Supplemental Digital Content is available in the text
Keywords: psoriasis, hypertension, adjusted-for-covariates, meta-analysis
Abstract
Background:
Several studies have shown a relationship between psoriasis and hypertension, but no meta-analysis has been restricted to studies that adjusted for confounders. The aim of the study was to estimate the association between psoriasis and hypertension with adjustment for covariates.
Methods:
A systematic literature search in the MEDLINE, Embase, Cochrane databases, and Google Scholar was conducted to identify relevant studies which reported the association of psoriasis with the risk of hypertension published up to November 2018 in English. Data analysis was performed with Stata V.12, and Begg adjusted rank correlation test and Egger regression asymmetry test were used to detect publication bias.
Results:
A total of 16 adjusted-for-covariates studies, involving 50,291 cases with hypertension in 255,132 psoriasis patients and 76,547 cases with hypertension in 814,631 controls (no psoriasis), were included in this meta-analysis. The results indicated that psoriasis was associated with an increased risk of hypertension compared to those without psoriasis, and the prevalence of hypertension in severe psoriasis patients was higher than that in mild psoriasis patients, and the risk of hypertension in psoriasis patients was higher than that in nonpsoriasis patients in Europe and Asia.
Conclusion:
We conducted this meta-analysis using the adjusted-for-covariates odds ratio, demonstrating that psoriasis was associated with an increased risk of hypertension compared to those without psoriasis.
1. Introduction
Psoriasis is a common, chronic inflammatory skin disease that affects approximately ranging from 2% to 4% of the world's population.[1–3] It impairs patients’ physical and psychologic well-being, leading to reduced health-related quality of life.[4] In recent years, a large number of studies in abroad have confirmed that psoriasis was associated with metabolic syndrome, cardiovascular disease, and it is believed that the concurrence of psoriasis, metabolic syndrome, and cardiovascular disease may be due to the nature of psoriasis itself as a chronic inflammatory process.[5]
Hypertension is an extremely common finding in the community, which is a major risk factor for myocardial infarction, stroke, peripheral vascular diseases, and cardiovascular death.[6] However, there is still a huge controversy about the association between psoriasis and hypertension, and elevated levels of endothelin-1, dysregulation of the renin–angiotensin system, and increased oxidative stress among patients with psoriasis are possible etiologic links.[7–9] Some studies showed that the prevalence of hypertension in psoriasis patients is higher than that in nonpsoriasis patients.[10–13] But the studies such as Huerta et al[14] and Chen et al[15] suggested that there was relation between psoriasis and hypertension. So far, the controversy about the relationship between psoriasis and hypertension has not been completely ended.
A great understanding about the association between psoriasis and hypertension in epidemiology would help in screening of cardiovascular risk factors in patients with psoriasis, and would help direct future clinical researches. A recent systematic review and meta-analysis by Armstrong et al[16] found the pooled odds ratio (OR) for hypertension among patients with psoriasis was 1.58 compared with the controls. However, such result has exhibited high heterogeneity (I2 = 98.4%) and has included few adjusted-for-covariates studies, to a certain extent, the result would be biased. However, no meta-analysis has been restricted to studies that adjusted for confounders heretofore in the literature. Considering the strength of the association between these 2 diseases is influenced by confounders (e.g., age, sex, smoking, alcohol, body mass index), we performed the systematic review and meta-analysis that every included study had adjusted-for-covariates to better understand the association between psoriasis and hypertension and provide more convincing evidence about that.
2. Methods
The meta-analysis was conducted and reported according to Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement.[17]
2.1. Data sources and searches
A systematic literature search in the MEDLINE, Embase, Cochrane databases, and Google Scholar was conducted to identify relevant studies which reported the association of psoriasis with the risk of hypertension published up to November 2018 in English. The Medical Subject Heading (Mesh) terms and/or key words and/or free words were psoriasis AND (hypertension OR high blood pressure). Additional manual searches were made using the reference lists from relevant studies to retrieve other papers relevant to our topic. We have tried to contact all corresponding authors when data were missing.
2.2. Study selection
Two reviewers (XD and JL) reviewed the full texts of the included studies which met the following inclusion criteria were included in the meta-analysis: Participants were female and male adults with psoriasis diagnosed with specific criteria and controls (participants without psoriasis). Studies had to evaluate the prevalence or incidence of hypertension by diagnostic codes, billing codes, clinician diagnosis, patient-self report, blood pressure measurement, or medical chart review; The risk estimate was reported as an adjusted OR or hazard ratio (AOR/AHR) with the 95% confidence interval (CI); The study had a cohort design or retrospective case–control design or cross-sectional or randomized control design; The study evaluated psoriasis with hypertension; The study language was published in English. We excluded studies lack of crude data after exhausting efforts to contact the authors for complete data. Overlapping studies were included after discarding the study with the smaller study population. Studies that did not meet the above criteria were excluded.
