Abstract
This study reports our experience, the therapeutic outcomes and complications of percutaneous sclerotherapy (PS) with polidocanol to treat venous malformations (VMs) in children.
A retrospective analysis was conducted of pediatric patients with VMs who underwent PS using polidocanol under continuous ultrasound (US) guidance between January 2015 and January 2018 at our department. Medical records were reviewed to record demographic information, lesion characteristics, treatment sessions, therapeutic outcomes and complications. χ2 analysis was employed to evaluate the effects of these characteristics on outcomes.
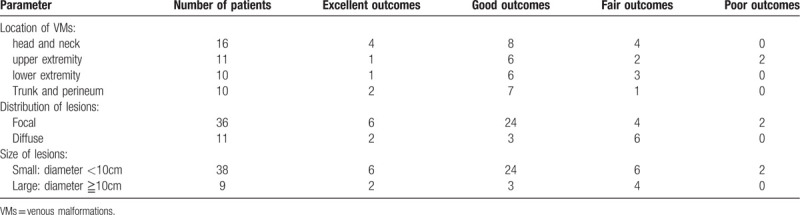
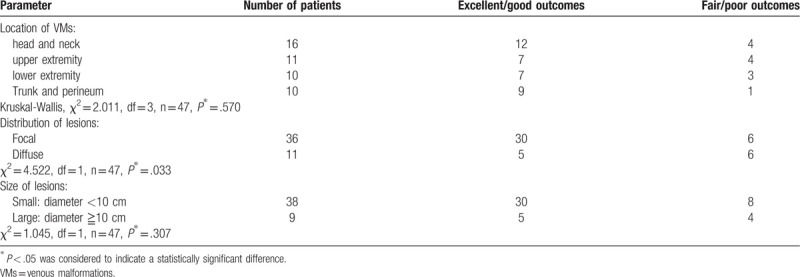
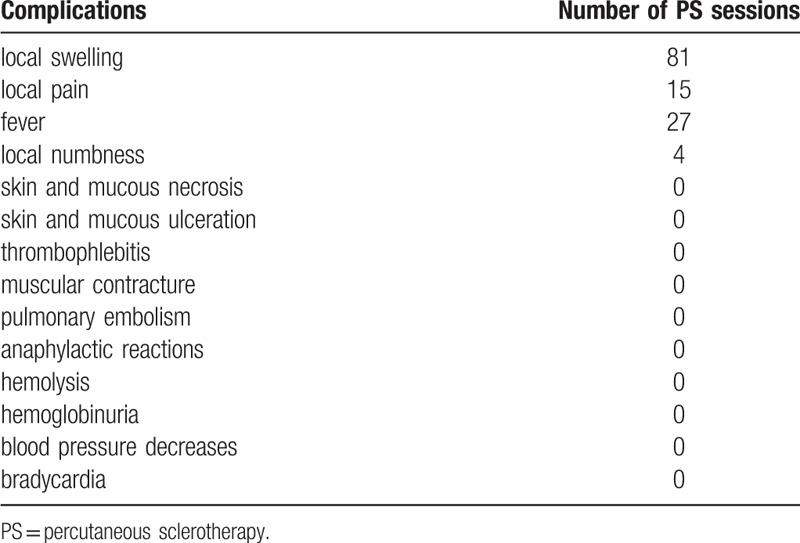
Hundred treatment sessions were performed for lesions in 47 patients. The mean age of the patients was 4.1 ± 3.6 years (mean ± SD). The female to male ratio was almost 2:1 (female 32, male 15). The location of the VMs included the head and neck in 16 cases (34.0%), upper extremity in 11 cases (23.4%), lower extremity in 10 cases (21.3%), and trunk and perineum in 10 cases (21.3%). The majority of the lesions were focal in 36 cases (76.6%), while 11 (23.4%) were diffuse. Seventeen patients (36.2%) underwent single PS session, 14 patients (29.8%) underwent 2 sessions, 10 patients (21.3%) underwent 3 sessions and 6 patients (12.7%) underwent ≧4 sessions. The mean PS session per patient was 2.1 ± 1.1. The mean follow-up duration was 11.4 ± 7.6 months. After the last PS session, 8 patients (17.0%) had excellent outcomes, 27 (57.4%) had good outcomes, 10 (21.3%) had fair outcomes, and 2 (4.3%) had poor outcomes. Focal lesions were more likely to have good or excellent outcomes than diffuse lesions (χ2 = 4.522, P = .033). No other lesion characteristic significantly affected the outcomes (good or excellent outcomes), including lesion location (χ2 = 2.011, P = .570) or lesion size (χ2 = 1.045, P = .307). After the PS procedure, temporary local swelling occurred in 81 sessions (81.0%), local pain occurred in 15 sessions (15.0%), fever occurred in 27 (27.0%) sessions, and transient local numbness occurred in four sessions (4.0%).
