Abstract
Background:
Type 2 diabetes (T2D) is a risk factor for cognitive dysfunction. The relationship between metformin therapy and cognitive function in patients with T2D is unknown. Therefore, we determined the relationship between metformin therapy and cognitive function in patients with T2D using a meta-analysis.
Methods:
We systematically searched the Cochrane library, PubMed, and Embase to identify studies showing correlations, and we calculated hazard ratios (HRs).
Results:
We identified 10 studies including 254,679 participants. Metformin significantly reduced the occurrence of cognitive dysfunction in patients with T2D (HR 0.90; 95% CI [0.88, 0.92]). Compared with other hypoglycemic drugs, sulfonylureas also improved cognitive dysfunction (HR 0.92; 95% CI [0.88, 0.95]). Thiazolidinediones gave no statistically significant improvement in cognitive dysfunction (HR 0.97; 95% CI [0.87, 1.07]). The use of insulin aggravated cognitive dysfunction (HR 1.34; 95% CI [1.24, 1.43]). In the subgroup analysis of various regions controlling for age, gender, education, diabetes course, complications, metformin administration and dosage, and follow-up time, metformin significantly improved cognitive dysfunction in patients in the Americas and Europe (HR 0.69; 95% CI [0.63, 0.74]), (HR 0.71; 95% CI [0.66, 0.76], respectively), while metformin did not significantly improve cognitive dysfunction in Asian patients (HR 0.99; 95% CI [0.96, 1.01]).
Conclusions:
Metformin significantly improved cognitive dysfunction in patients with T2D. Sulfonylureas also improved cognitive dysfunction. Thiazolidinediones had no significant effect on cognitive dysfunction. The use of insulin aggravated cognitive dysfunction. Metformin improved cognitive dysfunction more significantly in patients in the Americas and Europe than in Asia.
Keywords: cognitive dysfunction, meta-analysis, metformin, type 2 diabetes
1. Introduction
Type 2 diabetes (T2D) is a risk factor for cognitive dysfunction, including cognitive decline, mild cognitive impairment, and dementia.[1,2] Cognitive dysfunction is defined as the development of several cognitive deficits that cause severe social and occupational dysfunction, representing significant declines from previous functional levels.[3] Several investigators reported cognitive dysfunction in patients with T2D. A strong relationship between the two conditions has been demonstrated.[4] As many as 60% of patients with T2D suffer from cognitive dysfunction.[5,6]
In the previous studies, the relationship between metformin and cognitive dysfunction in patients with T2D is controversial. Several studies found that metformin improved cognitive abilities[7,8] and neurons survival rate.[9–12] The mechanism include activating the mTOR pathway and tau hyperphosphorylation, which thereby inhibits hepatic gluconeogenesis, reduces insulin resistance, and increases insulin sensitivity, while inhibiting the inflammatory status.[13]However, other studies have suggested that metformin in patients with T2D may increase the risk of cognitive dysfunction.[14,15] Therefore, we performed a meta-analysis aimed to evaluate the relationship between the use of metformin and cognitive outcomes in patients with T2D.
2. Materials and methods
2.1. Ethical approval
This study was performed based on guidelines governing the creation of meta-analyses of observational and epidemiological studies.[16] No ethics committee approval was required, because only published research data were analyzed.
2.2. Literature retrieval and research selection
Two investigators (Qing-Qing Zhang and Wen-Shan Li) independently searched the Cochrane Library, PubMed, and Embase for all studies that reported associations between metformin and cognitive dysfunction in patients with T2D (up to May 31, 2019). Three groups of keywords contain the Boolean operator “AND.” Within the groups employing the Boolean operator “OR,” no language restriction was applied. To ensure a comprehensive search of the literature, we also reviewed the full bibliography of relevant publications, as well as the relevant reviews. When the required data were not clear or missing, we contacted the author.
Inclusion criteria were as follows:
-
1.
all patients with T2D ≥18 years old, without a history of cognitive dysfunction;
-
2.
Interventions included metformin;
-
3.
the endpoint was cognitive dysfunction;
-
4.
the studies were randomized controlled studies, or observational (prospective or retrospective cohort) studies. We included detailed meeting summary information. For studies using identical populations, the study with the longest follow-up or the largest number of patients was selected;
-
5.
when the studies were conducted by the same author, different subjects were chosen.
The exclusion criteria were as follows:
-
1.
other types of diabetes or patients with T2D and a history of cognitive dysfunction;
-
2.
case–control, cross-sectional studies, case reports, case series, and letters;
-
3.
Hazard ratio (HR) data after metformin use could not be obtained.
