129:95–9. [DOI] [PubMed] [Google Scholar]
- [16].Smolen JS, Landewé R, Bijlsma J, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis 2017;76:960–77. [DOI] [PubMed] [Google Scholar]
- [17].Kim KI, Lee JH, Chang HJ, et al. Association between blood pressure variability and inflammatory marker in hypertensive patients. Circ J 2008;72:293–8. [DOI] [PubMed] [Google Scholar]
- [18].Bautista LE, Vera LM, Arenas IA, et al. Independent association between inflammatory markers (C-reactive protein, interleukin-6, and TNF-α) and essential hypertension. J Hum Hypertens 2005;19:149–54. [DOI] [PubMed] [Google Scholar]
- [19].Sandoo A, Panoulas VF, Toms TE, et al. Anti-TNFα therapy may lead to blood pressure reductions through improved endothelium-dependent microvascular function in patients with rheumatoid arthritis. J Hum Hypertens 2011;25:699–702. [DOI] [PubMed] [Google Scholar]
- [20].Yoshida S, Takeuchi T, Kotani T, et al. Infliximab, a TNF-alpha inhibitor, reduces 24-h ambulatory blood pressure in rheumatoid arthritis patients. J Hum Hypertens 2014;28:165–9. [DOI] [PubMed] [Google Scholar]
- [21].Desai RJ, Solomon DH, Schneeweiss S, et al. Tumor necrosis factor-α inhibitor use and the risk of incident hypertension in patients with rheumatoid arthritis. Epidemiology 2016;27:414–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Smolen JS, Beaulieu A, Rubbert-Roth A, et al. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomized trial. Lancet 2008;371:987–97. [DOI] [PubMed] [Google Scholar]
- [23].Jones G, Sebba A, Gu J, et al. Comparison of tocilizumab monotherapy versus methotrexate monotherapy in patients with moderate to severe rheumatoid arthritis: the AMBITION study. Ann Rheum Dis 2010;69:88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rao VU, Pavlov A, Klearman M, et al. An evaluation of risk factors for major adverse cardiovascular events during tocilizumab therapy. Arthritis Rheumatol 2015;67:372–80. [DOI] [PubMed] [Google Scholar]
- [25].Benucci M, Manfredi M, Saviola G, et al. Changes in atherosclerosis markers during tocilizumab treatment in rheumatoid arthritis: preliminary results. Clin Exp Rheumatol 2013;31:322–3. [PubMed] [Google Scholar]
- [26].Kim SC, Solomon DH, Rogers JR, et al. No difference in cardiovascular risk of tocilizumab versus abatacept for rheumatoid arthritis: a multi-database cohort study. Semin Arthritis Rheum 2018;48:399–405. [DOI] [PubMed] [Google Scholar]
- [27].Xie F, Yun H, Levitan EB, et al. Tocilizumab and the risk for cardiovascular disease: a direct comparison among biologic disease-modifying antirheumatic drugs for rheumatoid arthritis patients. Arthritis Care Res (Hoboken) 2019;71:1004–18. [DOI] [PubMed] [Google Scholar]
- [28].Kang EH, Jin Y, Brill G, et al. Comparative cardiovascular risk of abatacept and tumor necrosis factor inhibitors in patients with rheumatoid arthritis with and without diabetes mellitus: a multidatabase cohort study. J Am Heart Assoc 2018;7:e007393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ursini F, Russo E, Letizia Hribal M, et al. Abatacept improves whole-body insulin sensitivity in rheumatoid arthritis: an observational study. Medicine (Baltimore) 2015;94:e888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Mathieu S, Couderc M, Glace B, et al. Effects of 6 months of abatacept treatment on aortic stiffness in patients with rheumatoid arthritis. Biologics 2013;7:259–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ewing MM, Karper JC, Abdul S, et al. T-cell co-stimulation by CD28-CD80/86 and its negative regulator CTLA-4 strongly influence accelerated atherosclerosis development. Int J Cardiol 2013;168:1965–74. [DOI] [PubMed] [Google Scholar]
- [32].Roubille C, Richer V, Starnino T, et al. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: a systematic review and meta-analysis. Ann Rheum Dis 2015;74:480–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Mangoni AA, Baghdadi LR, Shanahan EM, et al. Methotrexate, blood pressure and markers of arterial function in patients with rheumatoid arthritis: a repeated cross-sectional study. Ther Adv Musculoskelet Dis 2017;9:213–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Navarro-Millán I, Yang S, DuVall SL, et al. Association of hyperlipidaemia, inflammation and serological status and coronary heart disease among patients with rheumatoid arthritis: data from the National Veterans Health Administration. Ann Rheum Dis 2016;75:341–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Majka DS, Vu TT, Pope RM, et al. Association of rheumatoid factors with subclinical and clinical atherosclerosis in African American women: the multiethnic study of atherosclerosis. Arthritis Care Res (Hoboken) 2017;69:166–74. [DOI] [PubMed] [Google Scholar]
- [36].Montes A, Corrales A, Calaza M, et al. Brief report: lack of replication of an association between anti-citrullinated fibrinogen and subclinical atherosclerosis in patients with rheumatoid arthritis. Arthritis Rheumatol 2015;67:2861–5. [DOI] [PubMed] [Google Scholar]
- [37].Jeong H, Baek SY, Kim SW, et al. Comorbidities of rheumatoid arthritis: results from the Korean National Health and Nutrition Examination Survey. PLoS One 2017;12:e0176260. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


