Abstract
Interleukin-10 (IL-10), a cytokine with anti-inflammatory effects, is produced by renal parenchymal cells and bone marrow derived cells. Both endogenous and exogenous IL-10 are protective in cisplatin-induced acute kidney injury. However, the source of endogenous IL-10 in cisplatin-induced nephrotoxicity is not clear. Bone marrow chimera experiments in IL10-KO mice indicated that bone marrow derived cells were the primary source of IL-10 in cisplatin nephrotoxicity. Cell specific deletion of IL-10 in T regulatory cells and dendritic cells was accomplished using Foxp3 and CD11c driven cre recombination in IL10flox/flox mice, respectively. Upon treatment with cisplatin, both the IL10flox/flox and the Foxp3YFP-Cre x IL10flox/flox mice developed similar degrees of kidney injury. However, mice with the dendritic cell deletion of IL-10 showed more severe structural and functional changes in the kidney compared to the IL10flox/flox mice. These results indicate that IL-10 from dendritic cells but not from T regulatory cells offers significant endogenous protection against cisplatin induced nephrotoxicity.
Introduction
Cisplatin is an effective chemotherapeutic agent used for treatment of various solid tumors. Acute kidney injury is a major, and sometimes limiting, toxicity of cisplatin [1]. A large body of evidence indicates the involvement of inflammatory mechanisms in cisplatin-induced nephrotoxicity [2–7]. Various mediators of inflammation including chemokines, cytokines, TLRs and damage associated molecular patterns produced by renal parenchymal cells in response to cisplatin-induced injury [1–7]. These proinflammatory mediators recruit and activate leukocytes from the circulation and further aggravate kidney injury. In addition to proinflammatory molecules, anti-inflammatory pathways are activated in response to injury which inhibit ongoing cell injury and/or facilitate repair after the initial tubular injury [8–10] Previous reports suggest that some cytokines such as IL-4, IL-10, IL-3 and TGFβ may protect against tissue injury but the source of these cytokines and their role in renal injury/repair are poorly defined [11–13].
IL- 10 is an anti-inflammatory cytokine produced by a variety of cells; mainly Th2 cells, dendritic cells, T regulatory (Tregs) cells (CD4+CD25+Foxp3+), macrophages and renal proximal tubule cells [10, 14–16]. IL-10 attenuates the production of proinflammatory cytokines and chemokines [16, 17]. In addition, IL-10 inhibits the activation of immune cells and ameliorates renal injury in several models of kidney disease including lupus nephritis, ischemia reperfusion injury and transplantation [18–21]. We reported that cisplatin enhances renal IL-10 signaling and that endogenous production of IL-10 reduced cisplatin-induced acute kidney injury [9]. However, the source of IL-10 in cisplatin-induced nephrotoxicity is not clear. Among bone marrow derived cells, both Tregs and dendritic cells have been reported to reduce cisplatin nephrotoxicity [8, 10]. Both cells are also known to produce IL-10 [10, 14, 22], suggesting the possibility that IL-10 production by either or both of these cells is an endogenous mechanism to mitigate cisplatin-induced kidney injury.
In this study, we examined the cellular sources of IL-10 and their functional relevance during cisplatin-induced acute kidney injury. First, we performed bone marrow chimera experiments in IL10-KO mice to determine the role of renal parenchymal cells vs bone marrow derived cells in cisplatin-induced nephrotoxicity. Next, to analyze the role of endogenous IL-10 produced by Tregs and dendritic cells, we generated Foxp3YFP-Cre x IL10flox/flox mice, in which IL-10 is selectively inactivated in Foxp3+Treg cells, and CD11c-Cre x IL10flox/flox mice, in which IL-10 is selectively deleted in CD11c+ dendritic cells.
Methods
Mice
All the experiments were performed using 8–10 week old C57BL/6, IL10 knockout, IL10flox/flox; IL10flox/floxCD11c-Cre and IL10flox/flox Foxp3YFP-Cre mice. All mice were on a C57BL/6 background. All procedures were approved by the Institutional Animal Care and Use Committee at University Texas Health Sciences, San Antonio (protocol 20160044AR)
Cisplatin induced acute kidney injury
Acute kidney injury was induced in mice by a single intraperitoneal injection of cisplatin (20 mg/kg body weight). Renal function was determined by measuring BUN and serum creatinine prior to the cisplatin injection and at 24h, 48h and 72h after injection. We used IL10flox/flox injected with cisplatin as a control group and compared with IL10flox/floxCD11c-Cre and IL10flox/flox Foxp3YFP-Cre mice injected with cisplatin. Based on previous studies, we included at least 5 mice in each group for cisplatin induced acute kidney injury experiments. Because of the variability of the litter size, we used different numbers of mice for each experiment. We performed concurrent controls within each experiment and only compare the experimental groups with the corresponding control group. After 72h of cisplatin injection mice were sacrificed by isoflurane anesthesia followed by cervical dislocation.