PS with polidocanol under the guidance of US appears to be safe and effective for the treatment of VMs in children, especially for focal lesions.
Keywords: percutaneous sclerotherapy, polidocanol, ultrasound guidance, venous malformations
1. Introduction
Venous malformations (VMs) are one of the most common vascular malformations, with an estimated incidence of 1 per 5000 to 10,000 individuals, and equal gender distribution.[1] VMs are highly variable in location and size. They may occur anywhere in the body and infiltrate any structure including the dermis, subcutaneous tissue, muscle, bone, joint, nerve, mucous membrane and viscera. They can be deep, superficial, localized, diffused, solitary or multiple.[2] Approximately 40% of VMs are located in the head and neck region, 40% in the extremities and 20% in the trunk, respectively.[3]
VMs are known to arise from the congenital disruption of vascular morphogenesis. They are composed of abnormal networks of venous channels, which have thin channel walls and abnormal smooth muscles.[4] VMs are a type of slow-flow vascular malformations. As a result of the slow flowing blood within the abnormal network of poorly draining veins, the VMs will expand gradually and contiguously.[2] VMs are usually clinically asymptomatic until they are large enough to cause a visible mass or symptoms. However, dramatic enlargement can occur as a result of hormonal changes, inappropriate therapy or trauma.[5] The symptoms depend on the location and infiltration depth of the VMs. Common symptoms of VMs include pain, dysfunction, swelling, bleeding, coagulopathy, disfigurement, nerve compression and airway obstruction.[6,7]
Various therapeutic options have been explored for VMs, including compressive wrapping, laser therapy, sclerotherapy, surgical excision or a combination of these.[8] Percutaneous sclerotherapy (PS), as a minimally invasive therapy, is widely used to reduce the size of VMs and relieve the symptoms.[8] Various sclerosing agents have been used to treat VMs, such as ethanol, sodium tetradecyl sulfate (STS), polidocanol and pingyangmycin. However, they may lead to complications, such as fever, anaphylactic reactions, tissue necrosis, and nerve injuries.[9] The main objective of this study was to report single center experience, therapeutic outcomes and complications of PS with polidocanol under the guidance of US to treat VMs in children.
2. Materials and methods
This study was approved by the Institutional Review Board of Sichuan Provincial People's Hospital. Written informed consent for PS with polidocanol was obtained from the guardians of all pediatric patients.
2.1. Patients
A retrospective analysis was performed of pediatric patients with VMs who underwent PS using polidocanol only between January 2015 and January 2018 at our department, pediatric surgery department of a major academic medical center.
The diagnosis of VMs was confirmed by clinical evaluation, ultrasonography (US) or magnetic resonance imaging (MRI) according to the International Society for the Study of Vascular Anomalies (ISSVA) classification.[10] In this research, all the pediatric patients with VMs were divided into several groups by 3 different ways, respectively. The VMs were classified as head and neck, trunk and perineum, upper or lower extremity by locations. The VMs were classified as focal or diffuse by anatomic distribution and morphologic characteristics.[11,12] The VMs were classified as small (max diameter <10 cm) or large (max diameter ≧10 cm) by max diameter.
Patients who underwent any previous treatment or were lost to follow-up (a minimum of three months after the last PS) were excluded from the study. All patients were evaluated with US or MRI before and after each treatment session.
2.2. Percutaneous sclerotherapy procedure
The aim of the treatment was not to eliminate the VMs, but to reduce the volume of them, alleviate the symptoms and improve functions with the least risk of complications. Therefore, the treatment was discontinued when the aim has been achieved, even if the mass remained. All patients were treated for pain, functional disorders, bleeding, discomfort or cosmetic concerns. All PS procedures used the same treatment protocol and were performed under general anesthesia. All patients were admitted for observation overnight after the PS procedures.
PS was performed under continuous US guidance with 22 to 27 gauge needles. When a typical US image of VMs[13] appeared and blood reflux was noted, the needle was confirmed to be within the lesion. Then, the polidocanol foam (1% polidocanol, maximal dose 2 mg/kg,[14,15] mixed with three times volume of air[16]) was gently injected. The injection was ceased when the lesion became less compressible, resistance was felt in the syringe or extravasation into normal skin was found. The outflow vein of VMs was manually pressed for >3 min during and after injection of the polidocanol foam. Multiple injections were performed for large or diffused lesions.
