Supplemental Digital Content is available in the text
Keywords: adenosquamous carcinoma, component, diagnosis, gastric cancer, survival
Abstract
For the diagnosis of gastric adenosquamous carcinoma (ASC), discrepancies regarding a rational diagnostic proportion of the squamous cell carcinoma (SCC) component exist among different organizations. Our study aimed to evaluate the impact of the SCC component on the survival of gastric cancer patients and identify the optimal cutoff value for the SCC component necessary for diagnosing gastric ASC.
Cases of gastric cancer with an SCC component were obtained from our center and from case reports and series extracted from Medline. Univariate and multivariate analyses were conducted to compare the overall survival between groups and examine the prognostic value of various clinical parameters.
We identified 45 qualified cases in published literature and 13 in our center. Forty-two of them were males and 16 females (M: F = 2.6:1). Thirty of them were Asian patients and the rest were mainly from the United States and Europe. The mean age was 61.1 years (median 64 years, range 32–84 years). The average tumor size was 6.9 cm (median 6.0 cm, range 2.0–16.0 cm). The most common location of the cancer was the lower third (39.7%). Although a statistical difference was not achieved, the Kaplan–Meier curve demonstrated that as the proportion of the SCC component in the primary lesion increased, the patients’ survival risk increased (P = .489), and the presence of the SCC component in metastatic lymph nodes also increased the risk of survival (P = .259); both of these findings indicated a negative impact of the SCC component on survival. Furthermore, we identified the optimal cutoff for the SCC component as 35% (χ2 = 6.544, P = .011), which was subsequently validated in a Cox regression model as an independent prognostic factor (P = .026).
An increased proportion of the SCC component is associated with worse survival in gastric cancer patients with an SCC component. The optimal cutoff for the proportion of the SCC component necessary for the diagnosis of gastric ASC is 35%.
1. Introduction
Primary gastric adenosquamous carcinoma (ASC) is a rare form of gastric cancer associated with dismal survival[1–8] and is characterized by variable combinations of 2 malignant components, namely, adenocarcinoma (AC) and squamous cell carcinoma (SCC) components. ASC accounts for less than 1% of gastric carcinomas.[4,6] According to the classification of tumors of the digestive system introduced by the World Health Organization (WHO), without a clear indication of the diagnostic threshold of the SCC component, ASC is defined as a malignant epithelial tumor with significant components of both glandular and squamous differentiation.[9] However, the Japanese Gastric Cancer Association (JGCA) suggests that the SCC component exceeds 25% of the primary tumor.[10] This discrepancy in diagnostic criteria also exists in ASC of other organs, such as the esophagus[11,12] and pancreas.[13] This divergence comes mainly from the unsure definition of rational thresholds.
Significantly, in the Johns Hopkins study of pancreatic ASC, although the presence of any SCC component was associated with a poorer prognosis than AC, the proportion of the SCC component with a cutoff of 30% according to the WHO classification was not associated with survival,[14] thus leading to the opposition to the diagnostic standard by some authors.[13–15] In our perspective, a plausible threshold should maximize the distinction between high-risk and low-risk patient groups.
However, the role of the SCC component in the survival of patients with gastric cancer is not well defined and exploration of a reasonable diagnostic threshold of the SCC component for diagnosing ASC is important. Few studies have yet been conducted to address this issue.
We have therefore conducted this retrospective study aiming to assess the impact of the SCC component on survival in resected gastric cancer patients and determine the optimal cutoff for the proportion of the SCC component necessary for the diagnosis of gastric ASC.
2. Materials and methods
2.1. Patient selection and data preparation
This study was approved by the Ethics Committee of West China Hospital. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Informed consent was obtained from all participants from our hospital who were included in our study.
Cases of gastric cancer with an SCC component were collected from our hospital and also from the literature. We screened patients who underwent surgery in our hospital from 2008 to 2020, and a literature search of Medline was conducted for articles[1,5,8,16–20] published from 1986 through 2020. We conducted a computerized search in Medline through internet in March 2020. Because of the rarity of this carcinoma, language limit was not set in the literature search. Terms of “adenosquamous AND stomach,” “adenosquamous AND gastric,” “carcinoma with squamous component AND stomach,” “carcinoma with squamous component AND gastric” were used independently. A combination of these search results was performed by inputting citations into the “clipboard” function of PubMed. Original articles were retrieved and reviewed, and additional cases were then identified through the review process.
Patients diagnosed on the basis of histology with gastric AC cancer with the squamous component were enrolled in the present study. Inclusion criteria were shown as follows: age at the diagnosis ≥18 years; the diagnosis must be confirmed by the tumor tissue sample from surgery but not by autopsy or death certificate; only 1 malignant primary tumor was diagnosed. We excluded patients who had incomplete survival data.