2.3. Data extraction and quality assessment
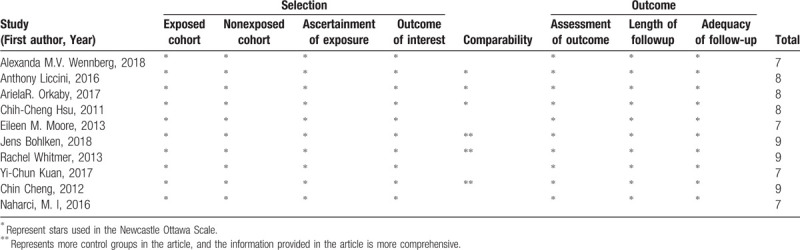
The abstractors independently screened titles and abstracts of the studies and reviewed the full texts of the selected titles and abstracts based on the selection criteria. For each study, the following data were recorded: first author, year of publication, geographical location, study design (observational cohort or randomized controlled study), participants (gender, age, sample size, and history of cognitive impairment), years of follow-up, presence or absence of diabetic complications, and maximum adjusted covariates and hazard risks (HRs) with 95% confidence intervals (CIs). If there was an adjustment of HRs, the most fully adjusted HRs would be extracted. Any disagreements or discrepancies were resolved through consensus. HR was a common indicator in this study, and relative risk (RR) was considered to be equal to HR. All results were expressed as HRs. Often, the original author was contacted to discuss any ambiguity or missing information. Newcastle–Ottawa Scale (NOS) items were employed to evaluate the quality of the article.[17] NOS score ≥6 stars was defined as high quality, and NOS score <6 stars was defined as low quality.
2.4. Statistical analysis
The HR was a common indicator in the studies. Forest plots were drawn to evaluate HRs and the corresponding 95% CIs. HR heterogeneity was evaluated using Cochrane Q statistics(P < .1 was considered statistical heterogeneity), and I2 statistics (25%, 50%, and 75% were considered to represent low, medium, and high heterogeneity, respectively).[18,19] To provide a more conservative estimation of the pooled HRs, random rather than fixed effect models were adopted, because the former can explain more about heterogeneity between studies. When heterogeneity was high, subgroup analysis and sensitivity analysis were conducted to explore the sources of heterogeneity. The Beggs test[20] was used to evaluate potential publication bias. P < .05 indicated statistical significance. All statistical analyses were carried out using Stata version 12.0.
3. Results
3.1. Data selection
There were 545 studies identified initially in the Cochrane Library (n = 7), PubMed (n = 26), and Embase (n = 512) (Fig. 1). Twelve articles were retrieved manually, none of which met the inclusion criteria. A total of 510 studies were excluded from 545 studies based on title or abstract, including 17 duplicate studies and eight case reports. Full texts of 35 eligible studies were subsequently reviewed, and 21 lacked the HRs indicators required for this study and were excluded. For the remaining 14 studies, we conducted a more detailed review, including four studies that were excluded for the following reasons:
Figure 1.
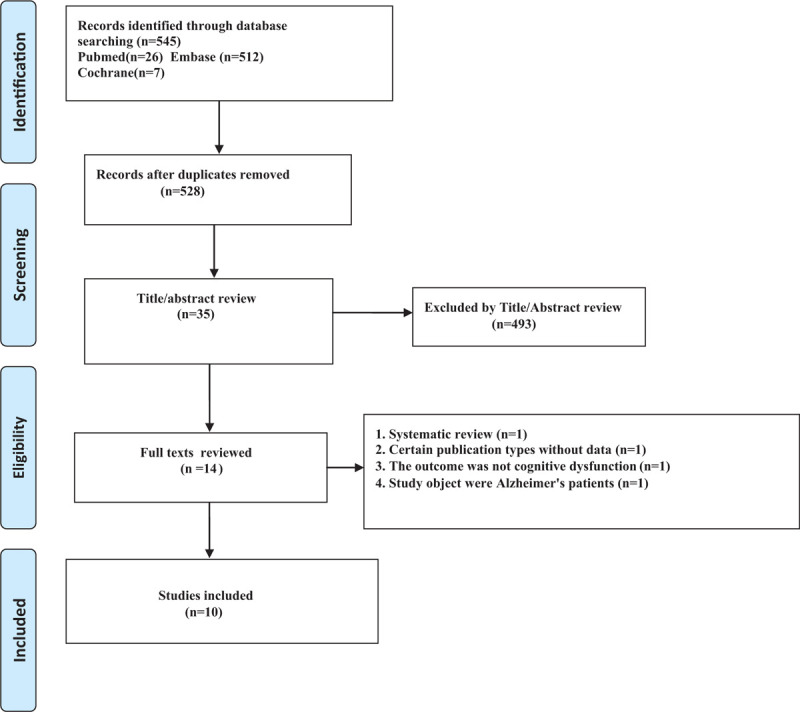
Flow chart of search result.
-
1.
the publication type (one was a systematic review);
-
2.
the study object (one was Alzheimer's patients);
-
3.
the outcome that was not cognitive dysfunction (one was associated with hypoglycemia causing cognitive dysfunction); and
-
4.
missing data (one was unable to provide raw data).
Finally, ten studies (nine retrospective cohort studies and one prospective cohort study) were included in this meta-analysis, including 254,679 participants.