2.3. Data extraction and quality assessment
Data were extracted via a standardized data extraction form, collecting information on the year of publication, country, study design, number of cases and controls, AOR/AHR, adjusted factors, and diagnosis approach of hypertension, when available. Each included article was appraised by 2 reviewers (MY And TL), who assessed the methodologic quality of selected studies independently with the quality of included studies using Newcastle–Ottawa scale (NOS) to assess the methodologic quality of selected studies independently. We defined scores as 6 to 9 being high methodologic quality and <6 being low quality. NOS quality scores were presented as part of descriptive summaries for each study and did not influence decisions to pool studies in meta-analysis.
2.4. Data synthesis and meta-analysis
The results of the meta-analysis are presented in forest plots. Heterogeneity, that is, the proportion of variability across studies was assessed using Cochran Q statistic and quantified using the I2 statistic. If high heterogeneity (I2 > 50) was found, subgroup analysis or influence analysis (sensitivity analysis) would be performed. Data analysis was performed with Stata V.12 (StataCorp, College Station, TX). Subgroup analysis was also used to evaluate the heterogeneity by dividing the group according to the patient geographic location and severity of psoriasis. We used Begg adjusted rank correlation test and Egger regression asymmetry test to detect publication bias, and P < .05 for both tests was considered to represent significant statistical publication bias.
3. Results
3.1. Literature search and study election
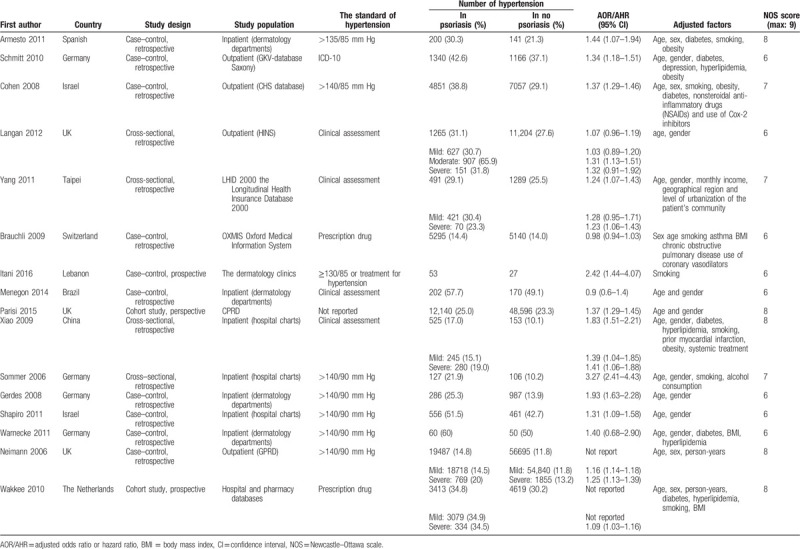
A PRISMA flow chart of screening and selection results is shown as Figure 1. Using prespecified search strategy, our initial search identified 1573 articles, and after application of the criteria selection, from 234 studies initially identified, 79 were considered potentially suitable. After a full-text review, 16 adjusted-for-covariates studies[5,10–13,18–28] were included in this meta-analysis. Table 1 provides a description of the 16 studies.[5,10–13,18–28] There are 50,291 cases with hypertension in 255,132 psoriasis patients and 76,547 cases with hypertension in 814,631 controls (no psoriasis). Among all 16 studies,[5,10–13,18–28] 2 was cohort study,[24,28] 10 were case–control studies,[10–12,18–21,23,25,26] and the rest 4 studies were cross-sectional.[5,13,22,27] Among the 16 studies,[5,10–13,18–28] 1 was performed in South America,[23] 10 in Europe,[5,10,11,18,20,22,24–26,28] and 5 in Asia.[12,13,19,21,27] The number of stars of studies assessed by NOS ranged from 6 to 9 (Table 1).
Figure 1.
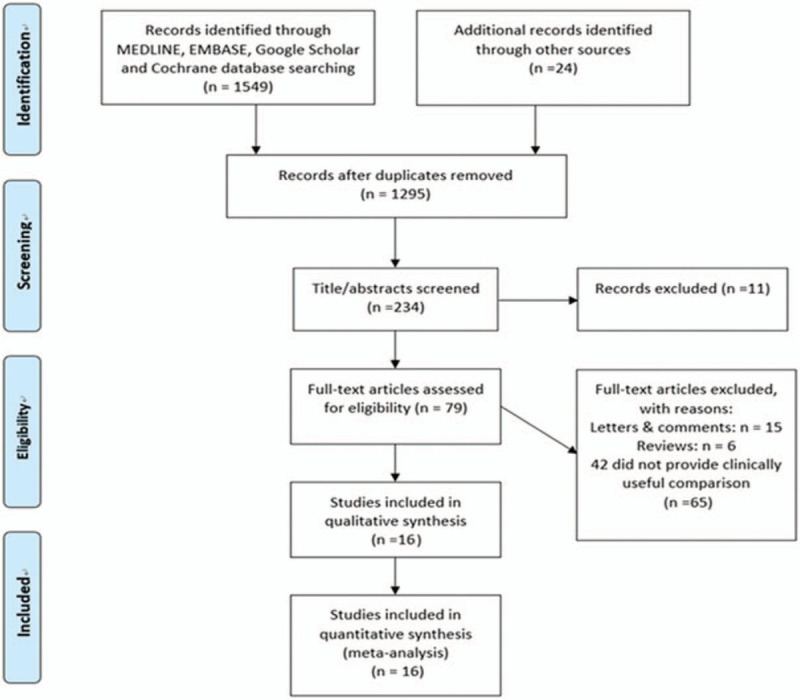
Flowchart for records selection process of the meta-analysis.