Data including sex, age, tumor site, tumor size, Borrmann classification, differentiation grade, lymphovascular invasion (LVI), tumor depth, nodal status, metastatic status, the proportion of the SCC component, nodal contents, adjuvant therapy, and survival data were collected. All cases were restaged based on the 8th edition classification system released by the American Joint Committee on Cancer.
Overall survival (OS) was applied as the primary outcome of our study, and defined as the time from diagnosis to the last follow-up or death. The cutoff date for follow-up was March 31, 2020. The follow-up time was censored if any primary end point occurred or if the patient was unavailable for follow-up.
2.2. Statistical analysis
The cutoff values for the proportion of the SCC component were analyzed using the X-tile program (http://www.tissuearray.org/rimmlab/), which identified the cutoff for the categorical proportion of the SCC component for survival using the minimum P values from log-rank χ2 statistics.[21] The relationship between various variables and survival was evaluated using the Kaplan–Meier method. Differences between survival curves were tested for statistical significance using the log-rank test. Significant prognostic predictors for patients identified by univariate analysis were further assessed by multivariate analysis using the Cox proportional hazards regression model. Missing attribute values were treated as special values. Data were processed using SPSS 23.0 (SPSS Inc., Chicago, IL). An α value of .05 was used as the examination standard.
3. Results
3.1. Patient selection and baseline characteristics
We identified a total of 301 articles in PubMed, including 146 articles by searching “”adenosquamous“ AND stomach,” 34 by “”adenosquamous“ AND gastric,” 74 by “”carcinoma with squamous component“ AND stomach,” and 47 by “”carcinoma with squamous component“ AND gastric,” respectively. Among all these articles identified by computerized search in PubMed as of March 2020, 4 articles that met criteria were included in this study. They provided information of 60 patients. Additional 6 qualified reports, each presented 1 patient, and were collected by manually reviewing those articles. Thus, with 22 additional patients in our center, a total of 88 patients were qualified. However, 58 patients were included in this study eventually because 30 cases were excluded due to lack of information on proportion of the squamous component or survival time (Fig. 1).
Figure 1.
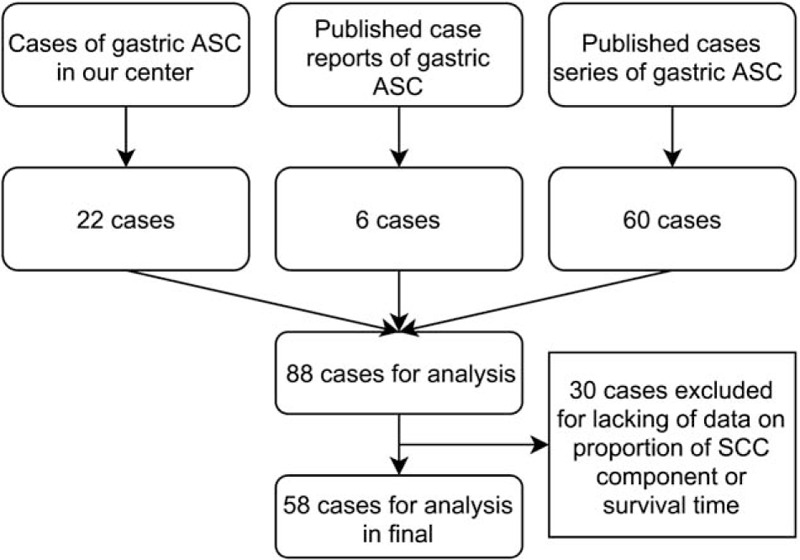
Flowchart of patient selection. ASC = adenosquamous carcinoma; SCC = squamous cell carcinoma.
