Abstract
Non-small cell lung cancer (NSCLC) is the most common type of lung cancer, accounting for 80–85% of cases. Epidermal growth factor receptor (EGFR) mutations are observed in approximately 40% and 20% of patients with NSCLC in Asian and non-Asian populations, respectively.
First-generation (gefitinib, erlotinib) and second-generation (afatinib, dacomitinib) EGFR-tyrosine kinase inhibitors (TKIs) have been standard-of-care (SoC) first-line treatment for patients with sensitizing EGFR mutation positive advanced NSCLC following Phase III trials versus platinum-based doublet chemotherapy. However, most patients treated with first-line first- or second-generation EGFR-TKIs develop resistance. Osimertinib, a third-generation, central nervous system active EGFR-TKI which potently and selectively inhibits both EGFR-TKI sensitizing (EGFRm) and the most common EGFR T790M resistance mutations, has shown superior efficacy versus first-generation EGFR-TKIs (gefitinib / erlotinib). Osimertinib is now a treatment option for patients with advanced NSCLC harboring EGFRm in the first-line setting, and treatment of choice for patients with T790M positive NSCLC following disease progression on first-line EGFR-TKIs. The second-generation EGFR-TKI dacomitinib has also recently been approved for the first-line treatment of EGFRm positive metastatic NSCLC.
There remains a need to determine appropriate sequencing of EGFR-TKIs in this setting, including EGFR-TKIs as monotherapy or in combination with other TKIs / signaling pathway inhibitors. This review considers the evolving role of sequencing treatments to maximize benefits for patients with EGFRm positive advanced NSCLC.
Keywords: non-small cell lung cancer, epidermal growth factor receptor, tyrosine kinase inhibitor, sequencing, exon 19 deletion, exon 21 mutation
Classification: systemic treatments
1. Introduction
Lung cancer is the leading cause of cancer-related mortality globally, with approximately 1.7 million deaths attributed to the disease each year [1]. Non-small cell lung cancer (NSCLC) is the most common type of lung cancer, accounting for approximately 80–90% of all lung cancers [2]. Historically, first-line treatment for advanced NSCLC was broadly confined to platinum-based chemotherapy. The discovery of targetable oncogenic mutations revolutionized treatment choices for NSCLC, yet refinement of NSCLC classification by biomarker target is still developing. ASCO recommends that all patients with advanced lung adenocarcinoma be screened for EGFR, ALK, ROS1 and BRAF mutations irrespective of clinical characteristics. Patients with advanced lung adenocarcinoma should also be screened for RET, HER2, KRAS, MET, and NTRK by multiplex genetic sequencing (next-generation sequencing) wherever feasible, which is quickly becoming a standard approach to screening for oncogenic targets [2,3].
Epidermal growth factor receptor (EGFR) mutations are observed in approximately 40% and 20% of patients with NSCLC in Asian and non-Asian populations, respectively [4]. EGFR mutations are located in the tyrosine kinase domain and result in increased kinase activity of the EGFR, leading to sustained activation of signaling pathways and continued cell proliferation [5]. The most common EGFR mutations are deletions in exon 19 (Ex19del) or exon 21 L858R point mutation [5].
Phase III trials comparing first-generation (gefitinib, erlotinib) and second-generation (afatinib, dacomitinib) EGFR-tyrosine kinase inhibitors (TKIs) with platinum-based doublet chemotherapy established first- and second-generation EGFR-TKIs as standard-of-care (SoC) for patients with EGFR-mutated advanced NSCLC [6-14]. More recently, osimertinib, a third-generation, central nervous system (CNS)-active EGFR-TKI which potently and selectively inhibits both EGFR-TKI sensitizing (EGFRm) and EGFR T790M resistance mutations [15-19], has shown superior progression-free survival (PFS) compared with standard EGFR-TKI (gefitinib / erlotinib) [19], resulting in osimertinib becoming an additional first-line treatment option for EGFRm positive advanced NSCLC [2,3,20,21]. In the second-line setting, following disease progression on first- or second-generation EGFR-TKIs, osimertinib is treatment of choice for patients whose tumors harbor acquired EGFR resistance mutation T790M [2,16,20].