Table 1 provides detailed characteristics of the study. In the 10 included studies, the effects of various types of hypoglycemic agents on cognitive dysfunction in patients with T2D and the effects of metformin on cognitive dysfunction in patients with T2D in various regions were compared. Table 2 shows that the quality of the included reports was acceptable, as all studies scored no <6 stars on NOS.
Table 1.
Detailed characteristics of the 10 studies included in this meta-analysis.
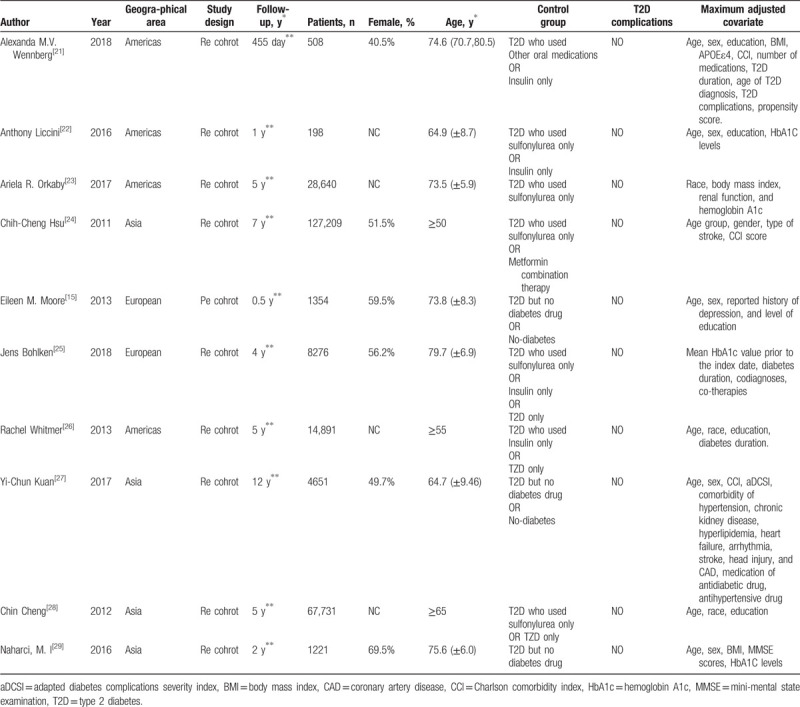
Table 2.
Quality assessment of the 10 cohort included studies.
3.2. Meta-analysis
For 10 articles including 254,679 patients, the forest plot showed that metformin significantly improved cognitive dysfunction in patients with T2D (HR 0.90, 95% CI [0.88, 0.92]) (Fig. 2). I2 = 95.3%, with high heterogeneity, possibly related to the small sample size. Sensitivity analysis and subgroup analysis were conducted.
Figure 2.
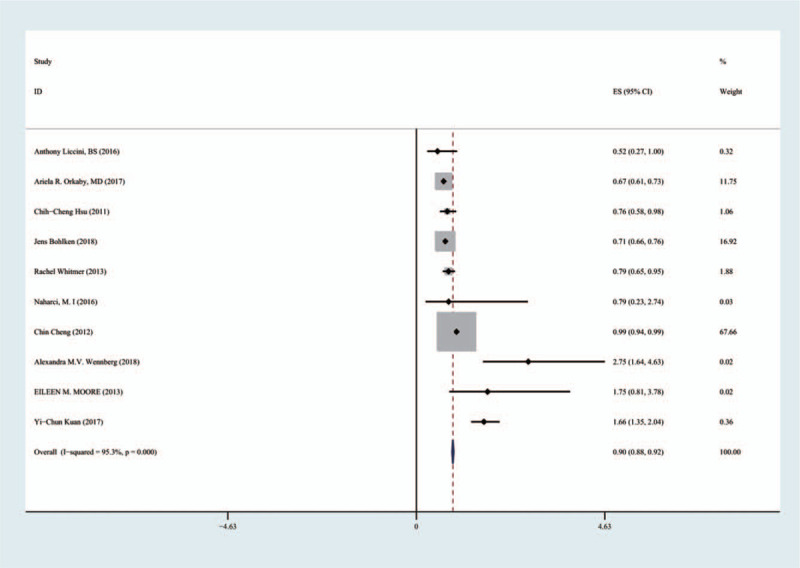
Effects of metformin on cognitive dysfunction in patients with type 2 diabetes.
3.3. Sensitivity analysis
After excluding each study one by one, the remaining results were combined to measure the extent of changes in the combined results, so as to conduct sensitivity analysis of the research results.
Sensitivity analysis results showed that the combined results of metformin on the relationship of cognitive dysfunction in T2D patients were highly stable (Fig. 3). After excluding each study one by one, no significant effect was found on the combined results.
Figure 3.