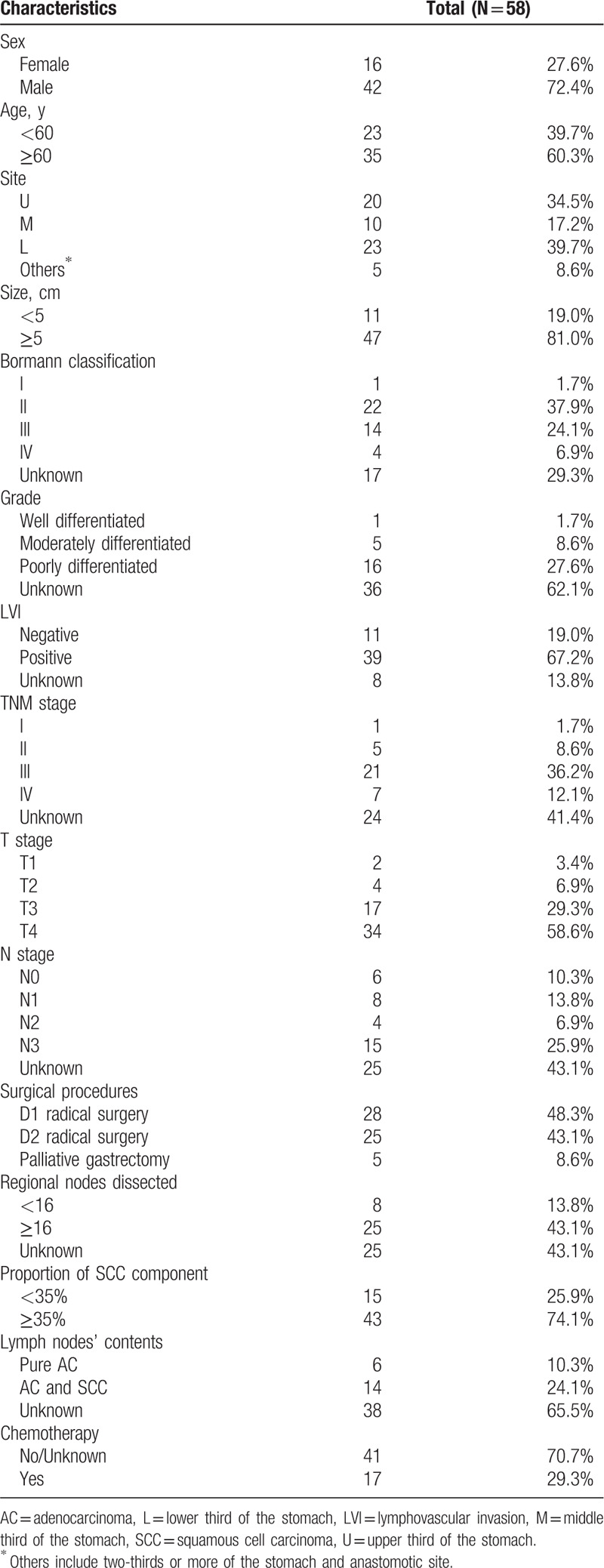
Patient clinicopathological features are summarized in Table 1. There were 42 male (72.4%) and 16 female (27.6%) patients. The median age was 64 years (range 32–84 years). The most common location of the cancer was the lower third (39.7%), followed by upper third (34.5%) and middle third (17.2%) of the stomach. The median tumor size was 6.0 cm (range 2–16 cm). Type II of the Borrmann classification was most frequently observed (37.9%). Most of the tumors were poorly differentiated (27.6%), followed by tumors that were moderately differentiated (8.6%) and well-differentiated (1.7%). Half of the cases (58.6%) were staged as T4. Most of the patients (46.6%) suffered from lymph node metastases. In terms of the components of the metastatic lymph nodes, a pure AC component was found in 10.3% of cases, both AC and SCC components were found in 24.1% of cases, and a pure SCC component was not found in any of the cases.
Table 1.
Characteristics of patients of gastric cancer patients with the squamous cell carcinoma component.
3.2. Identification of the cutoff for the minimum proportion of the SCC component
All 58 cases were included in the survival analysis. The median survival time was 14 months, and the median follow-up time was 60 months (range 1–118 months).
To assess the influence of different proportions of the SCC component on OS, we analyzed the individual results using different proportions of the SCC component ranging from 15% to 85%. Proportions less than 15% and greater than 85% were not analyzed to guarantee sufficient statistical power. The 5-year OS was calculated for patients with gastric cancer that had a certain percentage of the SCC component (P) or more and less than P (Table 2). It showed that when P ranged from 15% to 85%, the 5-year OS of patients with P or more than P decreased gradually; however, in patients with gastric cancer who had a proportion of the AC component (calculated by “1-P”) that ranged from 15% to 70%, the 5-year OS increased (range, 15.6–37.5%), although not consistently. Furthermore, the survival hazard plot (Fig. 2A) demonstrated that with the increase in the proportion of the SCC component in the primary lesion, the risk of death gradually increased (P = .489). In addition, we observed that the presence of the SCC component in metastatic lymph nodes indicated a higher risk of death (P = .259) (Fig. 2B), although a statistical difference was not achieved. X-tile plots were constructed, and the maximum χ2 log-rank value of 6.544 (P = .011) was produced, applying 35% as the optimal cutoff value to divide the cohort into high-risk and low-risk subsets in terms of OS (Fig. 3). We compared the baseline characteristics of the 2 subgroups of patients and no significant differences were found (see Table, Supplemental Content, which illustrates the characteristics of the 2 subgroups of patients).
Table 2.
Univariate analysis of the influence of different proportions of the squamous cell carcinoma component on survival in gastric cancer patients.