Treatment of EGFRm positive advanced NSCLC has more therapeutic options than ever before, particularly with regards to choice of first-line EGFR-TKI. Herein, we review first- and second-generation EGFR-TKIs, including acquired resistance mechanisms, and consider the evolving role of third-generation EGFR-TKIs, and other emerging therapeutic approaches in order to optimize the sequencing of EGFR-TKIs.
2. First- / second-generation EGFR-TKI therapy and resistance
2.1. Efficacy of first- and second-generation EGFR-TKIs
In patients diagnosed with EGFRm positive NSCLC, first-generation reversible EGFR-TKIs provide superior efficacy versus platinum-based doublet chemotherapy (Figure 1; Table A.1) [6-11]. Despite initial responses, most patients treated with first-generation EGFR-TKIs eventually develop resistance [22,23]. The second-generation EGFR-TKIs, afatinib and dacomitinib have shown, in vitro, that they bind selectively and irreversibly to the tyrosine kinase domain of EGFR (HER1), HER2, and HER4 receptors, and certain EGFR mutants (including, Ex19del, L858R and T790M) to inhibit proliferation and induce apoptosis in tumor cells that overexpress these receptors [24-26]. Afatinib has also demonstrated efficacy in patients with the uncommon EGFR mutations G719X, L861G and S768I [27,28]. As with first-generation EGFR-TKIs, afatinib treatment showed superior efficacy versus platinum-based doublet chemotherapy in patients with EGFRm positive advanced NSCLC (Figure 1; Table A.1) [13,29]. In head-to-head trials versus gefitinib, second-generation EGFR-TKIs showed improved PFS (Figure 1; Table A.1), and in the case of dacomitinib, improved overall survival (OS; median 34.1 months versus 26.8 months; hazard ratio [HR] 0.76, 95% confidence interval [CI]: 0.58–0.99; p = .044); OS was not considered to be statistically significant in this case due to the hierarchal approach to hypothesis testing in the study design, meaning that no formal testing of OS was conducted since the formal comparison of ORR between the treatment arms was not statistically significant [30,31]. However, the irreversible binding of second-generation EGFR-TKIs has led to increased toxicity, with dose reductions occurring in 39–52% and 66% of patients treated with afatinib and dacomitinib, respectively [14,32,33]. Despite exhibiting promising anti-EGFR T790M activity in vitro, afatinib and dacomitinib are unsuccessful in overcoming T790M-mediated resistance in the clinical setting [34-36] because the therapeutic threshold for clinical efficacy is unachievable in humans due to dose-limiting toxicity associated with non-selective inhibition of wild-type EGFR [15]. In line with resistance to first-generation EGFR-TKIs, T790M is the most common resistance mechanism, present in approximately 50% of progressive cases [32,37-44].
Figure 1.