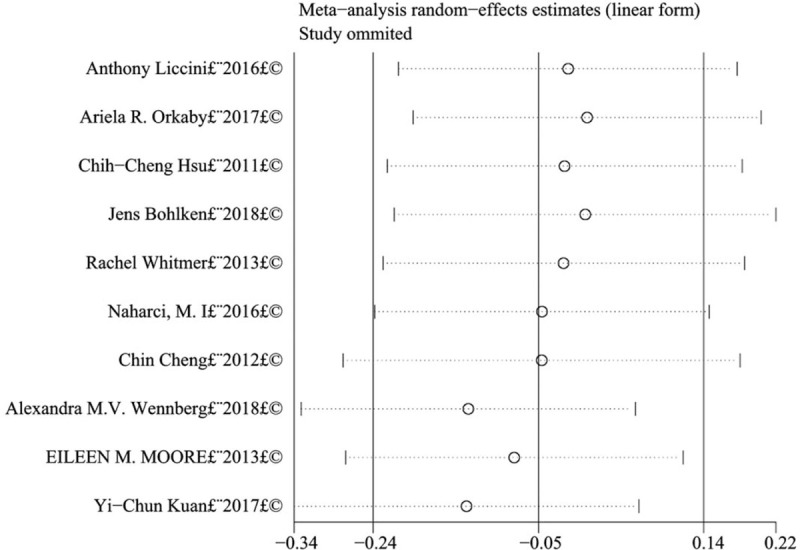
Sensitivity analysis of the effects of metformin on cognitive dysfunction in patients with type 2 diabetes.
3.4. Subgroup analysis
3.4.1. Metformin and other types of hypoglycemic agents
Ten articles included 254,679 patients treated with metformin, three articles included 90,898 patients treated with thiazolidinediones, and six articles included 235,505 patients treated with sulfonylureas. Sulfonylureas improved cognitive impairment (HR 0.92; (95% CI [0.88, 0.95]), metformin improved cognitive dysfunction slightly better than sulfonylureas. Thiazolidinediones had no significant effect on cognitive function (HR 0.97; 95% CI [0.87, 1.07]) (Fig. 4).
Figure 4.
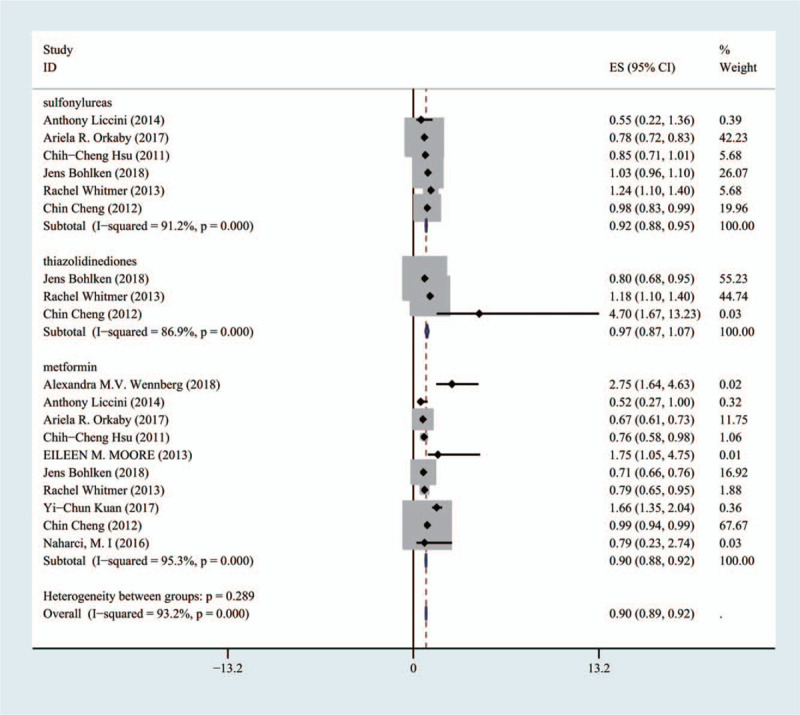
Comparison of the effects of metformin group, thiazolidinediones group and sulfonylureas group on cognitive dysfunction.
3.4.2. Metformin and insulin
In the four articles, including 23,873 patients, the use of insulin aggravated cognitive dysfunction associated with T2D (HR 1.34; 95% CI [1.24, 1.43]). Metformin improved cognitive function (HR 0.72; 95% CI [0.67, 0.76]) (Fig. 5).
Figure 5.