2.3. Postprocedural management
All the patients wore pressure garments or stockings continuously after PS, if possible. 5 mg/kg ibuprofen was taken orally if the patient had severe local pain after PS. No antibiotic was used before or after PS. Follow-up was recorded from the first and last PS session till date. Patients were clinically followed-up for ≧3 months after the last PS. Repeat PS was based on the residual size and symptoms of the lesions, 4 weeks after PS. Any improvements and complications were noted during follow-up.
In this research, the main indication for sclerotherapy is patient's symptoms and dysfunctions. However, symptom and functional assessment before or after PS treatment involve many different aspects and parameters, which are difficult to quantitate. Especially, many children cannot describe the subjective perception correctively. Therefore, the response to treatment was assessed only by Lesion volume changes. This approach was commonly used qualitative assessment measures that were described in many previous PS researches[17–20]: Based on physical examination, US or MRI measurement during follow-up, the response to treatment was assessed as excellent, good, fair or poor. Lesions with no remaining visible abnormality were considered to have excellent outcomes. Lesions that were visibly smaller than half their original sizes were considered to have good outcomes. Lesions that were visibly smaller but not less than half their original sizes were considered to have fair outcomes. Lesions that were visibly bigger than their original sizes or no changes were considered to have poor outcomes. Lesion volume was calculated using T2-weighted MRI or US.[21] (Young children did not have the control MRI to avoid unnecessary general anesthesia)
2.4. Statistical analysis
Microsoft Office Excel 2007 and SPSS statistical software were applied for data analysis. A χ2 analysis was employed to evaluate effects of the above-described characteristics on outcomes. The results were considered statistically significant if P < .05.
3. Results
3.1. Patient demographics
A total of 47 pediatric patients were performed by this retrospective analysis. The mean age of the patients was 4.1 ± 3.6 years (range 4 months – 13 years at the time of initial treatment, mean ± SD). The female to male ratio was almost 2:1 (female 32, male 15). Patient demographics are summarized in Table 1.
Table 1.
Patient demographics.
3.2. Distribution of lesions
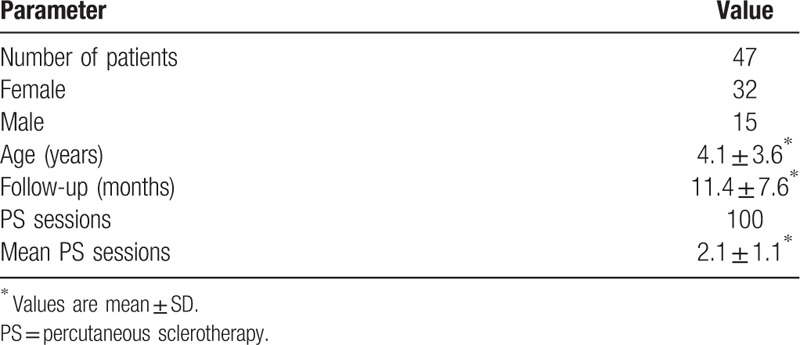
The location of the VMs was in the head and neck in 16 cases (34.0%, Figs. 1–3), upper extremity in 11 cases (23.4%), lower extremity in 10 cases (21.3%), and trunk and perineum in 10 cases (21.3%). The majority of the lesions were focal in 36 cases (76.6%, Fig. 2), while 11 (23.4%, Figs. 1 and 3) were diffuse. Lesion characteristics are listed in Table 2.
Figure 1.
A 6-year-old female with a large diffuse VM involving the right face and neck region. (A) Lateral view of VM during the first PS session. The lesion was superficial, blue, compressible and soft on palpation. The boundary of the lesion was not clearly defined. (B) One month after the first PS session, during the second PS session. The volume of the lesion did not significantly change. (C) One month after the second PS session, during the third PS session. The lesion was smaller and flat. (D) One month after the third PS session, during the fourth PS session. (E) Three weeks after the fourth PS session, significant reduction in size and color fading of the lesion were noticed (arrow). (F) 18 months after the fourth PS session, although partial VM remained, the desired goal of treatment was achieved. PS = percutaneous sclerotherapy, VM = venous malformation.