Table 1.
Characteristics of the 16 eligible studies.
3.2. Overall analysis
The relationship between psoriasis and the risk of hypertension using data from 16 studies[5,10–13,18–28] was examined, and total AOR/AHR reported by the 14 adjusted-for-covariates studies[5,10,12,13,18–27] were used to assess the associations between them. Overall, there was statistically significant association between psoriasis and hypertension risk (OR 1.43; 95% CI 1.25–1.64; P = .000; I2 = 94.1%) (Fig. 2). Despite the random effects model used, the studies appeared high heterogeneous (I2 = 94.1%).
Figure 2.
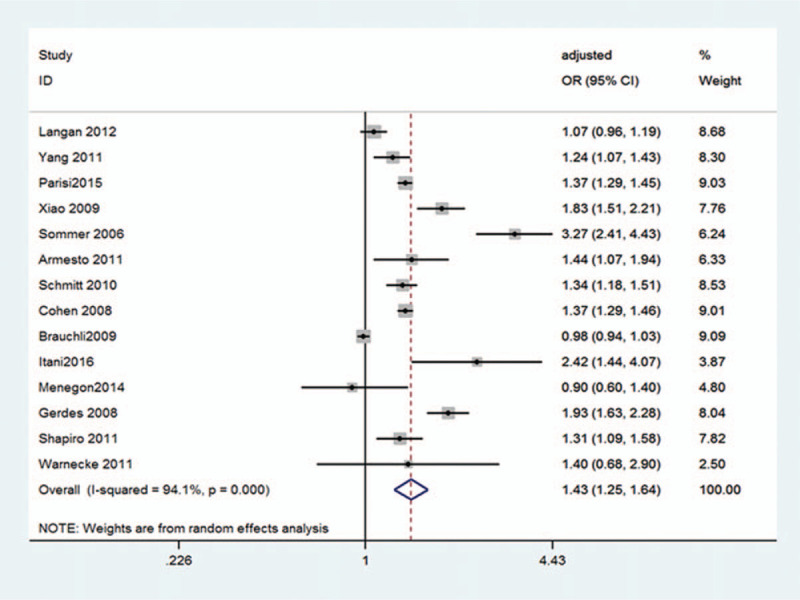
Pooled estimate of association between psoriasis and the risk of hypertension. CI = confidence interval, OR = odds ratio.
3.3. Subgroup analysis
We performed subgroup analysis to attempt to explain the high heterogeneity. Based on the different the severity of illness and geographic locations of these trials, 2 subgroup analyses were performed.
Among the 16 studies,[5,10–13,18–28] 4 studies[11,13,22,27] reported associations between hypertension and mild psoriasis and 5 reported associations between hypertension and severe psoriasis[11,13,22,27,28] (Fig. 3). The heterogeneity was acceptable among those studies with severe psoriasis (I2 = 49.5%) (Fig. 3). There was no significant association for studies between psoriasis and hypertension risk observed in the group with mild psoriasis (OR 1.09; 95% CI 0.98–1.22; P = .118; I2 = 51.5%) (Fig. 3), but a significant association between psoriasis and hypertension risk observed in the group with severe psoriasis (OR 1.13; 95% CI 1.03–1.25; P = .011; I2 = 49.5%) (Fig. 3). In addition, a significant association for studies between psoriasis and hypertension risk was observed basing on overall effect (OR 1.11; 95% CI 1.04–1.18; P = .002; I2 = 59.2%) (Fig. 3).
Figure 3.