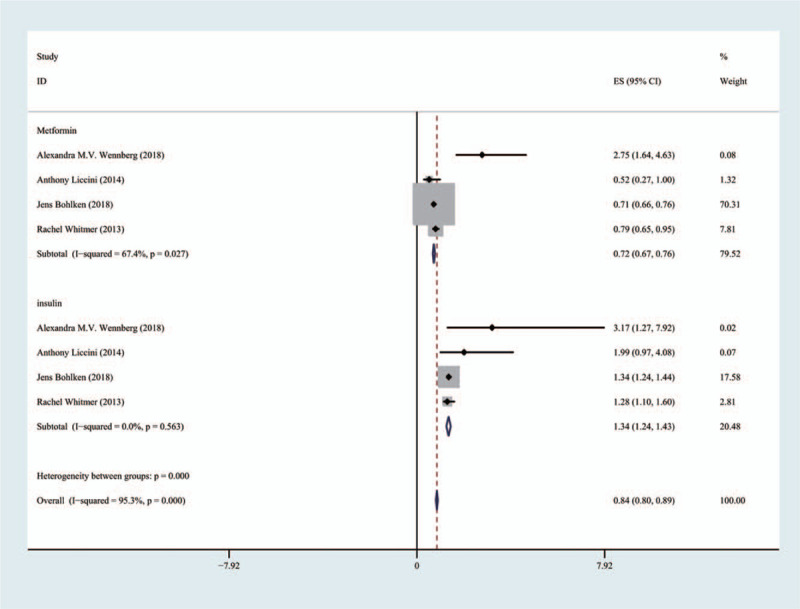
Comparison of the effects of metformin group and insulin group on cognitive dysfunction.
3.4.3. Patients taking metformin in different regions
The 10 articles included 44,237 in the Americas, 200,812 in Asia, and 9630 in Europe. Metformin significantly improved cognitive dysfunction in the patients in the Americas and Europe (HR 0.69;95% CI [0.63, 0.74]), (HR 0.71;95% CI [0.66, 0.76]), while metformin did not significantly improve cognitive dysfunction in Asian patients (HR 0.99;95% CI [0.96, 1.01]) (Fig. 6).
Figure 6.
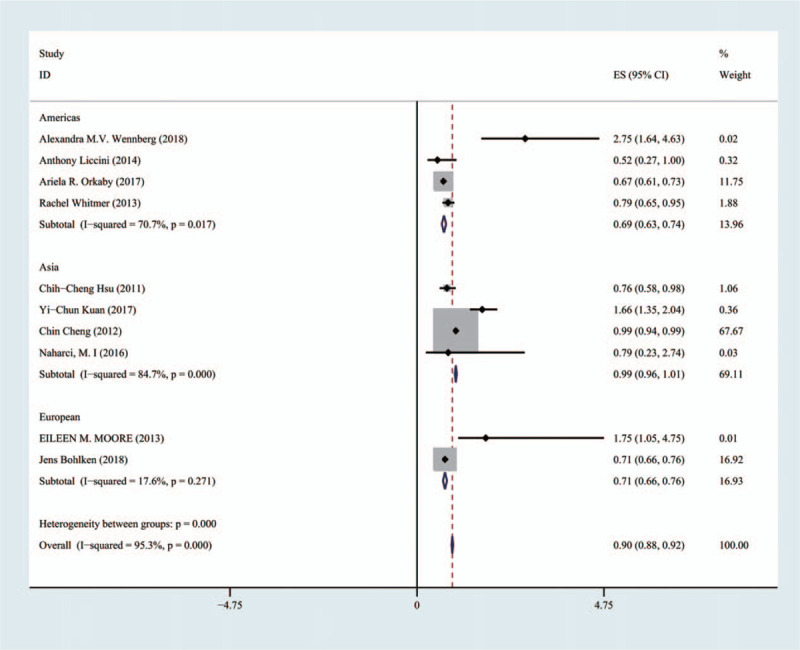
Comparison of the effects of patients taking metformin in different regions on cognitive dysfunction.
3.5. Publication bias
To check whether there was publication deviation in the included studies, the Begg test funnel graph was applied. In the analysis of the relationship between metformin and cognitive dysfunction in patients with T2D, no significant deviation was observed, and the distribution of funnel plot was approximately symmetrical (Fig. 7).
Figure 7.
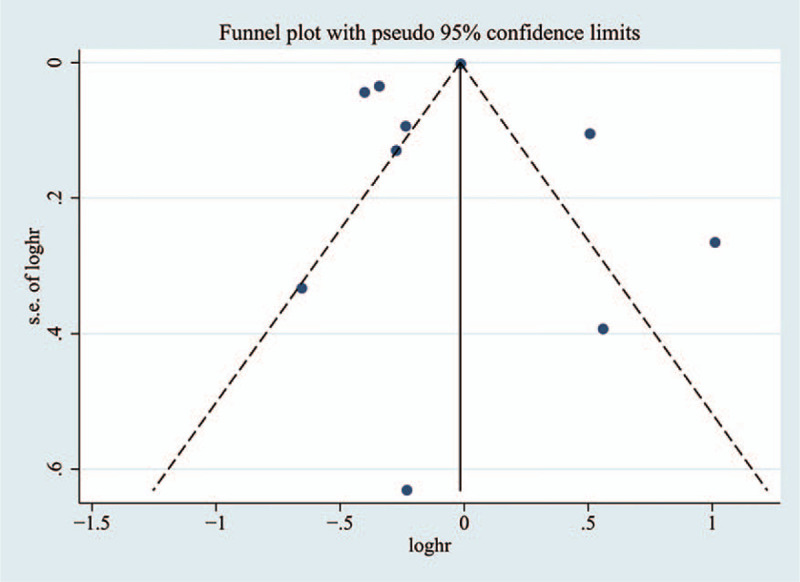
Funnel graph of the effects of metformin on cognitive dysfunction in patients with type 2 diabetes.