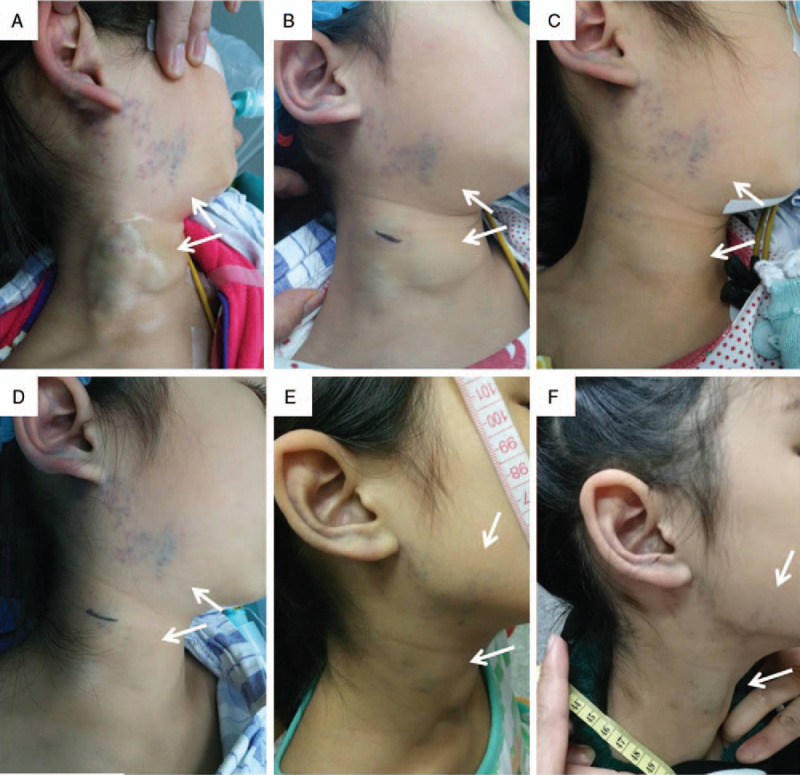
Figure 3.
A 20-month-old female with a small diffuse venous malformation involving the right face region. (A) Lateral view of the lesion when she was 1-month-old. (B) PS treatment with polidocanol was performed when she was 20-month-old. (C) One day after the PS session, swelling, skin blanching (poor capillary refill) and skin ecchymoses (arrow) were noticed on the right face. (D) One month after the PS session, significant reduction in size and color fading of the lesion were noticed (arrow). There was no skin necrosis or scarring (arrow). PS = percutaneous sclerotherapy.
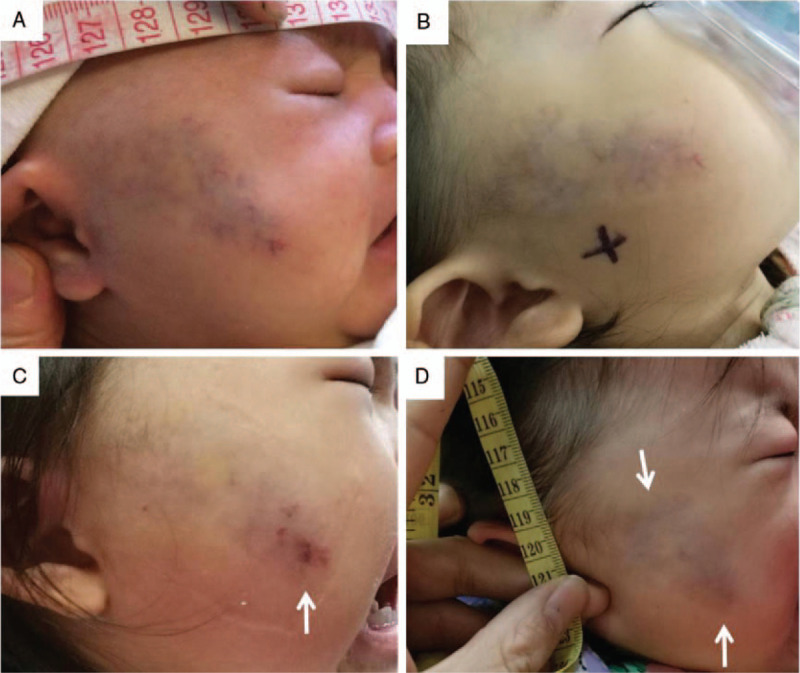
Figure 2.
A 5-month-old male with a small focal VM involving the left upper eyelid. (A) First visit when the patient was 5-month-old. He was born with the left upper eyelid VM, which partly occluding visual field (arrow). (B) PS treatment with polidocanol was performed when he was 5-month-old. One week after the first PS session, although abnormal networks of VM were still obvious (arrow), significant reduction in size of the lesion was noticed. (C, D) One month after the first PS session, significant reduction in size and color fading of the lesion were noticed. (E) One month after the second PS session, the lesion was smaller and flat, but abnormal networks of VM still can be found (arrow). (F) One month and (G) 3 months after the third PS session, the lesion was smaller and flat, but abnormal networks of VM still can be found (arrow). (H) Six months after the third PS session, VM lesion was almost invisible, thus achieving the excellent outcomes. PS = percutaneous sclerotherapy, VM = venous malformation.
Table 2.
Lesion characteristics and responses to completed treatments.