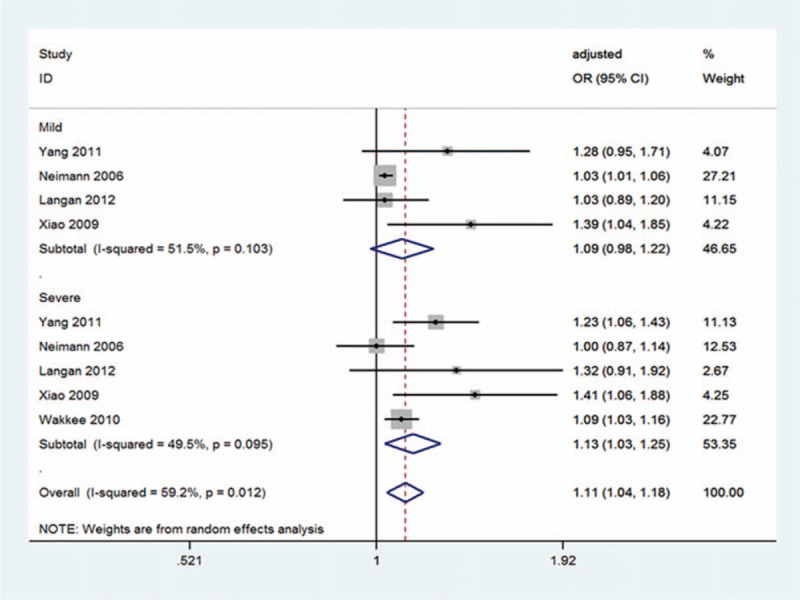
Pooled estimate of the subgroup analysis on the association between psoriasis and the risk of hypertension according to the severity of psoriasis. CI = confidence interval, OR = odds ratio.
With regard to geographic location, we grouped the studies, but the heterogeneous was still high, so the random effects model was used. And we observed European studies (OR 1.46; 95% CI 1.20–1.77; P = .000; I2 = 96.0%; random-effects model) and Asian studies (OR 1.46; 95% CI 1.26–1.68; P = .000; I2 = 73.9% random-effects model) exhibiting significant association between psoriasis and hypertension risk (Fig. 4), respectively. However, we found that the OR of South American study was 0.9 (95% CI 0.59–1.37; P = .626) (Fig. 4).
Figure 4.
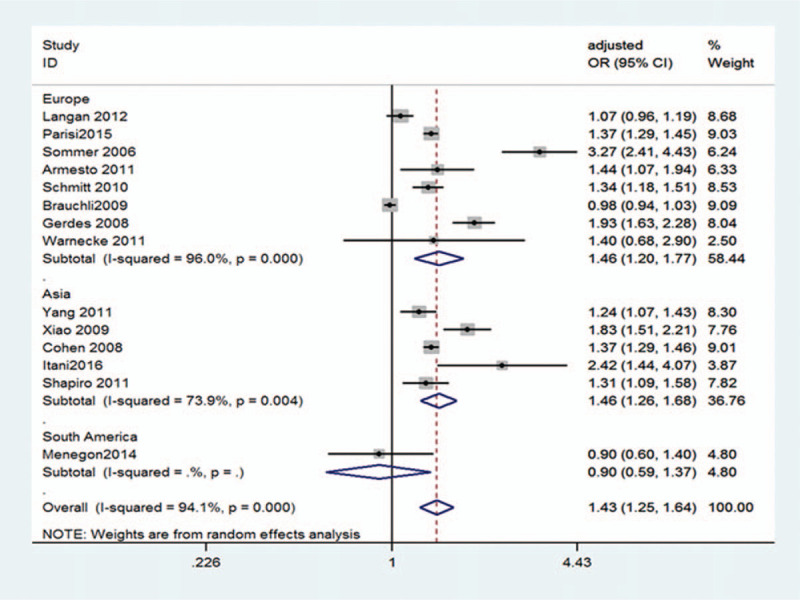
Pooled estimate of the subgroup analysis on the association between psoriasis and the risk of hypertension according to regional differences. CI = confidence interval, OR = odds ratio.
3.4. Sensitivity analysis
To further assess the heterogeneity in these pooled studies, a sensitivity analysis was performed to thereby evaluate the influence of individual studies on the overall risk of psoriasis and hypertension. The results are shown in Figure 5. We found that when each 1 or each 2 trials were excluded, the heterogeneity has not yet decreased from high to the level that we could accept. Finally, the heterogeneity was significantly reduced (I2 = 23.0%) when 5 trials[5,13,18,20,22] were excluded from the meta-analysis (Supplemental Table 1). Consequently, after analyzing the existing data, we finally attributed the high heterogeneity to the difference in diagnostic criteria for hypertension and psoriasis, instruments for measuring blood pressure, case source, and so on.
Figure 5.