Chimeric mice
Chimeric mice were created using C57BL/6 mice and mice with a global deletion of IL-10 as either bone marrow donors or recipients. Donor mice were killed with sodium pentobarbital and the femurs removed and flushed with RPMI medium containing 10% fetal calf serum to isolate bone marrow cells. Unfractionated bone marrow cells were washed and re-suspended in PBS at a concentration of 20 million cells/ml. Recipient C57BL/6 mice and IL-10 knockout mice were lethally irradiated using Gammacell irradiator (two doses of 600 rads, 4h apart). 8h after irradiation, 10 million-donor bone marrow cells were injected into the lateral tail vein of recipients. Three sets of chimeric mice were created: WT-WT (wild type donor and recipients; a control for the transplantation procedure) WT-KO (wild type donor and IL-10 knockout recipient; these mice express IL-10 only in the cells of bone marrow origin); KO-WT (IL10 knockout donor and wild type recipient, these mice express IL-10 in all tissue except cells of bone marrow origin). Mice were maintained in specific pathogen free conditions and were used around 8 weeks after the bone marrow transplantation. We used WT-WT chimeric mice as a control group.
Histology
Kidneys were fixed in 10% neutral-buffered formalin overnight, dehydrated, and embedded in paraffin. Tubular injury was assessed in Periodic acid-Schiff (PAS)-stained sections. The number of injured tubules (cast formation, sloughing of epithelial cells, apoptotic nuclei) were counted blindly in ten randomly selected 40X fields for each kidney and expressed as a percentage of total tubule profiles.
Western blotting
Kidneys homogenized in lysis buffer were separated on Nupage SDS-PAGE and then transferred to polyvinyllidene difluoride membranes. After blocking, membranes were incubated with rabbit anti-caspase 3 antibody (Cell Signaling) followed by HRP conjugated goat anti-rabbit antibody. Next, membranes were washed and proteins on the membrane were detected using enhanced chemiluminescence detection reagent.
Validation of CD11c-Cre mediated IL-10 deletion in dendritic cells
To confirm the deletion of the IL10 gene in dendritic cells, IL-10 transcripts were examined in CD11c cells purified from the spleens of IL10flox/flox and IL10flox/floxCD11c-Cre mice using the MagniSort CD11c positive selection kit (Thermo Fischer Scientific). Briefly, single cells suspensions were prepared from the spleen and labeled with biotinylated anti-mouse CD11c antibody. After washing, Magnisort positive selection beads were added to the pellet and incubated at room temperature. The bead-bound dendritic cells were separated with a magnet and used for qRT-PCR to measure the IL10 transcript levels.
Validation of IL10flox/flox Foxp3YFP-Cre mediated IL10 deletion in Tregs
To test the deletion of IL10 in T reg cells, we prepared single cell suspensions from spleens of Il10flox/flox and IL10flox/flox Foxp3YFP-Cre x mice and isolated CD4+CD25+ Treg cells using the Dynabeads FlowComp kit (Invitrogen Life Technologies). This method uses negative selection of CD4+ T cells, followed by positive selection of CD25+ regulatory T cells. First, an antibody mixture was added to the spleen single cell suspension to bind the non CD4+ T cells. Mouse Depletion Dynabeads were then added and the bound non CD4+ cells were removed with a magnet. The remaining untouched mouse CD4+ T-cells were then labeled with CD25 antibody and positively isolated using Dynabeads. The T reg cells adherent to Dynabeads were separated with a magnet and used for qRT-PCR to measure the IL10 transcript levels.
Statistical analyses
Statistical differences among groups were analyzed using Student’s t-test (two groups), and one-way ANOVA with Tukey's post hoc analysis; p < 0.05 was considered statistically significant. All data are expressed as mean ± SEM.