3.3. Procedure details
A total of 47 patients underwent 100 PS treatment sessions. 17 patients (36.2%, Fig. 3) underwent single PS session, 14 patients (29.8%) underwent 2 sessions, 10 patients (21.3%, Fig. 2) underwent 3 sessions and 6 patients (12.7%, Fig. 1) underwent ≧4 sessions. The mean PS sessions per patient were 2.1 ± 1.1. (Table 1)
3.4. Clinical outcomes
After the last PS procedure, 8 patients (17.0%) had excellent outcomes (Fig. 2), 27 (57.4%) had good outcomes (Figs. 1 and 3), 10 (21.3%) had fair outcomes, and 2 (4.3%) had poor outcomes. The mean follow-up duration from the last procedure was 11.4 ± 7.6 months (range: 3–33 months) (Table 1). No in situ recurrence was noticed. Focal lesions were more likely to have good or excellent outcomes than diffuse lesions (χ2 = 4.522, P = .033). The therapeutic outcomes (good or excellent outcomes) were not influenced by lesion location (χ2 = 2.011, P = .570) or lesion size (χ2 = 1.045, P = .307). Lesion locations, distributions, sizes, and response to completed treatment are summarized in Tables 2 and 3.
Table 3.
Lesion characteristics and responses to completed treatments.
3.5. Procedure-related complications
During the PS procedure, there was no transient blood pressure decrease or bradycardia. After the PS procedure, temporary local swelling occurred in 81 PS sessions (81.0%, Fig. 3), local pain occurred in 15 PS sessions (15.0%), fever occurred in 27 PS sessions (27.0%), and transient local numbness occurred in 4 PS sessions (4.0%). No skin and mucous necrosis, ulceration, infection, abscess, nerve injury, thrombophlebitis, muscular contracture, pulmonary embolism, anaphylactic reactions, hemolysis or hemoglobinuria occurred. There was no patient need a prolonged hospital stay or re-intake due to sclerotherapy complications. Complications are listed in Table 4.
Table 4.
Procedure-related complications.
4. Discussion
VMs are benign lesions, and not all of them require treatment. Only the symptomatic, disfiguring and life-threatening lesions need treatment.[22] PS can be used in a lot of VMs and easy to perform under imaging guidance. It can be used alone or combined with other therapeutic modalities.[23] Various sclerosing agents have been used to treat VMs and no superior agents has been identified.[21] The sclerosing agents are typically chosen based on the location, size, depth and invaded structure of VMs, the advantage and disadvantages of the agents, and the general experience of the doctors. Polidocanol is known to be safe, with high efficacy and fewer complications in the treatment of VMs.[9,14,24–26]
Polidocanol is a nonionic surfactant sclerosing agent, which consists of 95% hydroxypolyethoxydodecane and 5% ethanol.[16,26,27] Its intravascular administration can reduce the volume of VMs and alleviate the symptoms by directly damaging the vascular endothelial cells.[13,28] Polidocanol was invented as an anesthetic agent.[29] The dosage of polidocanol is recommended to not exceed 2 mg/kg daily for safety reasons.[14,15] Therefore, the maximum permitted dose of polidocanol may be insufficient to fill large VMs. Administering polidocanol in foam form is known to increase its volume and achieve a greater therapeutic effect than in liquid form.[16,30] That is because the foam dislodges blood from the VM vessel, maintains proper concentration without being diluted by blood, and remains in the VMs to achieve a maximal sclerosant effect.[16] According to the size of VMs and the concentration of polidocanol ranging from 0.25% to 4%,[16,26,27] various polidocanol: air admixtures were suggested for use.[16] In this study, polidocanol foam was produced by mixing 1% polidocanol with three times volume of air.
PS with polidocanol foam was performed under the guidance of US in all the patients in this study. Usually, US is initially used in the suspected VMs, which typically appear well-demarcated, hypoechoic, heterogeneous and compressible.[31] Due to the highly echogenic appearance of foam, when the polidocanol foam enters into the VMs, diffuse hyperechoic appearance is immediately seen by US.[32] Therefore, the extent and morphology of VMs are clearly defined, and the feeding vessels can be identified.[13]
Polidocanol is a weak and low dosage sclerosant. Therefore, manual compression of the VM outflow vein during and after injection of the polidocanol foam is important. This method may lead to polidocanol stasis causing greater polidocanol absorption by vascular endothelial cell membranes, which will improve the efficacy of PS,[24] and may prevent polidocanol leakage and damage to other vessels. Also, all the patients should wear pressure garments or stockings continuously if possible after PS, which is important for the improvement of symptoms and enhancement of concomitant therapies.[13,33]
This study indicated that PS with polidocanol had significant efficacy in improving VMs volumes, symptoms and functions. Complete relief or improvement (excellent and good outcomes) was obtained in 35 (74.5%) of 47 patients, during a follow-up of 11.4 ± 7.6 months. Previous studies reported excellent therapeutic outcomes using polidocanol to treat small, superficial or well-defined VMs,[24,34] which are consistent with the current study of a better therapeutic effect (excellent or good outcomes) in pediatric patients with focal VMs. Moreover, pediatric patients with large VMs may achieve similar therapeutic effects (excellent or good outcomes) as those with small VMs using PS with polidocanol, in this study.