4. Discussion
Type 2 diabetes is associated with cognitive dysfunction and the development of mild cognitive impairment. The mechanisms of this effect include inflammation, oxidative stress, vascular reaction (affecting the circulation of blood to the brain), increased cerebral β-amyloid peptide, cerebral insulin resistance, hyperinsulinemia and the formation of advanced glycosylation end products.[30–32] Scholars believe that several of these factors combine to cause cognitive dysfunction. Nevertheless, whether metformin reduces the incidence of cognitive dysfunction in patients with T2D remains controversial.
In our meta-analysis, we found that metformin reduced cognitive dysfunction in patients with T2D. Previous studies have also indicated that metformin may decrease the risk of developing cognitive dysfunction.[33] Similarly, a separate study in an Australian population showed that metformin alone predicted a reduction in the risk for cognitive dysfunction incidence in T2D.[14] There are several possible mechanisms explaining this effect. Studies have shown that metformin reduces insulin levels,[34] improves inflammation and reduces thrombosis,[35] reducing the risk of metabolic syndrome.[37] It also improves insulin sensitivity,[36] which has a potential protective effect on cognitive dysfunction. The most obvious mechanism by which metformin affects the development of cognitive dysfunction in patients with T2D is by preventing hyperinsulinemia that may lead to the formation of amyloid plaques in the brain and the onset of cognitive dysfunction.[37]
Among the various hypoglycemic agents, this meta-analysis suggested that, compared with metformin, sulfonylureas improved cognitive impairment; however, metformin improved cognitive dysfunction slightly better than sulfonylureas. Orkaby et al reported that metformin had more neuroprotective effects than sulfonylureas,[23] consistent with the results of the present study. One study reported that thiazolidinediones may improve cognitive performance.[38] Nevertheless, its potential cardiovascular adverse effects increase the risk of vascular cognitive dysfunction and Alzheimer's disease. We found that thiazolidinedione hypoglycemic agents had no significantly greater effect than metformin in terms of improving cognitive function,[39,40] possibly because their adverse cardiovascular effects outweighed the potential benefits. This meta-analysis suggested that the use of insulin increased the risk of cognitive dysfunction in patients with T2D. Bohlken's case–control study also showed that the use of insulin was a risk factor for exacerbation of cognitive dysfunction.[25]
Controlling for age, gender, education, diabetes course, complications, metformin administration and dosage, and follow-up time, metformin significantly improved cognitive dysfunction in patients in the Americas and Europe, while there was no significant effect in Asians. We speculate that this discrepancy may be attributable to varying lifestyles, eating habits, economic differences and education levels. There are no studies on the effect of metformin on cognitive dysfunction in patients with T2D in different regions.
We conclude that metformin therapy improves cognitive dysfunction in patients with T2D. It has potential to prevent cognitive dysfunction.
The following points support the stability of our conclusions. First, we conducted a comprehensive search of literature outside language constraints, requesting additional data from authors in cases of ambiguity. Second, data extraction was done by two independent investigators, and disputes were resolved via consensus. Third, we performed sensitivity analyses to assess the stability of the results. Fourth, to the best of our knowledge, this was the first study to analyze the relationship between metformin and cognitive impairment in patients with T2D in a large population. Fifth, this study included comparisons of metformin and insulin, as well as the comparison of metformin among people in various geographical regions, increasing the robustness of the conclusion. Sixth, to make the results more stable, the random model was adopted. Finally, objective criteria were used to evaluate the quality of the study. Our study scores were at least 6 (high quality).
5. Limitations
There are several potential limitations in our study. First, in patients with T2D, the relationship between metformin and cognitive function prognosis can change with age and duration of diabetes mellitus. Although the HRs collected were the result of multivariate adjustment, some confounding factors could not be excluded. Second, there was significant heterogeneity in the study, possibly due to differences in study design, sample analysis, strategy, and participant characteristics. Nevertheless, these factors cannot be carried out in a subgroup analysis based on limited data. Third, this study mainly focused on the effects of various types of hypoglycemic drugs on cognitive impairment; unfortunately, we lacked data on dose, frequency and duration of various types of hypoglycemic drugs, as well as the age and gender of the study subjects. Further studies are needed to address these factors. Finally, the population we studied mainly came from Asia, the Americas and Europe; there was a lack of relevant studies in other regions; therefore, further studies are needed to validate our conclusions.
6. Conclusions
Metformin significantly reduces the incidence of cognitive dysfunction in patients with T2D. Sulfonylureas also improve cognitive dysfunction, while thiazolidinediones had no significant effect. The use of insulin aggravates cognitive dysfunction. Metformin improved cognitive dysfunction more significantly in patients in the Americas and Europe than in patients in Asia. More randomized controlled trials are essential to validate and support this conclusion.