Results
IL-10 from bone marrow derived cells plays an important role in protection against cisplatin-induced nephrotoxicity
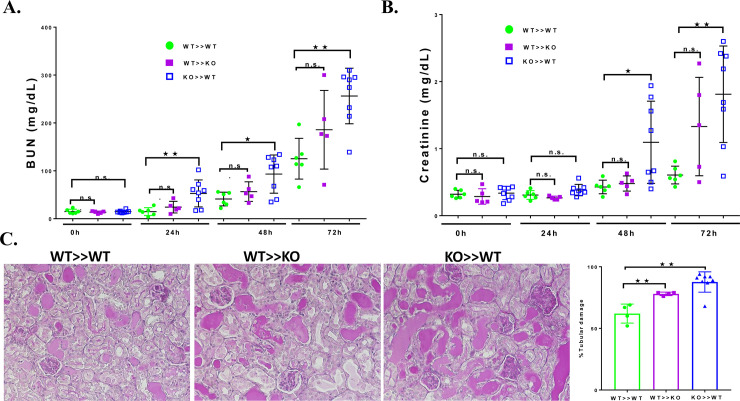
To investigate the source of IL-10 which mediates protection in cisplatin toxicity, we performed bone marrow transplantation to create three sets of chimeric mice; WT to WT; WT- to KO; and KO to WT and tested the differential role of bone marrow cell-derived versus parenchyma-derived IL-10 in cisplatin induced nephrotoxicity. We previously reported that bone marrow transplantation results in a high degree of chimerism and does not alter the distribution of circulating leukocyte subtypes and the ability of circulating immune cells to produce TNFα [5]. Bone marrow chimeric mice were treated with cisplatin (20 mg/kg/body weight) or saline and evaluated for renal dysfunction by measuring serum creatinine and BUN. WT-WT chimeric mice developed significant renal failure at 48 and 72h (Fig 1A&1B). The serum creatinine and BUN values at 72h are similar to what we observed previously in non-transplanted WT mice [3, 4, 8, 9, 23, 24]. These results indicate that bone marrow transplantation does not alter the susceptibility to cisplatin nephrotoxicity. Similarly, WT-KO mice also exhibited increasing BUN and serum creatinine values at 48h and 72h (Fig 1A&1B). Although the BUN and creatinine values were numerically greater than in WT-WT mice, the difference was not statistically significant. However, KO-WT mice had a significantly greater increase in the BUN and serum creatinine values at 72h compared to the other groups. This result indicates that IL10 from bone marrow derived cells is important in ameliorating cisplatin kidney injury. The functional changes in the chimeric mice were also reflected in structural changes in kidney. At 72h after cisplatin treatment, kidneys with a wild type background had obvious tubular injury as evidenced by cast formation, loss of brush border membrane, sloughing of tubular epithelial cells, and dilation of tubules. These changes were more pronounced in the KO-WT mice (Fig 1C).
Fig 1. Cisplatin effect on kidney function and structure in chimeric mice.
BUN (A) and creatinine (B) were measured before and at 24-hour intervals after injection of cisplatin in WT>>WT (n = 6), WT>>KO (n = 5) and KO>>WT (n = 8). C. PAS-stained sections of kidney 72 hours after cisplatin injection. D. Summary of the tubular damage score for each group of chimeric mice. Statistical analysis: WT>>WT was compared with WT>>KO and KO>>WT for each time point using one-way ANOVA with Tukey's post hoc analysis; Mean ± SEM. *p < 0.05, **p < 0.01, ns, not-significant.