Complications resulting from sclerotherapy of VMs vary greatly depending on the type and dose of sclerosing agent used, and the type, location, extent and invaded structure of the VMs. Furthermore, patients with VMs undergoing sclerotherapy combined with other therapies could have more complex complications.[2] Fever, pain and swelling were the most common complications. Other complications include anaphylactic reactions, skin necrosis, nerve impairment, thrombophlebitis, muscular contracture and pulmonary embolism.[2] However, polidocanol does not actuate as much VMs endothelial impairment as ethanol, STS, or ethanolamine oleate.[33] Therefore, local complications, such as skin necrosis or nerve impairment, were relatively few.[16,30] But, this milder effect of polidocanol may reduce its effectiveness and increase the number of treatment sessions. In the present study, temporary local swelling occurred in 81 PS sessions, local pain occurred in 15 PS sessions, fever occurred in 27 PS sessions, and transient local numbness occurred in 4 PS sessions. However, because of the anesthetic properties of polidocanol, transient local numbness is not presumably nerve injury in this research. This result seems lower than sclerotherapy with STS which has 1.1% nerve injury rate.[35] But, local pain occurred in children seems higher than previous studies that polidocanol is almost painless when used as a sclerosing agent in adults.[32]
No infection, abscess, thrombophlebitis, muscular contracture or pulmonary embolism occurred. No skin and mucous necrosis, or ulceration occurred; even extravasation of polidocanol was found in several procedures. Systemic complications such as decrease in blood pressure, bradycardia, hemolysis, and hemoglobinuria are reported.[14,24] Due to the anesthetic properties of polidocanol, more significant and rare events, such as reversible cardiac arrest, can occur.[14] Allergic and anaphylactic reactions are also rare but possible causes of cardiac complications, with a reported incidence of 0% to 0.3%.[15] So, the excessive usage of polidocanol should be avoided. In the present study, no transient blood pressure decrease, hemolysis, hemoglobinuria or bradycardia occurred. Only several minor complications occurred, such as temporary local swelling, local pain, fever and transient local numbness, which were treatment-related effects rather than typical complications.[23] These results suggested that PS with polidocanol is safe for treating VMs in children.
This retrospective study had several limitations. First, the aim of the treatment was not to eliminate the VMs, but to reduce the volume of them, alleviate the symptoms and improve functions. However, symptom and functional assessments before or after PS treatment involve many different aspects, which are difficult to quantitate and to obtain correct description of the subjective perception from young children. Hence, response to PS treatment was assessed only by lesion volume changes in the current study. The outcomes (excellent, good, fair, and poor) may not truly reflect the PS treatment response. Second, data retrospection was challenging since complications resulting from PS of VMs vary greatly, and it was very hard to obtain precise verbally description from young children. Third, the number of pediatric VM patients was limited and the mean follow-up duration was only 11.4 ± 7.6 months which may underrate the number of recurrence. Fourth, in this study, we did not include huge, deep, visceral and intraosseous VMs, which are more suit for interventional radiology sclerotherapy.
5. Conclusion
According to these mid-term results, PS with polidocanol foam under the guidance of US seems to be safe and efficacious for the treatment of VMs in children, especially for focal lesions.
Author contributions
Conceptualization: Fang Hou, Wenying Liu.
Data curation: Fang Hou, Jidong Chen.
Formal analysis: Fang Hou, Jidong Chen, Meng Xia, Ke Ding, Qiang Zeng.
Investigation: Meng Xia, Ke Ding, Qiang Zeng.
Supervision: Wenying Liu.
Validation: Wenying Liu.
Writing – original draft: Fang Hou.
Writing – review & editing: Jidong Chen, Meng Xia, Ke Ding, Qiang Zeng, Wenying Liu.
Fang Hou orcid: 0000-0002-2463-7087.
Footnotes
Abbreviations: ISSVA = International Society for the Study of Vascular Anomalies, MRI = magnetic resonance imaging, PS = percutaneous sclerotherapy, STS = sodium tetradecyl sulfate, US = ultrasound, VMs = venous malformations.
How to cite this article: Hou F, Chen J, Xia M, Ding K, Zeng Q, Liu W. Percutaneous sclerotherapy with polidocanol under the guidance of ultrasound for venous malformations in children – A retrospective cohort study from a single tertiary medical center. Medicine. 2020;99:9(e18839).