Author contributions
Conceptualization: Qing-Qing Zhang.
Formal analysis: Qing-Qing Zhang, Wen-Shan Li, Zhou Liu.
Investigation: Qing-Qing Zhang, Wen-Shan Li.
Methodology: Qing-Qing Zhang, Zhou Liu.
Project administration: Qing-Qing Zhang.
Resources: Rui-Xia Zhang.
Software: Qing-Qing Zhang, Wen-Shan Li, Zhou Liu.
Supervision: Rui-Xia Zhang, Hui-Li Zhang, Ying-Gui Ba
Validation: Rui-Xia Zhang, Qing-Qing Zhang
Writing – original draft: Qing-Qing Zhang, Wen-Shan Li.
Writing – review & editing: Rui-Xia Zhang.
Footnotes
Abbreviations: CIs = confidence intervals, HR = hazard ratio, NOS = newcastle–ottawa scale, RR = relative risk, T2D = type 2 diabetes.
How to cite this article: Zhang QQ, Li WS, Liu Z, Zhang HL, Ba YG, Zhang RX. Metformin therapy and cognitive dysfunction in patients with type 2 diabetes: A meta-analysis and systematic review. Medicine. 2020;99:10(e19378).
QQZ, WSLi, and ZL have contributed equally to this work.
Basic research program of Qinghai Science and Technology Department (2019-zj-7079).
General guiding topic of Qinghai health and family planning commission (2018-wjzdx-114).
The authors have no conflicts of interest to disclose.
References
- [1].Roberts RO, Knopman DS, Geda YE, et al. Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Arch Neurol 2004;61:661–6. [DOI] [PubMed] [Google Scholar]
- [2].Roberts RO, Knopman DS, Geda YE, et al. Association of diabetes with amnestic and nonamnestic mild cognitive impairment. Alzheimers Dement 2014;10:18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Mckhann G. Diagnostic and Statistical Manual of Mental Disorders International Journal of Offender Therapy & Comparative Criminology 2000;189:39–44. [Google Scholar]
- [4].Gaspar JM, Baptista FI, Macedo MP, et al. Inside the diabetic brain: role of different players involved in cognitive decline. ACS Chem Neurosci 2016;7:131–42. [DOI] [PubMed] [Google Scholar]
- [5].Chatterjee S, Peters SA, Woodward M, et al. Type 2 diabetes as a risk factor for dementia in women compared with men: a pooled analysis of 2.3 million people comprising more than 100,000 cases of dementia. Diabetes Care 2016;39:300–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Li W, Huang E. An update on type 2 diabetes mellitus as a risk factor for dementia. J Alzheimers Dis 2016;53:393–402. [DOI] [PubMed] [Google Scholar]
- [7].Yokoyama H, Ogawa M, Honjo J, et al. Risk factors associated with abnormal cognition in Japanese outpatients with diabetes, hypertension or dyslipidemia. Diabetol Int 2015;6:268–74. [Google Scholar]
- [8].Zhou Y, Fang R, Liu LH, et al. Clinical characteristics for the relationship between type-2 diabetes mellitus and cognitive impairment: a cross-sectional study. Aging Dis 2015;6:236–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ahmed S, Mahmood Z, Javed A, et al. Effect of metformin on adult hippocampal neurogenesis: comparison with donepezil and links to cognition. J Mol Neurosci 2017;62:88–98. [DOI] [PubMed] [Google Scholar]
- [10].El-Mir MY, Detaille D, R-Villanueva G, et al. Neuroprotective role of antidiabetic drug metformin against apoptotic cell death in primary cortical neurons. J Mol Neurosci 2008;34:77–87. [DOI] [PubMed] [Google Scholar]
- [11].Kickstein E, Krauss S, Thornhill P, et al. Biguanide metformin acts on tau phosphorylation via mTOR/protein phosphatase 2A (PP2A) signaling. Proc Natl Acad Sci USA 2010;107:21830–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mielke JG, Taghibiglou C, Wang YT. Endogenous insulin signaling protects cultured neurons from oxygen-glucose deprivation-induced cell death Neuroscience 2006;143:165–73. [DOI] [PubMed] [Google Scholar]
- [13].Rojas LB, Gomes MB. Metformin: an old but still the best treatment for type 2 diabetes. Diabetol Metab Syndr 2013;5:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Imfeld P, Bodmer M, Jick SS, et al. Metformin, other antidiabetic drugs, and risk of Alzheimer's disease: a population-based case-control study. J Am Geriatr Soc 2012;60:916–21. [DOI] [PubMed] [Google Scholar]
- [15].Moore EM, Mander AG, Ames D, et al. Increased risk of cognitive impairment in patients with diabetes is associated with metformin. Diabetes Care 2013;36:2981–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- [17].Wells GA, Shea B, O Connell D, et al. The Newcastle Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses; 2014. [Google Scholar]