T regulatory cell-derived IL-10 does not significantly reduce cisplatin-induced nephrotoxicity
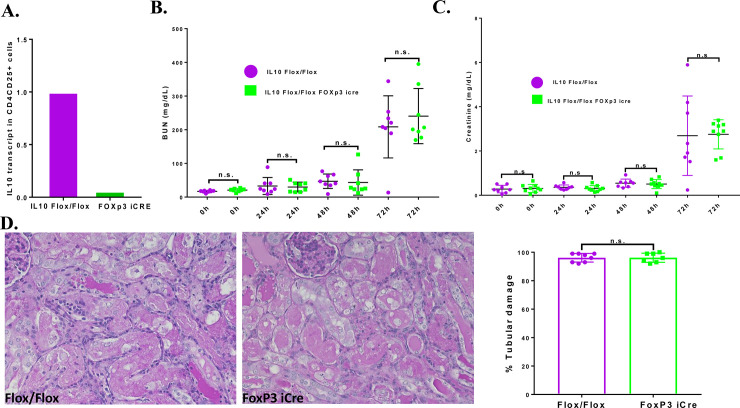
Since the chimera results indicated that bone marrow derived cells were important sources of IL-10 during cisplatin nephrotoxicity, we explored the role of Treg cells by producing a Treg specific IL-10 knockout. First, we validated the deletion of IL10 in CD4+CD25+ cells by qRT-PCR. The results showed that IL-10 transcript levels in CD4+CD25+ cells from Il10flox/flox Foxp3YFP-Cre mice were reduced by 87% compared to the cells from IL10flox/flox mice (Fig 2A). These results indicate effective iCre recombination in CD4+CD25+ Treg cells. IL10flox/flox and IL10flox/flox mice Foxp3YFP-Cre were treated with cisplatin and renal function was assessed by measuring the levels of BUN and serum creatinine. Both the IL10flox/flox mice and the IL10flox/flox Foxp3YFP-Cre mice treated with cisplatin showed minimal increases in the levels of BUN and serum creatinine at 24h; with progressive increases at 48h and 72h (Fig 2B&2C). There was no significant difference in the serum creatinine and BUN levels between the two genotypes. IL10flox/flox and Foxp3YFP-Cre mice treated with saline also exhibited comparable levels of BUN and serum creatinine. Structurally, IL10flox/flox mice sacrificed 72h after cisplatin treatment showed severe tubular injury as characterized by cast formation, loss of brush border membranes, sloughing of epithelial cells, and dilation of tubules (Fig 2D). IL10flox/flox Foxp3YFP-Cre mice showed similar structural changes as the IL10flox/flox mice. Semi quantification of tubule damage in both groups showed no significance difference. These results suggest that CD4+CD25+ Treg cells are not a major source of IL-10 in offering protection against cisplatin-induced nephrotoxicity.
Fig 2. Effect of IL-10 depletion in Treg cells on cisplatin nephrotoxicity: A. Validation of Foxp3icre mediated IL-10 deletion in Treg cells.
A. CD4/CD25+ cells isolated from spleens of both IL10flox/flox and IL10flox/flox Foxp3icre mice and IL-10 transcript levels measured by qRT-PCR. B. BUN and creatinine levels in IL10flox/flox (n = 8) and Il10flox/flox Foxp3icre (n = 9) mice injected with cisplatin. C. Histology of kidneys 72 hours after cisplatin injection. D. Summary of the tubular damage score for each group. Statistical analysis: Student’s unpaired t test; Mean ± SEM. *p < 0.05, ns, not-significant.
Dendritic cell derived IL-10 plays a significant role in protection against cisplatin-induced nephrotoxicity
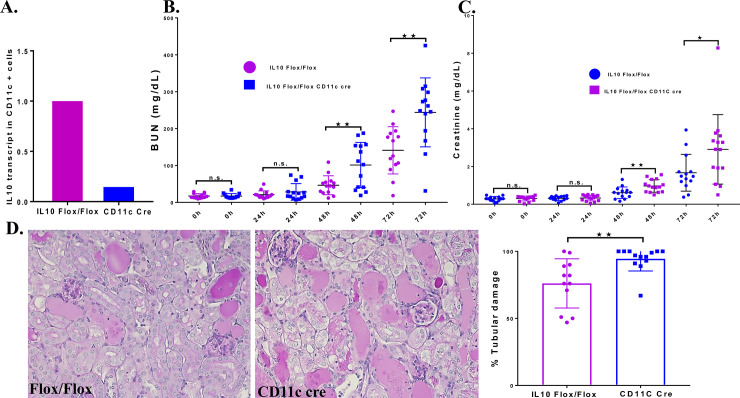
Previously, we reported that dendritic cells, which are the most abundant immune cells in the normal kidney, are protective against cisplatin-induced nephrotoxicity [8]. This conclusion was based on studies performed in CD11c-DTRtg mice in which the expression of the simian diphtheria toxin receptor driven by the CD11c promoter targets dendritic cells for DT mediated cell death. Based on these results, we hypothesized that IL-10 from dendritic cells might offer protection from cisplatin induced nephrotoxicity. To test this, we created CD11cCre xIL10flox/flox mice and confirmed the deletion of l10 in CD11c splenic cells by qRT-PCR. The results showed that CD11c splenic cells isolated from CD11cCre xIl10flox/flox mice had >95% reduction in Il10 transcript levels compared to cells from Il10flox/flox mice (Fig 3A). These results indicate effective CD11c cre recombination of IL10 in dendritic cells. Analysis of renal function revealed that Il10flox/flox mice treated with cisplatin showed minimal increases in the levels of BUN and serum creatinine at 24h, with large increases at 48h and 72h. Of note, CD11cCre xIl10flox/flox mice showed significantly greater BUN and serum creatinine levels at 48h and 72h compared to Il10flox/flox mice (Fig 3B & 3C). Analysis of structural changes in the kidney 72h after the cisplatin injection showed more severe tubular damage in the CD11cCre xIl10flox/flox kidneys compared to Il10flox/flox mice (Fig 3D). These results suggest that CD11c cells are an important source of IL-10 in offering protection against cisplatin-induced nephrotoxicity.