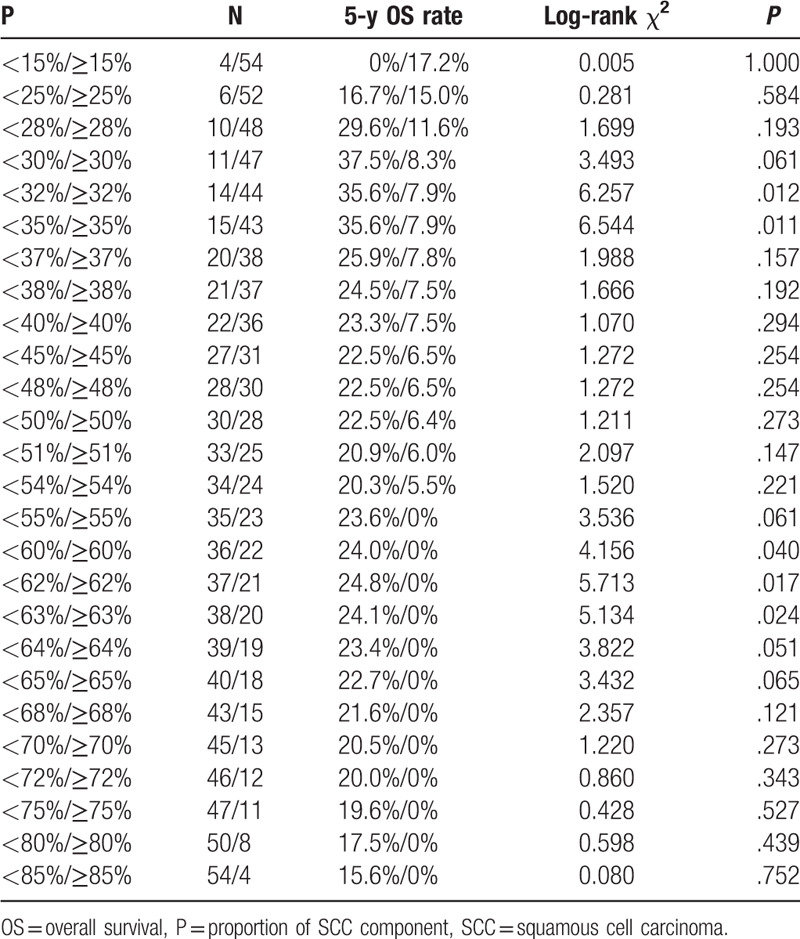
Figure 2.
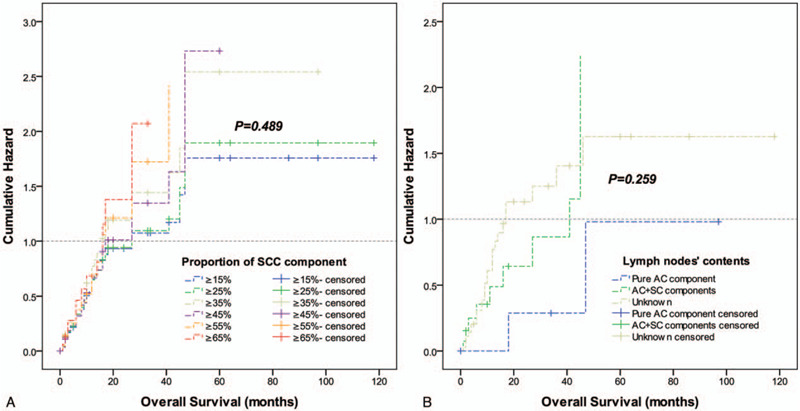
Log-rank tests of overall survival comparing patients with different proportions of the SCC component (χ2 = 4.435, P = .489) (A) and comparison of patients with different contents in the metastatic lymph nodes (χ2 = 2.703, P = .259) (B). AC = adenocarcinoma; SCC = squamous cell carcinoma.
Figure 3.
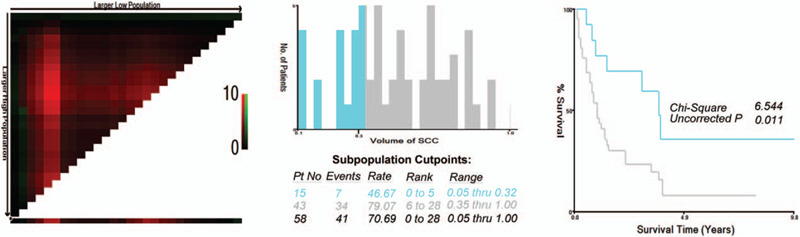
X-tile analysis of survival data in our study. The optimal cutoff value for the SCC component is shown as 35% (χ2 = 6.544, P = .011). SCC = squamous cell carcinoma.