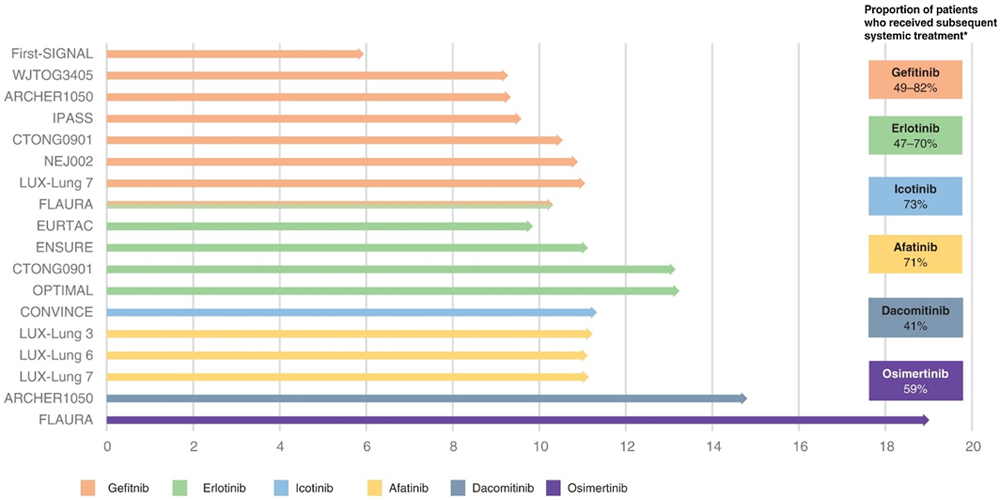
Median PFS with first-line EGFR-TKI treatment in EGFR mutation-positive NSCLC and the proportion of patients who received subsequent systemic treatment [6-12,19,29,32,33,102,103,138-142]
*As a proportion of patients who discontinued EGFR-TKI at data cut-off; treatment presumed systemic when details not provided; however, some references are not clear in this respect. Note, due to the differences in trial designs and patient populations, cross-trial comparisons should be interpreted with caution.
2.2. Mechanisms of resistance to first- and second-generation EGFR-TKIs
Acquired mutations in the EGFR tyrosine kinase domain, the activation of bypass signaling pathways, and phenotypic or histologic transformation have been identified as mechanisms of acquired resistance to first- and second-generation EGFR-TKIs [45,46]. Of the acquired EGFR mutations that can desensitize tumors to erlotinib, gefitinib, afatinib and dacomitinib, the EGFR T790M mutation is the most common, ranging from 36–69% in resistant cases [22,38,45,47-50]. T790M mutation results in the substitution of threonine to methionine at the “gatekeeper” amino acid position 790 on exon 20 of EGFR, causing conformational change, which leads to steric hindrance and reduces the binding activity of first- and second-generation EGFR-TKIs [23,51]. Furthermore, the T790M mutation of EGFR may restore the affinity of the mutant receptor for ATP, thus reducing the potency of competitive EGFR-TKIs [52].
De novo T790M mutations have been found to co-exist at a low frequency in EGFR-TKI treatment-naïve patients with EGFRm positive NSCLC [53,54]; these are rarely seen by standard genotyping methods, occurring in 3.5% of patients [55]. However, with the introduction of highly sensitive assays, the frequency of de novo T790M mutation in EGFR-TKI-naïve patients has been shown to range from 22% to 80% [56-60]. In a meta-analysis of 1462 patients with EGFRm positive advanced NSCLC across 22 studies, pre-treatment de novo T790M mutation-positive status in TKI-naïve patients, was associated with decreased PFS (HR 2.23, p < .001) and OS (HR 1.55, p = .003) with EGFR-TKI therapy (erlotinib or gefitinib) compared with pretreatment T790M mutation-negative status [61]. There is evidence that germline T790M mutations may be present in up to 50% of all patients with de novo T790M [62]. This has implications both around appropriate treatment sequencing and the screening of family members, but must also be considered with the caveat that false positive results may occur [63]. The prevalence of germline T790M mutations is currently being studied in the INHERIT trial (NCT01754025) [64].
Bypass resistance mechanisms utilize alternative cellular pathways and activating downstream signal transduction, thereby facilitating tumor cell growth and survival. MET gene amplification, HER2 gene amplification, and PI3KCA gene mutations are most frequently observed [22,38,65,66]. Changes in tumor phenotype at disease progression have also been reported, with up to 14% of EGFR-TKI resistant tumors showing transformation to small-cell lung cancer (SCLC) [22]. Rarely, epithelial-to-mesenchymal transition can occur [67]. Other rare mechanisms of resistance to EGFR-TKIs include acquired receptor tyrosine kinase fusions and BRAF kinase fusions [68-70].