Fig 3. Effect of IL-10 depletion in dendritic cells on cisplatin nephrotoxicity.
: A. Validation of CD11c-Cre mediated Il-10 deletion in dendritic cells. CD11c + cells were isolated from spleens of both Il10flox/flox and CD11cCre xIl10flox/flox and Il-10 transcript levels measured by qRT-PCR. B. BUN and C. creatinine levels in Il10flox/flox (n = 14) and CD11cCre xIl10flox/flox (n = 14) mice injected with cisplatin. D. Histology of kidneys 72 hours after cisplatin injection. Summary of the tubular damage score for each group of mice. Statistical analysis: Student’s unpaired t test;. Mean ± SEM. *p < 0.05, **p < 0.01, ns, not-significant.
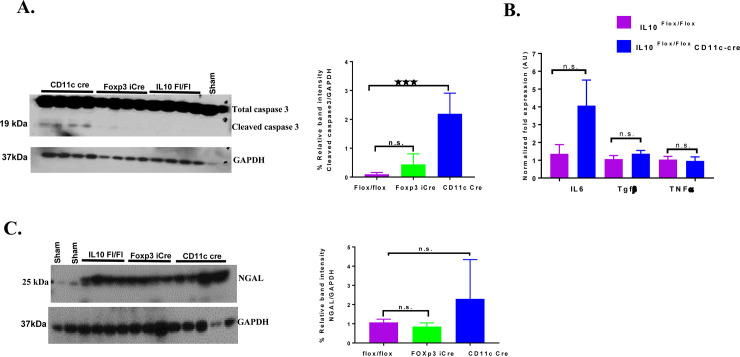
IL-10 depletion in dendritic cells increases apoptosis, inflammatory gene expression and kidney injury markers
Inflammation and tubular cell apoptosis are mechanisms underlying cisplatin induced AKI [3]. We found that deletion of IL-10 in dendritic cells resulted in a significant increase in cleaved caspase 3 in the kidney compared to Foxp3YFP-Crex Il10flox/flox and Il10flox/flox mice (Fig 4A). Cisplatin increases a number of proinflammatory cytokines in the kidney which contribute to renal injury [3]. IL-10 is known to inhibit the production of certain cytokines, chemokines and adhesion receptors [16, 17]. Therefore, we investigated the impact of deletion of Il-10 from dendritic cells on the expression of pro-inflammatory cytokines. Dendritic cell IL-10 knockout mice treated with cisplatin showed a trend towards increased expression of Il6, though not statistically significant, but similar expression of tgfβ, and tnfα in comparison with Il10flox/flox mice treated with cisplatin (Fig 4B). Finally, Dendritic cell IL-10 knockout mice treated with cisplatin showed a trend, though not statistically significant, towards increased levels of NGAL (Neutrophil gelatinase associated lipocalin) when compared with Foxp3YFP-Crex Il10flox/flox and Il10flox/flox mice treated with cisplatin (Fig 4C).
Fig 4. Increased apoptosis and proinflammatory gene expression in CD11cCre xIl10flox/flox mice injected with cisplatin.
A. Cisplatin-induced cleaved of caspase 3 levels measured by western blot. Cleaved capase 3 was measured in kidneys from Il10flox/flox, Foxp3YFP-Crex Il10flox/flox CD11cCre xIl10flox/flox mice, 72 hr after cisplatin injection. B. Cytokine gene expression measured 72h after injection of cisplatin by qRT-PCR. The expression levels normalized to expression of GAPDH and expressed relative to Il10flox/flox mice injected with cisplatin as a control. C. Kidney injury marker NGAL levels. NGAL was measured in kidneys from Il10flox/flox, Foxp3YFP-Crex Il10flox/flox CD11cCre xIl10flox/flox mice 72 hr after cisplatin injection by Western blot. Statistical analysis: one-way ANOVA with Tukey's post hoc analysis comparing flox/flox mice with the other genotypes. Mean ± SEM. *p < 0.05, **p < 0.01, ns, not-significant.