3.3. Prognostic value of the proportion of the SCC component
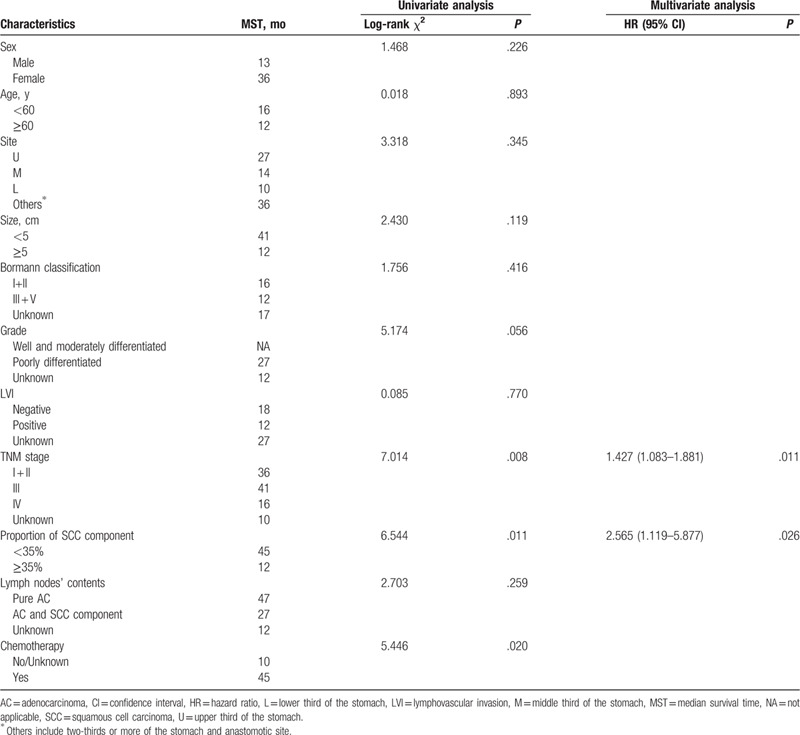
The proportion of the SCC component (P = .011), advanced TNM stage (P = .008), and not receiving chemotherapy (P = .020) were significant risk factors for poor survival according to the univariate analysis (Table 3). In the multivariate analysis, the TNM stage and proportion of the SCC component (with an optimal cutoff of 35%) were independently and significantly associated with OS, and a higher proportion (≥35%) of the SCC component demonstrated a negative effect on survival [hazard ratio (HR) 2.565; 95% confidence interval (95% CI) 1.119–5.877, P = .026] (Table 3).
Table 3.
Univariate and multivariate analyses for evaluating the influence of proportion of the squamous cell carcinoma component in gastric cancer patients.
4. Discussion
Although numerous discussions have occurred in previous articles,[5,7,8,19,22,23] the pathogenesis of the SCC component in gastric cancer remains obscure since the first report of this scarce disease in 1905 by Rolleston and Trevor.[24] The most convincing hypothesis regarding the pathogenesis of the SCC component is squamous metaplasia of a preexisting AC, which is widely accepted by most authors.[19,23,25,26] The main evidence supporting this includes the following: the presence of almost identical expression patterns of RB, p16, and p53 proteins, not only in the AC and SCC components of the primary tumor, but in the metastatic lesions as well[25]; the observation of a close transitional picture of a glandular to squamous element[19] and the demonstration of individual cells containing both tonofibrils and mucous vacuoles[27]; and positive expression of CK7 and CEA in the SCC component.[6,8] Another non-negligible hypothesis is stem cell differentiation toward both cellular lines, which is also supported by several scholars.[17,24,28] Moreover, other possible theories including cancerization of ectopic squamous epithelium, cancerization of metaplastic non-neoplastic squamous cells, and concurrent occurrence of AC and SCC cannot be ruled out.