Yu and colleagues performed targeted next-generation sequencing in EGFR-TKI-naïve EGFRm positive lung cancer. Concurrent HER2 amplification, MET amplification, or TP53 mutations were associated with a shorter time to progression and OS on EGFR-TKI therapy [71]. Thus, identification of these concurrent mutations early in the treatment pathway may help tailor personalized treatment options for these patients by adopting new therapeutic strategies from the outset to overcome primary resistance.
3. Third-generation EGFR-TKI therapy at acquired resistance
3.1. T790M resistance mutation
Third-generation EGFR-TKIs can effectively target both EGFRm and T790M resistance mutations, while sparing activity of wild-type EGFR [15,17-19,72-80]. Osimertinib is now globally recommended for the treatment of patients with T790M NSCLC following disease progression on EGFR-TKI [20,21]. Table A.2 summarizes third-generation EGFR-TKIs that are approved and in clinical development.
In the AURA program of clinical trials, once-daily dosing of 80 mg osimertinib consistently showed clinical benefit in patients with T790M NSCLC following disease progression on first or second-generation EGFR-TKIs [47,79,81]. In the Phase III AURA3 trial, objective response rate (ORR) by investigator assessment was 71% (95% CI: 65–76) and median PFS was significantly longer with osimertinib (10.1 months) than platinum-based doublet chemotherapy (4.4 months) (HR 0.30, 95% CI: 0.23–0.41; p < .001) [79]. These clinical trial data are supported by a large, global observational study of more than 3000 patients with T790M NSCLC and disease progression on prior EGFR-TKI (ASTRIS), in which osimertinib treatment showed an ORR of 57% (95% CI: 55–58) and a median PFS of 11.0 months (95% CI: 10.6–11.1). Furthermore, in AURA3, osimertinib demonstrated clinically meaningful and durable CNS responses; CNS ORR 54–70% [17,82].
3.2. T790M testing and failure rates
Current guidelines recommend T790M testing at clinical progression on a first-line EGFR-TKI, using tissue biopsy, plasma circulating tumor DNA (ctDNA) testing or both [3,21]. Because successful tissue biopsy is often not feasible, plasma testing should be considered a viable approach as the method is time effective and has little impact on patient morbidity. Unlike conventional tissue biopsies, liquid biopsies can circumvent tumor heterogeneity and quantify the proportion of mutated gene copies, which can be beneficial when monitoring disease response and in predicting early treatment failure [83,84]. However, T790M detection rates are often lower with plasma samples than with adequate tissue or cytology samples as ctDNA testing relies on DNA being shed from the tumor [85-88]. Indeed, high rates of false negative ctDNA T790M have been observed across the AURA clinical trial program: positive percentage agreement (PPA) was 51% in AURA3 [44,79,89,90]. This highlights the need for high DNA concentrations in plasma samples and for assays with greater sensitivity when analyzing plasma ctDNA samples for T790M, such as droplet digital polymerase chain reaction assay [91]. For patients with a negative plasma T790M result, it is recommended to reflex test a tissue-based specimen [92].
There is some preliminary evidence to suggest that T790M detection may be lower in the real-world setting compared with the clinical setting [93], possibly due to poor quality specimens, pre-analytical issues or limited access to liquid biopsies. Further research and development are therefore required to improve the quality and utility of liquid biopsies in clinical practice.
Tissue re-biopsy is not feasible for up to 20% of patients with progression on an EGFR-TKI, due to the risk of complications, lack of consent for the procedure, poor performance status of the patient or inaccessibility of the tumor [94-96]. In patients for whom T790M testing (plasma or tissue) is performed, not all will receive a result for reasons such as insufficient tissue, false-negative on plasma test or test limitations [97]. Recently, in a prospective Japanese study in 236 patients with EGFRm positive NSCLC and disease progression on first- or second-generation EGFR-TKI, 13% of patients (n = 31) had not been tested for T790M mutations after disease progression. Of the 199 patients who were tested for T790M, 31% of patients tested positive. Of note, 50 patients underwent second re-biopsy and eight had third re-biopsy, which led to T790M mutation detected in an additional 12 patients [93]. In line with these findings, a US study of electronic health records showed that following first- or second-generation EGFR-TKIs, only 28% of those tested for EGFR mutations had a T790M positive status [98]. In both of these studies, the majority (>90%) of patients who were T790M positive, received osimertinib as their subsequent treatment [93,98]. There remains limited evidence to guide treatment for patients who are T790M negative, or do not receive a valid result and therefore their T790M status remains unknown.