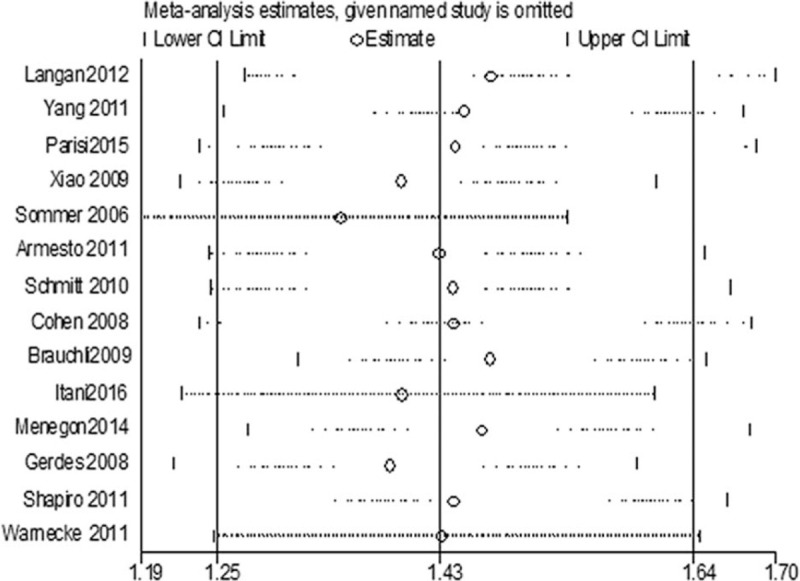
Forest plot for sensitivity analysis. CI = confidence interval.
3.5. Publication bias
There was no evidence of publication bias to be observed by visual inspection of the funnel plots (Fig. 6) or by the application of Begg test (P = .743) or Egger test (P = .072).
Figure 6.
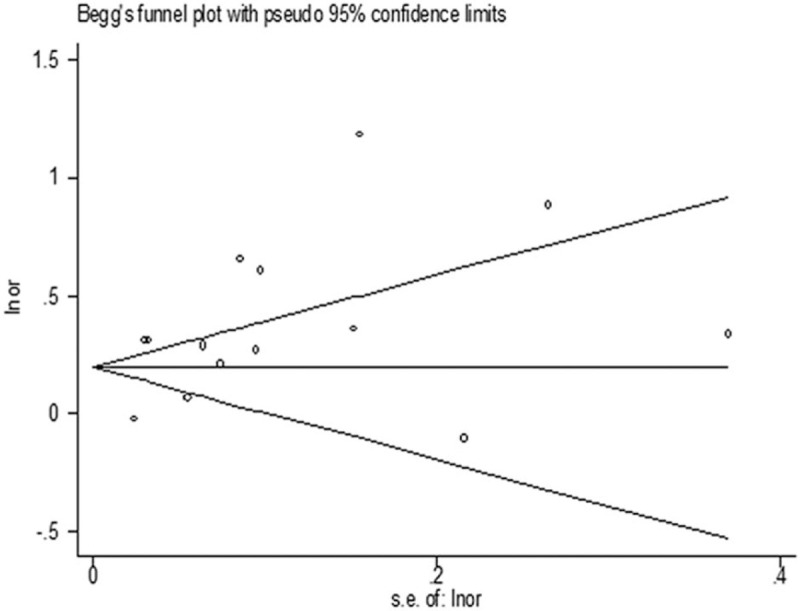
Funnel plot to detect publication bias.
4. Discussion
The present meta-analysis results demonstrated that psoriasis was positively associated with an increased risk of hypertension compared to those without psoriasis. Our study was the 1st meta-analysis to examine the relationship between psoriasis and risk of hypertension with adjustment for covariates in the literature to date with 255,132 psoriasis patients and 814,631 controls. Over the included studies in our meta-analysis, 16 were all of high quality based on NOS quality scores.
There were many confounding factors between psoriasis and hypertension. First of all, psoriasis itself might lead to stress, anxiety, depression, more likely to smoke, drink, lack of exercise, and lead to abnormal blood pressure, and moreover, drugs for psoriasis, such as cyclosporine and compound glycyrrhizin, might affect blood pressure,[29] and diabetes mellitus and metabolic syndrome were also confounding factors associated with psoriasis and hypertension.[30] In the literature, there was one previous meta-analysis[16] assessing the effect of psoriasis on hypertension, they identified 24 observational studies with a total of approximately 2.7 million study participants fulfilling their inclusion criteria in that meta-analysis. However, the meta-analysis[16] had some limitations. In that meta-analysis,[16] only some studies adjusted for confounders and most studies did not completely adjust for important confounders such as obesity and smoking. Given that observational studies might have a high potential for bias due to confounding variables, adjusted-for-covariates ORs were preferred to avoid the heterogeneity and bias.[31,32] Therefore, it is more possible to get a convincing result after more thorough adjustment for confounders of the association between psoriasis and hypertension. All studies adjusted for confounders to get more convincing evidence, in our study, to illustrate the link between both.