The role of the SCC component in the survival of patients with gastric cancer is not well construed. Usually, the SCC component is associated with less favorable survival than the conventional gastric AC component,[2–6,8,17,29,30] which, theoretically, proves that the SCC component is more malignant than the AC component. However, it appears that the AC component plays a leading role, even when it is not superior to the SCC component in quantity.[5,20,31,32] The AC component is predominantly found in hematogenous and hepatic metastases[4,6,32]; however, the SCC component is not. Mori et al[19] performed a clinicopathologic analysis of 28 cases and reported that the variations in distribution and quantity of each component had no effect on the survival of patients diagnosed with gastric cancer with the SCC component. However, these conclusions are too empirical and lack statistical validation. With a relatively large sample size, however, our findings revealed that the SCC component had an influence on survival. Our data demonstrated that with the increase in the proportion of the SCC component (range, 15–85%), the 5-year survival rate gradually decreased (range, 17.2% to 0%), which was not the case with the AC component. Moreover, the hazard plot showed that with the increase in the proportion of the SCC component in the primary lesion, the risk of death gradually increased, although no significant difference was revealed due to an inadequate number of cases. Most importantly, excluding the influence of confounding factors, such as the differentiation grade, LVI, and chemotherapy, the multivariate analysis suggested that the proportion of the SCC component (cutoff value of 35%) was independently related to the prognosis. Regardless of the primary organ, an earlier study used human cholangiocellular carcinoma cells in animal models to demonstrate that the SCC component grew more aggressively than the AC component.[33] Moreover, several studies reported significantly shorter doubling times of the SCC component than the AC component in lung cancer.[34,35]
The existence of the AC or SCC component in the metastatic lymph nodes remains controversial in the literature.[5,8] Some studies showed only the AC[36] or SCC component,[20,25,27] and others demonstrated both components. Mori et al[19] demonstrated that histologically, both components could be found in almost all (9 cases) of the metastatic areas examined, and the quantitative ratio of the AC to SCC component in the metastatic lesions roughly paralleled that of the primary lesions. Our study suggested no association between the proportion of the SCC component in primary lesions and the presence of the SCC or AC component in the metastatic lymph nodes. Therefore, it appears that it is difficult to predict which type of component will be present in metastatic sites. Some authors[5,37] believe that the ratio of the AC to SCC component in metastatic lymph nodes might influence the prognosis of gastric ASC. Despite insufficient information about the metastatic lymph nodes, the presence of the SCC component in metastatic lymph nodes seemed to be related to worse survival in our study. However, owing to the rarity of this disease, the number of cases is too small to statistically support this claim.
Currently, surgical resection represents the only potentially curative treatment choice for gastric cancer with the SCC component, and the benefit of chemotherapy and radiotherapy as adjuvant treatments remains unclear.[6–8] In our present study, chemotherapy did not prove to be a prognostic factor in the multivariate analysis. Interestingly, our ongoing population-based study (174 cases) (Li HS, Zhang MY, in preparation) also suggested that adjuvant chemotherapy did not improve survival for resected gastric cancer patients with an SCC component, which is consistent with the results of a study by Li et al (85 cases).[29] Several possible reasons include the following: the high malignancy of this disease and insensitivity to chemotherapy; not enough statistical power due to the limited sample; the statistical effect of chemotherapy for survival is overshadowed by surgery; and the resistance of cytotoxic drugs caused by the multipotential stem cells.[38]
Considering the significant differences in clinicopathological characteristics,[2–6] molecular characteristics,[19,25,31] treatment patterns,[2,3] and prognosis,[2–5,32] a clear and reasonable boundary between gastric ASC and AC is warranted. Different diagnostic criteria increase the heterogeneity between studies, making research on gastric ASC more difficult and contributing to more confusion about the appropriate treatments for ASC patients. Considering the limited data in the literature and from our institution, our study managed to provide a reasonable cutoff value for the proportion of the SCC component necessary for the diagnosis of gastric ASC, which may provide an additional reference for pathologists and clinicians. Our cutoff seemed more stringent than the 25% suggested by the JGCA, but the results of the present study showed that 25% is not an ideal dividing line for ASC. Last but not least, although accurate evaluation of the percentages is sometimes too subjective to be reliably reproduced based on the amount of tumor sectioned, the specific area selected, and the method used to determine the tumor proportions, we believe, by using more objective, uniformed, quality-controlled evaluation standards, the final result has clinical value. Certainly, exploration and verification of a more effective diagnostic standard requires further research.
Several limitations must be considered when interpreting the results of the present analysis. First, as this is not a prospective, controlled, single-site study, there are inconsistencies in the data collection. Second, because the sample size was not large enough, the results of our present study should be explained cautiously. Third, the completeness of the data is limited due to data acquisition factors. We expect that our results will shed light on the study of ASC in the future, and ongoing research will continue to be conducted in our own hospital to learn more about this rare disease.
5. Conclusion
Our study showed that the increased proportion of the SCC component was associated with worse survival in resected gastric cancer patients with an SCC component. The biological behavior may be controlled by the SCC component. A reasonable cutoff value for the proportion of the SCC component necessary for the diagnosis of gastric ASC is 35%, which may provide an additional reference for pathologists and clinicians.
Acknowledgment
We would like to thank the Statistical Department of Sichuan University for their efforts in checking the data.
Author contributions
Conceptualization: Ji-Yan Liu, Hong-Shuai Li.
Data collection: Ye Chen, Ke Cheng, Ming-Yi Zhang.
Formal analysis: Ye Chen, Yu-Wen Zhou.
Writing – original draft: Hong-Shuai Li.
Writing – review & editing: Ji-Yan Liu, Ye Chen.