This study was approved by the Institutional Review Board of Sichuan Provincial People's Hospital.
Written informed consent for PS with polidocanol was obtained from the guardians of all pediatric patients.
Consent for publication was obtained from the guardians of the pediatric patients (Figs. 1–3).
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
The authors declare that they have no competing interests.
Funding: This study was supported by National Natural Science Foundation of China (Grant No. 81300238), Young Scientists Foundation of Sichuan Provincial Science and Technology Department (Grant No. 2016JQ0059), Key research Project of Sichuan Provincial Science and Technology Department (Grant No. 2017FZ0040), Research Foundation of Sichuan Provincial Health and Family Planning Commission (Grant No. 16PJ449), and Research Foundation of Central Universities (Grant No. ZYGX2016J171).
The authors have no funding and conflicts of interests to disclose.
References
- [1].Vikkula M, Boon LM, Mulliken JB. Molecular genetics of vascular malformations. Matrix Biol 2001;20:327–35. [DOI] [PubMed] [Google Scholar]
- [2].Fishman SJ, Young AE. Mulliken JB, Burrows PE, Fishman SJ. Slow-flow vascular malformations. Vascular Anomalies: Hemangiomas and Malformations 2nd ed.New York: Oxford University Press; 2013. 562–94. [Google Scholar]
- [3].Dubois J, Soulez G, Oliva VL, et al. Soft-tissue venous malformations in adult patients: imaging and therapeutic issues. Radiographics 2001;21:1519–31. [DOI] [PubMed] [Google Scholar]
- [4].Richter GT, Braswell L. Management of venous malformations. Facial Plast Surg 2012;28:603–10. [DOI] [PubMed] [Google Scholar]
- [5].Hassanein AH, Mulliken JB, Fishman SJ, et al. Venous malformation: risk of progression during childhood and adolescence. Ann Plast Surg 2011;68:198–201. [DOI] [PubMed] [Google Scholar]
- [6].Mathes EF, Haggstrom AN, Dowd C, et al. Clinical characteristics and management of vascular anomalies: findings of a multidisciplinary vascular anomalies clinic. Arch Dermatol 2004;140:979–83. [DOI] [PubMed] [Google Scholar]
- [7].Zhuo KY, Russell S, Wargon O, et al. Localised intravascular coagulation complicating venous malformations in children: associations and therapeutic options. J Paediatr Child Health 2017;53:737–41. [DOI] [PubMed] [Google Scholar]
- [8].Dompmartin A, Vikkula M, Boon LM. Venous malformation: update on aetiopathogenesis, diagnosis and management. Phlebology 2010;25:224–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Castrén E, Aronniemi J, Klockars T, et al. Complications of sclerotherapy for 75 head and neck venous malformations. Eur Arch Otorhinolaryngol 2015;273:1027–36. [DOI] [PubMed] [Google Scholar]
- [10]. ISSVA Classification of Vascular Anomalies 2018 International Society for the Study of Vascular Anomalies Available at: “issva.org/classification” Accessed April 06, 2019. [Google Scholar]
- [11].North PE, Mihm MC., Jr Histopathological diagnosis of infantile hemangiomas and vascular malformations. Facial Plast Surg Clin North Am 2001;9:505–24. [PubMed] [Google Scholar]
- [12].Burrows PE, Paltiel HJ. Mulliken JB, Burrows PE, Fishman SJ. Radiological imaging of vascular malformations. Vascular Anomalies: Hemangiomas and Malformations 2nd ed.New York: Oxford University Press; 2013. 392–479. [Google Scholar]
- [13].Legiehn GM, Heran MK. Venous malformations: classification, development, diagnosis, and interventional radiologic management. Radiol Clin North Am 2008;46:545–97. vi. [DOI] [PubMed] [Google Scholar]
- [14].Marrocco-Trischitta MM, Guerrini P, Abeni D, et al. Reversible cardiac arrest after polidocanol sclerotherapy of peripheral venous malformation. Dermatol Surg 2002;28:153–5. [DOI] [PubMed] [Google Scholar]
- [15].Feied CF, Jackson JJ, Bren TS, et al. Allergic reactions to polidocanol for vein sclerosis. Two case reports. J Dermatol Surg Oncol 1994;20:466–8. [DOI] [PubMed] [Google Scholar]
- [16].Pascarella L, Bergan JJ, Yamada C, et al. Venous angiomata: treatment with sclerosant foam. Ann Vasc Surg 2005;19:457–64. [DOI] [PubMed] [Google Scholar]