At present, the etiology of psoriasis is unclear, and it is considered as an inflammatory skin disease mediated by T lymphocyte, and psoriasis is a common and frequently occurring disease in dermatology, which cannot be absolutely cured.[33] Hypertension is a clinical syndrome caused by many factors, such as heredity and environment, characterized by persistent elevation of systemic and circulatory arterial blood pressure, which is also a major risk factor for cardiovascular and cerebrovascular diseases such as coronary heart disease and stroke, and it is one of the most common cardiovascular diseases.[34] Hypertension and psoriasis are chronic diseases with high incidence, which seriously affect the quality of life and health of patients. These findings have led to the recommendation that all patients with psoriasis should undergo detailed screening and management of hypertension. However, the biologic mechanism underlying the association of psoriasis and risk of hypertension are uncertain.
The study of the correlation between psoriasis and hypertension has been a hot topic in dermatology and internal medicine in recent years, and many literatures have elucidated the correlation from the epidemiologic point of view and the biologic mechanism by means of basic research, cohort studies, cross-sectional studies, and case–control studies.[5,7,9–13,18–28,35] Some people[7,9,35] have done basic researches on the relationship between psoriasis and hypertension, and their conclusions indicated that shared pathways including systemic endothelial dysfunction, increased oxidative stress, and the altered renin–angiotensin system in psoriasis patients might link the pathogenesis of psoriasis with the development of hypertension. The result of a meta-analysis performed by Armstrong et al[16] indicated that psoriasis was associated with greater prevalence of hypertension, and it was put forward that both of mild and severe psoriasis were associated with hypertension. In addition, the result of Al-Metairie's study[36] supported that the correlation between severe psoriasis and hypertension was significantly higher than that of mild psoriasis, suggesting that the more severe the psoriasis was, the higher the risk of hypertension might be. Meanwhile, Langan's study[22] suggested that this trend was not obvious. Five of the articles[11,13,22,27,28] that we included studied the relationship between psoriasis and hypertension based on the severity of psoriasis, and our result, that the prevalence of hypertension in severe psoriasis patients was higher than that in mild psoriasis patients, was roughly the same as that of Al-Metairie's study.[36] But our result was somewhat different from that of previous study,[16] therefore, it was necessary to further study the correlation between the severity of psoriasis and hypertension and its related mechanisms in the future.
The prevalence of hypertension in the world ranged from 10% to 20%.[37] Generally speaking, hypertension was more common among white people, followed by yellow race and less black race, moreover the morbidity and prevalence of hypertension are often higher in economically developed areas, high altitude areas, and high salt diet areas.[38] At present, there was no related research about the effect of the incidence of hypertension in psoriasis based on regional difference. Fourteen of the studies[5,10,12,13,18–27] that we included studied the relationship between psoriasis and hypertension based on regional difference, and our result showed that psoriasis was associated with hypertension, and the risk of hypertension in psoriasis patients was higher than that in nonpsoriasis patients in Europe and Asia. Because the included literature of our meta-analysis did not classify the population's ethnic origins, we could not do the analysis of the correlation between psoriasis and hypertension among different ethnic groups. The better well-designed studies should be carried out to obtain more evidence in this regard in the future.
There were several limitations in our meta-analysis. First, we did not obtained missing data despite repeated attempts to contact the authors. Secondly, the included studies of our meta-analysis were mostly case–control studies and cross-sectional studies, which had some limitations owing to its weakness to determine cause and effects,[39] and we recorded the data on each participant only once. Thirdly, this was a heterogeneous trial. The difference in diagnostic criteria for hypertension and psoriasis, instruments for measuring blood pressure, and case source might contribute to the heterogeneity. In an attempt to reduce or explain the high heterogeneity, we performed subgroup analysis and sensitivity analysis. When 5 trials[5,13,18,20,22] were excluded from the meta-analysis, the heterogeneity was within acceptable. And in the future, the better well-designed, large-scale nationwide and the sufficient power studies will be needed to offer the more comprehensive evidence to clarify the association of psoriasis and hypertension.
5. Conclusion
We conducted this meta-analysis using the adjusted-for-covariates OR, demonstrating that psoriasis was associated with an increased risk of hypertension compared to those without psoriasis. Based on this result, we suggest that, in our clinical work, physicians should pay attention to screening whether psoriasis patients suffered from hypertension, and carry out appropriate intervention and health education for patients with hypertension or high blood pressure. It not only helps to improve the prognosis of primary diseases, but also prevents adverse events such as coronary heart disease and stroke.
Author contributions
Conceptualization: Xi Duan, Junbo Liu.
Data curation: Yunzhu Mu, Ting Liu, Yujuan Chen, Ruichao Yu, Xincai Xiong.
Formal analysis: Xi Duan, Junbo Liu, Yunzhu Mu, Ting Liu, Yujuan Chen.
Funding acquisition: Tao Wu.
Investigation: Xi Duan, Junbo Liu.
Methodology: Xi Duan, Junbo Liu, Tao Wu.
Resources: Xi Duan, Junbo Liu, Ruichao Yu, Xincai Xiong.
Writing – original draft: Xi Duan, Junbo Liu.
Writing – review & editing: Tao Wu.
Junbo Liu orcid: 0000-0003-2442-0950.