Supplementary Material
Footnotes
Abbreviations: AC = adenocarcinoma, ASC = adenosquamous carcinoma, CI = confidence interval, HR = hazard ratio, JGCA = Japanese Gastric Cancer Association, LVI = lymphovascular invasion, OS = overall survival, SCC = squamous cell carcinoma, WHO = World Health Organization.
How to cite this article: Li HS, Chen Y, Zhang MY, Cheng K, Zhou YW, Liu JY. Increased proportion of the squamous cell carcinoma component is associated with worse survival in resected gastric adenosquamous carcinoma: A STROBE compliant cohort study. Medicine. 2020;99:36(e21980).
H-SL and YC are contributed equally to this work.
The authors report no conflicts of interest.
All data generated or analyzed during this study are included in this published article [and its supplementary information files]; The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
We would like to thank the Statistical Department of Sichuan University for their efforts in checking the data. This study was supported by the Sichuan Science and Technology Department Key Research and Development Project (2019YFS0539), 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (ZYJC18022) and the National Clinical Research Center for Geriatrics (West China Hospital, Z2018B12).
References
- [1].Ajoodhea H, Zhang RC, Xu XW, et al. Fever as a first manifestation of advanced gastric adenosquamous carcinoma: a case report. World J Gastroenterol 2014;20:10193–201.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ge Y, Lin L, Ma X, et al. Adenosquamous carcinoma of the stomach: a population-based study from the SEER Database. J Cancer 2019;10:5705–13.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Akce M, Jiang R, Alese OB, et al. Gastric squamous cell carcinoma and gastric adenosquamous carcinoma, clinical features and outcomes of rare clinical entities: a National Cancer Database (NCDB) analysis. J Gastrointest Oncol 2019;10:85–94.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Feng F, Zheng G, Qi J, et al. Clinicopathological features and prognosis of gastric adenosquamous carcinoma. Sci Rep 2017;7:4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chen YY, Li AF, Huang KH, et al. Adenosquamous carcinoma of the stomach and review of the literature. Pathol Oncol Res 2015;21:547–51.. [DOI] [PubMed] [Google Scholar]
- [6].Chen H, Shen C, Yin R, et al. Clinicopathological characteristics, diagnosis, treatment, and outcomes of primary gastric adenosquamous carcinoma. World J Surg Oncol 2015;13:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Quan J, Zhang R, Liang H, et al. The clinicopathologic and prognostic analysis of adenosquamous and squamous cell carcinoma of the stomach. American Surg 2013;79:E206–8.. [PubMed] [Google Scholar]
- [8].Saito S, Hosoya Y, Morishima K, et al. A clinicopathological and immunohistochemical study of gastric cancer with squamous cell carcinoma components: a clinically aggressive tumor. J Dig Dis 2012;13:407–13.. [DOI] [PubMed] [Google Scholar]
- [9].Bosman F, Carneiro F, Hruban R, et al. WHO Classification of Tumours of the Digestive System. 4th ed. IARC Press, Lyon; 2010. [Google Scholar]
- [10].Association JGC. Japanese Classification of Gastric Carcinoma – 2nd English edition. Gastric Cancer 1998;1:10–24.. [DOI] [PubMed] [Google Scholar]
- [11].Schizas D, Kapsampelis P, Mylonas KM. Adenosquamous carcinoma of the esophagus: a literature review. J Transl Int Med 2018;6:70–3.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Evans M, Liu Y, Chen C, et al. Adenosquamous carcinoma of the esophagus: an NCDB-based investigation on comparative features and overall survival in a rare tumor. Oncology 2017;93:336–42.. [DOI] [PubMed] [Google Scholar]
- [13].Kardon DE, Thompson LD, Przygodzki RM, et al. Adenosquamous carcinoma of the pancreas: a clinicopathologic series of 25 cases. Mod Pathol 2001;14:443–51.. [DOI] [PubMed] [Google Scholar]
- [14].Voong KR, Davison J, Pawlik TM, et al. Resected pancreatic adenosquamous carcinoma: clinicopathologic review and evaluation of adjuvant chemotherapy and radiation in 38 patients. Hum Pathol 2010;41:113–22.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Katz MH, Taylor TH, Al-Refaie WB, et al. Adenosquamous versus adenocarcinoma of the pancreas: a population-based outcomes analysis. J Gastrointest Surg 2011;15:165–74.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bansal RK, Sharma P, Kaur R, et al. Primary gastric adenosquamous carcinoma in an Indian male. Indian J Pathol Microbiol 2013;56:416–8.. [DOI] [PubMed] [Google Scholar]