Discussion
In this mouse model of cisplatin induced nephrotoxicity, cisplatin increases IL-10 levels in plasma, renal IL-10 mRNA and IL-10R1 levels and STAT signaling, and this endogenous IL-10 offers protection against AKI [8, 9]. Thus, IL-10 deficient mice exhibit much more severe kidney injury than do wild type mice treated with cisplatin [9]. However, the source of IL-10 which accounts for this renal protection during acute kidney injury is not known. In this study, we demonstrate that IL-10 from bone marrow derived cells is critical for protection against cisplatin-induced nephrotoxicity. More specifically, Il-10 from dendritic cells plays a significant protective role against cisplatin-induced nephrotoxicity, whereas IL-10 from Treg cells is not a major factor. Further, deletion of IL-10 production by dendritic cells is associated with increased cleaved caspase 3 and proinflammatory cytokines.
IL-10 is produced by a broad range of tissues and cells, including immune cells, and glomerular and tubular epithelial cells [14, 15]. Cisplatin induced nephrotoxicity is associated with the influx of bone marrow derived inflammatory cells [8, 9, 23]. To differentiate the contribution of bone marrow derived vs renal parenchymal derived IL10 production, we created bone marrow chimeric mice. These experiments revealed a significant role for bone marrow derived IL-10 in mitigating cisplatin induced nephrotoxicity. We previously used this approach to demonstrate the role of TNFα and TLR4 in cisplatin induced nephrotoxicity [5, 6]. In those studies, we found that TNFα and TLR4 expression by non-leukocytes was key to the development of nephrotoxicity whereas the current studies identified leukocytes as the important source of IL-10. A number of immune cells produce IL-10, including neutrophils, macrophages, B cells, dendritic cells and several T cell subsets, notably T reg cells [14, 22]. Based on the existing literature, we focused our investigation on Treg cells and dendritic cells. Treg cells suppress inflammation and reduce kidney injury in models of both acute and chronic kidney disease [25]. Using adoptive transfer in immune deficient mice, two groups have shown a protective role for Tregs in cisplatin nephrotoxicity [10, 26]. In the most recent study [26] the protection was shown to require TLR9 expression on the Treg cell. However, neither study determined the role of IL-10 production by Tregs in protection. Since Tregs cells are known to produce IL-10 and since both endogenous [9] and exogenous [18] IL-10 reduce cisplatin injury, we examined the possibility that IL-10 produced by Treg cells mediated protection against cisplatin nephrotoxicity. Interestingly, deletion of IL-10 in Treg cells, driven by a Foxp3-specific cre recombinase, did not affect either the functional or the histologic injury induced by cisplatin (Fig 3). We conclude that the protective actions of Treg cells noted by other groups are mediated through other pathways. As a corollary, we conclude that Treg cells are not a major source of endogenous IL-10, which mediates protection from cisplatin injury (Fig 1). In this regard, Foxp3YFP-Cre x Il10flox/flox mice still possess other IL-10+ regulatory immune cells. Dendritic cells are known to produce IL-10 and are the most abundant immune cells in the normal kidney. We had previously shown that CD11c+ dendritic cells (or mononuclear phagocytes) are protective in cisplatin nephrotoxicity [8] and that cisplatin increased IL-10 expression in kidney [9]. Using a cell ablation model, we provided evidence that IL-10 production by dendritic cells mediated a portion of the protection provided by dendritic cells [9]. In the current study, we used a genetic approach to assess the significance of IL-10 production by dendritic cells in cisplatin toxicity. IL-10 was deleted from dendritic cells using a DC-specific (CD11c) cre recombinase. Compared to mice with an intact IL-10 gene, mice with the dendritic cell deletion of IL-10 showed significantly worse kidney function and more severe histologic kidney damage (Fig 3). These results support a significant role for dendritic cell-derived Il-10 in cisplatin-induced nephrotoxicity. These results are in support of our previous studies performed in CD11c-DTRtg mice in which the expression of the simian diphtheria toxin receptor driven by the CD11c promoter was used to target dendritic cells for DT mediated cell death [8, 9]. We note that the distinction between kidney dendritic cells and macrophages is not clear cut as many cells exhibit overlapping expression of classical dendritic cell and macrophage markers such as CD11c, CD11b and F4/80 [8, 27, 28], prompting some investigators to refer to the cells as renal mononuclear macrophages [29]. Accordingly, although we use the term dendritic cell here, we acknowledge that the CD11c-cre may have also deleted IL-10 from cells which may have macrophage features. We also note that the deletion of IL-10 from dendritic cells, while increasing injury, did not result in as severe a phenotype as global IL-10 deletion, in which BUN and creatinine values are dramatically elevated by 24 hours after cisplatin injection [9]. Thus, there may be other sources of IL-10 production which are important in mitigating cisplatin kidney injury. Likewise, the deletion of IL-10 from dendritic cells did not result in as severe a phenotype as did deletion of dendritic cells themselves using the CD11c-DTR [8]. This finding would suggest that dendritic cells may be modulating injury though multiple pathways, some of which do not require IL-10.