Supplementary Material
Footnotes
Abbreviations: AOR/AHR = adjusted odds ratio or hazard ratio, CI = confidence interval, Mesh = Medical Subject Heading, NOS = Newcastle–Ottawa scale, OR = odds ratio, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-analysis.
How to cite this article: Duan X, Junbo L, Mu Y, Liu T, Chen Y, Yu R, Xiong X, Wu T. A systematic review and meta-analysis of the association between psoriasis and hypertension with adjustment for covariates. Medicine. 2020;99:9(e19303).
XD and JL contributed equally to the work.
No ethical approval and patient consent were needed for this study because no intervention was carried out for any patient in the study, and the data were collected from relevant clinical studies for statistical analysis.
This work was supported by the Natural Science Foundation of Sichuan Provincial Department of Education (16ZB0227), Scientific Research Foundation of Health and Family Planning Commission of Sichuan Province (17PJ155), and City of Nanchong Strategic Cooperation with Local Universities Foundation of Technology (NSMC20170421, NSMC20170111, 18SXHZ0581, 18SXHZ0128).
References
- [1].Christophers E. Psoriasis: epidemiology and clinical spectrum. Clin Exp Dermatol 2001;26:314–20. [DOI] [PubMed] [Google Scholar]
- [2].Gelfand JM, Weinstein R, Porter SB, et al. Prevalence and treatment of psoriasis in the United Kingdom: a population-based study. Arch Dermatol 2005;141:1537–41. [DOI] [PubMed] [Google Scholar]
- [3].Parisi R, Symmons DP, Griffiths CE, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol 2013;133:377–85. [DOI] [PubMed] [Google Scholar]
- [4].Schmitt J, Ford DE. Understanding the relationship between objective disease severity, psoriatic symptoms, illness-related stress, health-related quality of life and depressive symptoms in patients with psoriasis - a structural equations modeling approach. Gen Hosp Psychiatry 2007;29:134–40. [DOI] [PubMed] [Google Scholar]
- [5].Sommer DM, Jenisch S, Suchan M, et al. Increased prevalence of the metabolic syndrome in patients with moderate to severe psoriasis. Arch Dermatol Res 2006;298:321–8. [DOI] [PubMed] [Google Scholar]
- [6].Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003;42:1206–52. [DOI] [PubMed] [Google Scholar]
- [7].Huskić J, Alendar F. Tissue angiotensin-converting enzyme in patients with various clinical forms of psoriasis. Bosn J Basic Med Sci 2007;7:103–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ryder KW, Epinette WW, Jay SJ, et al. Serum angiotensin converting enzyme activity in patients with psoriasis. Clin Chim Acta 1985;153:143–6. [DOI] [PubMed] [Google Scholar]
- [9].Ena P, Madeddu P, Glorioso N, et al. High prevalence of cardiovascular diseases and enhanced activity of the reninangiotensin system in psoriatic patients. Acta Cardiol 1985;40:199–205. [PubMed] [Google Scholar]
- [10].Armesto S, Coto-Segura P, Osuna CG, et al. Psoriasis and hypertension: a case-control study. J Eur Acad Dermatol Venereol 2011;26:785–8. [DOI] [PubMed] [Google Scholar]
- [11].Neimann AL, Shin DB, Wang X, et al. Prevalence of cardiovascular risk factors in patients with psoriasis. J Am Acad Dermatol 2006;55:829–35. [DOI] [PubMed] [Google Scholar]
- [12].Shapiro J, Cohen AD, Weitzman D, et al. Psoriasis and cardiovascular risk factors: a case-control study on inpatients comparing psoriasis to dermatitis. J Am Acad Dermatol 2011;66:252–8. [DOI] [PubMed] [Google Scholar]
- [13].Xiao J, Chen LH, Tu YT, et al. Prevalence of myocardial infarction in patients with psoriasis in central China. J Eur Acad Dermatol Venereol 2009;23:1311–5. [DOI] [PubMed] [Google Scholar]
- [14].Huerta C, Rivero E, Rodri’guez LA. Incidence and risk factors for psoriasis in the general population. Arch Dermatol 2007;143:1559–65. [DOI] [PubMed] [Google Scholar]
- [15].Chen YJ, Shen JL, Wu CY, et al. Elevated plasma osteopontin level is associated with occurrence of psoriasis and is an unfavorable cardiovascular risk factor in patients with psoriasis. J Am Acad Dermatol 2009;60:225–30. [DOI] [PubMed] [Google Scholar]
- [16].Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and hypertension: a systematic review and meta-analysis of observational studies. J Hypertens 2013;31:433–42. [DOI] [PubMed] [Google Scholar]
- [17].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009;62:1006–12. [DOI] [PubMed] [Google Scholar]