- [17].Berenguer B, Burrows PE, Zurakowski D, et al. Sclerotherapy of craniofacial venous malformations: complications and results. Plast Reconstr Surg 1999;104:1–1. 12-15. [PubMed] [Google Scholar]
- [18].Kaji N, Kurita M, Ozaki M, et al. Experience of sclerotherapy and embolosclerotherapy using ethanolamine oleate for vascular malformations of the head and neck. Scand J Plast Reconstr Surg Hand Surg 2009;43:126–36. [DOI] [PubMed] [Google Scholar]
- [19].Burrows PE, Mitri RK, Alomari A, et al. Percutaneous sclerotherapy of lymphatic malformations with doxycycline. Lymphat Res Biol 2008;6:209–16. [DOI] [PubMed] [Google Scholar]
- [20].Yoo JC, Ahn Y, Lim YS, et al. OK-432 sclerotherapy in head and neck lymphangiomas: long-term follow-up result. Otolaryngol Head Neck Surg 2009;140:120–3. [DOI] [PubMed] [Google Scholar]
- [21].Alexander MD, McTaggart RA, Choudhri OA, et al. Quantitative volumetric analysis of venous and lymphatic malformations of the head and neck to assess response to percutaneous sclerotherapy. Acta Radiologica 2016;57:205–9. [DOI] [PubMed] [Google Scholar]
- [22].Hou F. Richter GT, Suen JY. Basic tenants and interventions. Head and Neck Vascular Anomalies: A Practical Case-Based Approach 1st ed.San Diego: Plural Publishing; 2014. 141–3. [Google Scholar]
- [23].Alexander MD, McTaggart RA, Choudhri OA, et al. Percutaneous sclerotherapy with ethanolamine oleate for venous malformations of the head and neck. J Neurointerv Surg 2013;6:695–8. [DOI] [PubMed] [Google Scholar]
- [24].Mimura H, Fujiwara H, Hiraki T, et al. Polidocanol sclerotherapy for painful venous malformations: evaluation of safety and efficacy in pain relief. Eur Radiol 2009;19:2474–80. [DOI] [PubMed] [Google Scholar]
- [25].Kumar S, Bhavana K, Kumar S, et al. Ultrasound-guided polidocanol foam sclerotherapy for treating venous malformations. J Clin Ultrasound 2018;46:23–31. [DOI] [PubMed] [Google Scholar]
- [26].Mimura H, Kanazawa S, Yasui K, et al. Percutaneous sclerotherapy for venous malformations using polidocanol under fluoroscopy. Acta Med Okayama 2003;57:227–34. [DOI] [PubMed] [Google Scholar]
- [27].Nitecki S, Bass A. Ultrasound-guided foam sclerotherapy in patients with Klippel-Trenaunay syndrome. Isr Med Assoc J 2007;9:72–5. [PubMed] [Google Scholar]
- [28].Cacciola E, Giustolisi R, Musso R, et al. Activation of contact phase of blood coagulation can be induced by the sclerosing agent polidocanol: possible additional mechanism of adverse reaction during sclerotherapy. J Lab Clin Med 1987;109:225–6. [PubMed] [Google Scholar]
- [29].Guex JJ. Indications for the sclerosing agent polidocanol (aetoxisclerol dexo, aethoxisklerol kreussler). J Dermatol Surg Oncol 1993;19:959–61. [DOI] [PubMed] [Google Scholar]
- [30].Cabrera J, Cabrera J, Garcia-Olmedo MA, et al. Treatment of venous malformations with sclerosant in microfoam form. Arch Dermatol 2003;139:1409–16. [DOI] [PubMed] [Google Scholar]
- [31].Trop I, Dubois J, Guibaud L, et al. Soft-tissue venous malformations in pediatric and young adult patients: diagnosis with Doppler US. Radiology 1999;212:841–5. [DOI] [PubMed] [Google Scholar]
- [32].Jain R, Bandhu S, Sawhney S, et al. Sonographically guided percutaneous sclerosis using 1% polidocanol in the treatment of vascular malformations. J Clin Ultrasound 2002;30:416–23. [DOI] [PubMed] [Google Scholar]
- [33].Rautio R, Saarinen J, Laranne J, et al. Endovascular treatment of venous malformations in extremities: results of sclerotherapy and the quality of life after treatment. Acta Radiol 2004;45:397–403. [DOI] [PubMed] [Google Scholar]
- [34].Goyal M, Causer PA, Armstrong D. Venous vascular malformations in pediatric patients: comparison of results of alcohol sclerotherapy with proposed MR imaging classification. Radiology 2002;223:639–44. [DOI] [PubMed] [Google Scholar]
- [35].Stuart S, Barnacle AM, Smith G, et al. Neuropathy after sodium tetradecyl sulfate sclerotherapy of venous malformations in children. Radiology 2015;274:897–905. [DOI] [PubMed] [Google Scholar]