In summary, we have demonstrated that the production of IL-10 by bone marrow derived immune cells is important for offering protection from cisplatin-induced nephrotoxicity. Specifically, IL-10 from dendritic cells, but not from Treg cells, plays a significant role in reducing cisplatin-induced nephrotoxicity. Further work is required to elucidate the targets of IL-10 in cisplatin nephrotoxicity and other potential sites of IL-10 production.
Supporting information
(PDF)
(DOCX)
(DOCX)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was supported in part by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (DK-081876 and DK-108185) to W.B.R. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. There was no additional external funding received for this study.
References
- 1.Miller RP, Tadagavadi RK, Ramesh G, Reeves WB. Mechanisms of Cisplatin Nephrotoxicity. Toxins. 2010;2(11):2490–518. 10.3390/toxins2112490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramesh G, Kimball SR, Jefferson LS, Reeves WB. Endotoxin and cisplatin synergistically stimulate TNF-{alpha} production by renal epithelial cells. Am J Physiol Renal Physiol. 2007;292:F812–F9. 10.1152/ajprenal.00277.2006 [DOI] [PubMed] [Google Scholar]
- 3.Ramesh G, Reeves WB. TNF-a mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. J Clin Invest. 2002;110:835–42. 10.1172/JCI15606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramesh G, Reeves WB. TNFR2-mediated apoptosis and necrosis in cisplatin-induced acute renal failure. Am J Physiol: Renal Physiol. 2003;285:F610–F8. [DOI] [PubMed] [Google Scholar]
- 5.Zhang B, Ramesh G, Norbury C, Reeves WB. Cisplatin-induced nephrotoxicity is mediated by tumor necrosis factor-a produced by renal parenchymal cells. Kidney International. 2007;72(1):37–44. 10.1038/sj.ki.5002242 [DOI] [PubMed] [Google Scholar]
- 6.Zhang B, Ramesh G, Uematsu S, Akira S, Reeves WB. TLR4 signaling mediates inflammation and tissue injury in nephrotoxicity. J Am Soc Nephrol. 2008;19:923–32. 10.1681/ASN.2007090982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faubel S, Lewis EC, Reznikov L, Ljubanovic D, Hoke TS, Somerset H, et al. Cisplatin-Induced Acute Renal Failure Is Associated with an Increase in the Cytokines Interleukin (IL)-1beta, IL-18, IL-6, and Neutrophil Infiltration in the Kidney. J Pharmacol Exp Ther. 2007;322(1):8–15. 10.1124/jpet.107.119792 [DOI] [PubMed] [Google Scholar]
- 8.Tadagavadi RK, Reeves WB. Renal dendritic cells ameliorate nephrotoxic acute kidney injury. J Am Soc Nephrol. 2010;21(1):53–63. 10.1681/ASN.2009040407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tadagavadi RK, Reeves WB. Endogenous IL-10 attenuates cisplatin nephrotoxicity: role of dendritic cells. J Immunol. 2010;185(8):4904–11. 10.4049/jimmunol.1000383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee H, Nho D, Chung HS, Lee H, Shin MK, Kim SH, et al. CD4+CD25+ regulatory T cells attenuate cisplatin-induced nephrotoxicity in mice. Kidney Int. 2010;78(11):1100–9. 10.1038/ki.2010.139 [DOI] [PubMed] [Google Scholar]
- 11.Kitching AR, Katerelos M, Mudge SJ, Tipping PG, Power DA, Holdsworth SR. Interleukin-10 inhibits experimental mesangial proliferative glomerulonephritis. Clin Exp Immunol. 2002;128(1):36–43. 10.1046/j.1365-2249.2002.01793.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laouar Y, Town T, Jeng D, Tran E, Wan Y, Kuchroo VK, et al. TGF-beta signaling in dendritic cells is a prerequisite for the control of autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2008;105(31):10865–70. 10.1073/pnas.0805058105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bamboat ZM, Ocuin LM, Balachandran VP, Obaid H, Plitas G, DeMatteo RP. Conventional DCs reduce liver ischemia/reperfusion injury in mice via IL-10 secretion. The Journal of Clinical Investigation. 2010;120:559–64. 10.1172/JCI40008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saraiva M O 'Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10(3):170–81. 10.1038/nri2711 [DOI] [PubMed] [Google Scholar]