- [18].Brauchli YB, Jick SS, Miret M, et al. Psoriasis and risk of incident myocardial infarction, stroke or transient ischaemic attack: an inception cohort study with a nested case-control analysis. Br J Dermatol 2009;160:1048–56. [DOI] [PubMed] [Google Scholar]
- [19].Cohen AD, Sherf M, Vidavsky L, et al. Association between psoriasis and the metabolic syndrome. A cross-sectional study. Dermatology 2008;216:152–5. [DOI] [PubMed] [Google Scholar]
- [20].Gerdes S, Zahl VA, Knopf H, et al. Comedication related to comorbidities: a study in 1203 hospitalized patients with severe psoriasis. Br J Dermatol 2008;159:1116–23. [DOI] [PubMed] [Google Scholar]
- [21].Itani S, Arabi A, Harb D, et al. High prevalence of metabolic syndrome in patients with psoriasis in Lebanon: a prospective study. Int J Dermatol 2016;55:390–5. [DOI] [PubMed] [Google Scholar]
- [22].Langan SM, Seminara NM, Shin DB, et al. Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United Kingdom. J Invest Dermatol 2012;132:556–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Menegon DB, Pereira AG, Camerin AC, et al. Psoriasis and comorbidities in a southern Brazilian population: a case-control study. Int J Dermatol 2014;53:e518–25. [DOI] [PubMed] [Google Scholar]
- [24].Parisi R, Rutter MK, Lunt M, et al. Psoriasis and the risk of major cardiovascular events: cohort study using the clinical practice research datalink. J Invest Dermatol 2015;135:2189–97. [DOI] [PubMed] [Google Scholar]
- [25].Schmitt J, Ford DE. Psoriasis is independently associated with psychiatric morbidity and adverse cardiovascular risk factors, but not with cardiovascular events in a population-based sample. J Eur Acad Dermatol Venereol 2010;24:885–92. [DOI] [PubMed] [Google Scholar]
- [26].Warnecke C, Manousaridis I, Herr R, et al. Cardiovascular and metabolic risk profile in German patients with moderate and severe psoriasis: a case control study. Eur J Dermatol 2011;21:761–70. [DOI] [PubMed] [Google Scholar]
- [27].Yang YW, Keller JJ, Lin HC. Medical comorbidity associated with psoriasis in adults: a population-based study. Br J Dermatol 2011;165:1037–43. [DOI] [PubMed] [Google Scholar]
- [28].Wakkee M, Meijer W, Neumann HA, et al. Psoriasis may not be an independent predictor for the use of cardiovascular and antidiabetic drugs: a 5-year prevalence study. Acta Derm Venereol 2010;89:476–83. [DOI] [PubMed] [Google Scholar]
- [29].Qureshi AA, Choi HK, Setty AR, et al. Psoriasis and the risk of diabetes and hypertension: a prospective study of US female nurses. Arch Dermatol 2009;145:379–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sanz LP. Psoriasis, a systemic disease? Actas Dermo-Sifiliográficas (English Edition) 2007;98:396–402. [PubMed] [Google Scholar]
- [31].Rodriguez-Zuniga MJM, Garcia-Perdomo HA. Systematic review and meta-analysis of the association between psoriasis and metabolic syndrome. J Am Acad Dermatol 2017;77:657–66. [DOI] [PubMed] [Google Scholar]
- [32].Voils CI, Crandell JL, Chang Y, et al. Combining adjusted and unadjusted findings in mixed research synthesis. J Eval Clin Pract 2011;17:429–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Gaspari AA. Innate and adaptive immunity and the pathophysiology of psoriasis. J Am Acad Dermatol 2006;54:S67–80. [DOI] [PubMed] [Google Scholar]
- [34].Sowers JR. Hypertension, angiotensin II, and oxidative stress. N Engl J Med 2002;346: 1999-1200a. [DOI] [PubMed] [Google Scholar]
- [35].Armstrong AW, Voyles SV, Armstrong EJ, et al. Angiogenesis and oxidative stress: common mechanisms linking psoriasis with atherosclerosis. J Dermatol Sci 2011;63:1–9. [DOI] [PubMed] [Google Scholar]
- [36].Al-Mutairi N, Al-Farag S, Al-Mutairi A, et al. Comorbidities associated with psoriasis: an experience from the Middle East. J Dermatol 2010;37:146–55. [DOI] [PubMed] [Google Scholar]
- [37].Rasmussen CB, Glisson JK, Minor DS. Dietary supplements and hypertension: potential benefits and precautions. J Clin Hypertens (Greenwich) 2012;14:467–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Stolarz-Skrzypek K, Kuznetsova T, Thijs L, et al. Fatal and nonfatal outcomes, incidence of hypertension, and blood pressure changes in relation to urinary sodium excretion. JAMA 2011;305:1777–85. [DOI] [PubMed] [Google Scholar]
- [39].Sedgwick P. Cross sectional studies: advantages and disadvantages. BMJ 2014;348:g2276. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