- [17].Faria GR, Eloy C, Preto JR, et al. Primary gastric adenosquamous carcinoma in a Caucasian woman: a case report. J Med Case Rep 2010;4:351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Fukuda Y, Takeshima F, Ogihara K, et al. Successful management of liver metastasis from gastric adenosquamous carcinoma with adjuvant chemotherapy and radiofrequency ablation. Nihon Shokakibyo Gakkai Zasshi 2012;109:606–14.. [PubMed] [Google Scholar]
- [19].Mori M, Iwashita A, Enjoji M. Adenosquamous carcinoma of the stomach. A clinicopathologic analysis of 28 cases. Cancer 1986;57:333–9.. [DOI] [PubMed] [Google Scholar]
- [20].Yoshida K, Manabe T, Tsunoda T, et al. Early gastric cancer of adenosquamous carcinoma type: report of a case and review of literature. Jpn J Clin Oncol 1996;26:252–7.. [DOI] [PubMed] [Google Scholar]
- [21].Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res 2004;10:7252–9.. [DOI] [PubMed] [Google Scholar]
- [22].Miyake H, Miyasaka C, Ishida M, et al. Simultaneous gastric adenosquamous carcinoma and gastric carcinoma with lymphoid stroma: a case report. Mol Clin Oncol 2019;11:77–80.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bae HI, Seo AN. Early gastric adenosquamous carcinoma resected using endoscopic submucosal dissection. Case Rep Gastroenterol 2019;13:165–72.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Straus R, Heschel S, Fortmann DJ. Primary adenosquamous carcinoma of the stomach. A case report and review. Cancer 1969;24:985–95.. [DOI] [PubMed] [Google Scholar]
- [25].Lee WA, Woo DK, Kim YI, et al. p53, p16 and RB expression in adenosquamous and squamous cell carcinomas of the stomach. Pathol Res Pract 1999;195:747–52.. [DOI] [PubMed] [Google Scholar]
- [26].Blazquez S, Raventos A, Diaz ML, et al. Adenosquamous gastric carcinoma in Caucasian patient. Rev Espan Enferm Dig 2005;97:211–2.. [DOI] [PubMed] [Google Scholar]
- [27].Mori M, Fukuda T, Enjoji M. Adenosquamous carcinoma of the stomach. Histogenetic and ultrastructural studies. Gastroenterology 1987;92:1078–82.. [DOI] [PubMed] [Google Scholar]
- [28].Manna ED, Seixas AA, de Araujo RP, et al. Primary adenosquamous carcinoma of the stomach. Rev Assoc Med Brasil 1998;44:152–4.. [DOI] [PubMed] [Google Scholar]
- [29].Li Y, Wang Z, Luo F. Clinic pathological features and survival of gastric adenocarcinoma. Chin J Clin Res 2018;31:738–40.. [Google Scholar]
- [30].Li B, Sun L, Wang X, et al. Analysis of clinicopathological characteristics and prognosis on 42 patients with primary gastric adenosquamous cell carcinoma. Zhonghua Wei Chang Wai Ke Za Zhi 2017;20:207–12.. [PubMed] [Google Scholar]
- [31].Kim YS, Heo WS, Chae KH, et al. Clinicopathological features and differences of p53 and Ki-67 expression in adenosquamous and squamous cell carcinomas of the stomach. Korean J Gastroenterol 2006;47:425–31.. [PubMed] [Google Scholar]
- [32].Endo K, Kohnoe S, Okamura T, et al. Gastric adenosquamous carcinoma producing granulocyte-colony stimulating factor. Gastric Cancer 2005;8:173–7.. [DOI] [PubMed] [Google Scholar]
- [33].Iemura A, Yano H, Mizoguchi A, et al. A cholangiocellular carcinoma nude mouse strain showing histologic alteration from adenocarcinoma to squamous cell carcinoma. Cancer 1992;70:415–22.. [DOI] [PubMed] [Google Scholar]
- [34].Honda O, Johkoh T, Sekiguchi J, et al. Doubling time of lung cancer determined using three-dimensional volumetric software: comparison of squamous cell carcinoma and adenocarcinoma. Lung Cancer 2009;66:211–7.. [DOI] [PubMed] [Google Scholar]
- [35].Wilson DO, Ryan A, Fuhrman C, et al. Doubling times and CT screen-detected lung cancers in the Pittsburgh Lung Screening Study. Am J Respir Crit Care Med 2012;185:85–9.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Terada T. Adenosquamous carcinoma of the stomach: report of two cases. Gastroenterol Res 2009;2:54–6.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kadowaki S, Yatabe Y, Nitta S, et al. Durable response of human epidermal growth factor receptor-2-positive gastric adenosquamous carcinoma to trastuzumab-based chemotherapy. Case Rep Oncol 2014;7:210–6.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ohkuma M, Haraguchi N, Ishii H, et al. Absence of CD71 transferrin receptor characterizes human gastric adenosquamous carcinoma stem cells. Ann Surg Oncol 2012;19:1357–64.. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