- 15.Dhande I, Ali Q, Hussain T. Proximal Tubule Angiotensin AT2 Receptors Mediate an Anti-Inflammatory Response via Interleukin-10. Hypertension. 2013;61(6):1218–26. 10.1161/HYPERTENSIONAHA.111.00422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Couper KN, Blount DG, Riley EM. IL-10: the master regulator of immunity to infection. J Immunol. 2008;180(9):5771–7. 10.4049/jimmunol.180.9.5771 [DOI] [PubMed] [Google Scholar]
- 17.Murray PJ. Understanding and exploiting the endogenous interleukin-10/STAT3-mediated anti-inflammatory response. Curr Opin Pharmacol. 2006;6(4):379–86. 10.1016/j.coph.2006.01.010 [DOI] [PubMed] [Google Scholar]
- 18.Deng J, Kohda Y, Chiao H, Wang Y, Hu X, Hewitt S, et al. Interleukin-10 inhibits ischemic and cisplatin-induced acute renal injury. Kidney International. 2001;60:2118–28. 10.1046/j.1523-1755.2001.00043.x [DOI] [PubMed] [Google Scholar]
- 19.Sakai K, Nozaki Y, Murao Y, Yano T, Ri J, Niki K, et al. Protective effect and mechanism of IL-10 on renal ischemia-reperfusion injury. Lab Invest. 2019;99(5):671–83. 10.1038/s41374-018-0162-0 [DOI] [PubMed] [Google Scholar]
- 20.Mu W, Ouyang X, Agarwal A, Zhang L, Long DA, Cruz PE, et al. IL-10 Suppresses Chemokines, Inflammation, and Fibrosis in a Model of Chronic Renal Disease. Journal of the American Society of Nephrology. 2005;16(12):3651–60. 10.1681/ASN.2005030297 [DOI] [PubMed] [Google Scholar]
- 21.Yin Z, Bahtiyar G, Zhang N, Liu L, Zhu P, Robert ME, et al. IL-10 Regulates Murine Lupus. The Journal of Immunology. 2002;169(4):2148–55. 10.4049/jimmunol.169.4.2148 [DOI] [PubMed] [Google Scholar]
- 22.Hedrich CM, Bream JH. Cell type-specific regulation of IL-10 expression in inflammation and disease. Immunol Res. 2010;47(1–3):185–206. 10.1007/s12026-009-8150-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tadagavadi RK, Gao G, Wang WW, Gonzalez MR, Reeves WB. Dendritic Cell Protection from Cisplatin Nephrotoxicity Is Independent of Neutrophils. Toxins. 2015;7(8):3245–56. 10.3390/toxins7083245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramesh G, Zhang B, Uematsu S, Akira S, Reeves WB. Endotoxin and cisplatin synergistically induce renal dysfunction and cytokine production in mice. Am J Physiol Renal Physiol. 2007;293(1):F325–32. 10.1152/ajprenal.00158.2007 [DOI] [PubMed] [Google Scholar]
- 25.Alikhan MA, Huynh M, Kitching AR, Ooi JD. Regulatory T cells in renal disease. Clin Transl Immunology. 2018;7(1):e1004–e. 10.1002/cti2.1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alikhan MA, Summers SA, Gan PY, Chan AJ, Khouri MB, Ooi JD, et al. Endogenous Toll-Like Receptor 9 Regulates AKI by Promoting Regulatory T Cell Recruitment. Journal of the American Society of Nephrology. 2016;27(3):706–14. 10.1681/ASN.2014090927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao Q, Wang Y, Wang XM, Lu J, Lee VW, Ye Q, et al. Renal F4/80+ CD11c+ mononuclear phagocytes display phenotypic and functional characteristics of macrophages in health and in adriamycin nephropathy. J Am Soc Nephrol. 2015;26(2):349–63. 10.1681/ASN.2013121336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu L, Faubel S, He Z, Andres Hernando A, Jani A, Kedl R, et al. Depletion of macrophages and dendritic cells in ischemic acute kidney injury. Am J Nephrol. 2012;35(2):181–90. 10.1159/000335582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nelson PJ, Rees AJ, Griffin MD, Hughes J, Kurts C, Duffield J. The renal mononuclear phagocytic system. Journal of the American Society of Nephrology: JASN. 2012;23(2):194–203. 10.1681/ASN.2011070680 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.