- [18].Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- [19].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- [21].Wennberg AMV, Hagen CE, Edwards K, et al. Association of antidiabetic medication use, cognitive decline, and risk of cognitive impairment in older people with type 2 diabetes: Results from the population-based Mayo Clinic Study of Aging. Int J Geriatr Psychiatry 2018;33:1114–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Liccini A, Malmstrom TK, Morley JE. Metformin use and cognitive dysfunction among patients with diabetes mellitus. J Am Med Dir Assoc 2016;17:1063–5. [DOI] [PubMed] [Google Scholar]
- [23].Orkaby AR, Cho K, Cormack J, et al. Metformin vs sulfonylurea use and risk of dementia in US veterans aged ≥65 years with diabetes. Neurology 2017;89:1877–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hsu CC, Wahlqvist ML, Lee MS, et al. Incidence of dementia is increased in type 2 diabetes and reduced by the use of sulfonylureas and metformin. J Alzheimer's Dis 2011;24:485–93. [DOI] [PubMed] [Google Scholar]
- [25].Bohlken J, Jacob L, Kostev K. Association between the use of antihyperglycemic drugs and dementia risk: a case-control study. J Alzheimer's Dis 2018;66:725–32. [DOI] [PubMed] [Google Scholar]
- [26].Whitmer R, Quesenberry C, Allison J, et al. Anti-hyperglycemic therapy and risk of dementia: a new user cohort study. Alzheimer's Dementia 2013;9:136. [Google Scholar]
- [27].Kuan YC, Huang KW, Lin CL, et al. Effects of metformin exposure on neurodegenerative diseases in elderly patients with type 2 diabetes mellitus. Prog Neuro-Psychopharmacol Biol Psychiatry 2017;79:77–83. [DOI] [PubMed] [Google Scholar]
- [28].Cheng C. Anti-diabetic medications in relation to dementia. Abstracts of Young Psychiatrists Sessions. Asia-Pacific Psychiatry 2012;4:63–82. [Google Scholar]
- [29].Naharci MI, Cintosun U, et al. Association of metformin therapy with the risk of dementia in older adults with type 2 diabetes mellitus. Eur Geriatr Med 2016;40:62–3. [Google Scholar]
- [30].Ahtiluoto S, Polvikoski, Peltonen M, et al. Diabetes, Alzheimer disease, and vascular dementia: a population-based neuropathologic study. Neurology 2010;75:1195–202. [DOI] [PubMed] [Google Scholar]
- [31].Craft S. Insulin resistance and Alzheimer s disease pathogenesis: potential mechanisms and implications for treatment. Curr Alzheimer Res 2007;4:147–52. [DOI] [PubMed] [Google Scholar]
- [32].Haus JM, Kashyap SR, Kasumov T, et al. Plasma ceramides are elevated in obese subjects with type2 diabetes and correlate with these verity of insulin resistance. Diabetes 2009;58:337–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ng TP, Feng L, Yap KB, et al. Long-term metformin usage and cognitive function among older adults with diabetes. J Alzheimers Dis 2014;41:61–8. [DOI] [PubMed] [Google Scholar]
- [34].Kitabchi AE, Temprosa M, Knowler WC, et al. Role of insulin secretion and sensitivity in the evolution of type 2 diabetes in the diabetes prevention program: effects of lifestyle intervention and metformin. Diabetes 2005;54:2404–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Haffner S, Temprosa M, Crandall J, et al. Intensive lifestyle intervention or metformin on inflammation and coagulation in participants with impaired glucose tolerance. Diabetes 2005;54:1566–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Orchard TJ, Temprosa M, Goldberg R, et al. The effect of metformin and intensive lifestyle intervention on the metabolic syndrome: the Diabetes Prevention Program randomized trial. Ann Intern Med 2005;142:611–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Qiu WQ, Folstein MF. Insulin, insulin-degrading enzyme and amyloid-beta peptide in Alzheimer's disease: review and hypothesis. Neurobiol Aging 2006;27:190–8. [DOI] [PubMed] [Google Scholar]
- [38].Watson GS, Cholerton BA, Reger MA, et al. Preserved cognition in patients with early Alzheimer disease and amnestic mild cognitive impairment during treatment with rosiglitazone: a preliminary study. Am J Geriatr Psychiatry 2005;13:950–8. [DOI] [PubMed] [Google Scholar]
- [39].Risner ME, Saunders AM, Altman JF, et al. Efficacy of rosiglitazone in a genetically defined population with mild-to-moderate Alzheimer's disease. Pharmacogenomics J 2006;6:246–54. [DOI] [PubMed] [Google Scholar]
- [40].Tzimopoulou S, Cunningham VJ, Nichols TE, et al. A multi-center randomized proof-of-concept clinical trial applying FDG-PET for evaluation of metabolic therapy with rosiglitazone XR in mild to moderate Alzheimer's disease. J Alzheimers Dis 2010;22:1241–56. [DOI] [PubMed] [Google Scholar]