3.2. Uncommon EGFR mutations
Uncommon EGFR mutations in exons 18, 20 and 21, including L861X, G719X and S768I, represent approximately 10% of EGFR mutations [99]. In an open-label Phase II study (n = 35), osimertinib demonstrated efficacy in patients with NSCLC with uncommon EGFR mutations other than exon 19 deletion, L858R, T790M and insertion in exon 20; partial responses were reported in seven (78%) patients with L861Q mutation, ten (53%) patients with G719X mutation and three (38%) patients with S768I mutation [100].
3.3. Proportion of patients who receive second-line treatment after first- / second-generation EGFR-TKIs
We reviewed the rates of subsequent therapies following discontinuation reported in randomized control trials (RCTs) of EGFR-TKIs in the first-line EGFRm positive advanced NSCLC setting (Table A.1).
For first-generation EGFR-TKIs (gefitinib / erlotinib / icotinib), the second-line systemic treatment rate was 47–82% (Table A.1). For second-generation EGFR-TKIs (afatinib / dacomitinib), the rate was 58–78%. Among RCTs that reported the types of subsequent treatment received, the majority received chemotherapy after EGFR-TKI therapy [6,7,10-13,101-103]. In the NEJ002 trial, 20 of 114 patients did not receive any subsequent regimens after first-line gefitinib due to a poor performance status, interstitial lung disease, exacerbation of co-morbidities, and patient preference [7]. There were very limited reports of T790M testing rates.
RCTs provide a good indicator of the rate of subsequent treatment for patients who discontinue first-line EGFR-TKIs. However, these are not representative of the real world, as patients enrolled into RCTs are usually healthier (performance status <2 and good organ function), do not have symptomatic brain metastases and are monitored well as per protocol. As a result, in real-world practice, the proportion of patients receiving second-line treatment can be lower. In a German study investigating patients with EGFRm positive advanced NSCLC, 30% of patients did not reach second-line therapy [104]. However, in a US study only 38% of patients with NSCLC treated with a first- or second-generation EGFR-TKI received a subsequent treatment [98]. In a recent analysis of treatment patterns from the US Flatiron Electronic Health Record-derived database, 44% of patients with EGFRm positive advanced NSCLC received second-line treatment [55].
It should be noted that when many of the RCTs listed here were undertaken, T790M testing was not routine and third-generation EGFR-TKIs were unavailable as a post-first-line EGFR-TKI treatment option. In fact, among patients in the RCT chemotherapy arms, a higher proportion received subsequent treatment, mainly cross-over to second-line EGFR-TKI therapy [8-12,102,103].
In the best-case scenario, approximately 50% of patients with EGFRm positive advanced NSCLC who start with first- or second-generation EGFR-TKIs will be T790M positive at disease progression and eligible to receive second-line osimertinib. However, some of these patients would not receive valid results, which may reduce the T790M positive rate to as low as 30%, as reported by Seto and colleagues [93]. Furthermore, 18–53% of patients (based on RCT data), may not receive any second-line treatment. A composite estimate of the proportion of patients who receive first- or second-generation EGFR-TKIs but do not receive osimertinib after progression could be as high as approximately 70%, meaning only 30% of patients with EGFRm positive advanced NSCLC may ever receive the clinical benefit of osimertinib if treated in the second line (Figure 2).
Figure 2.
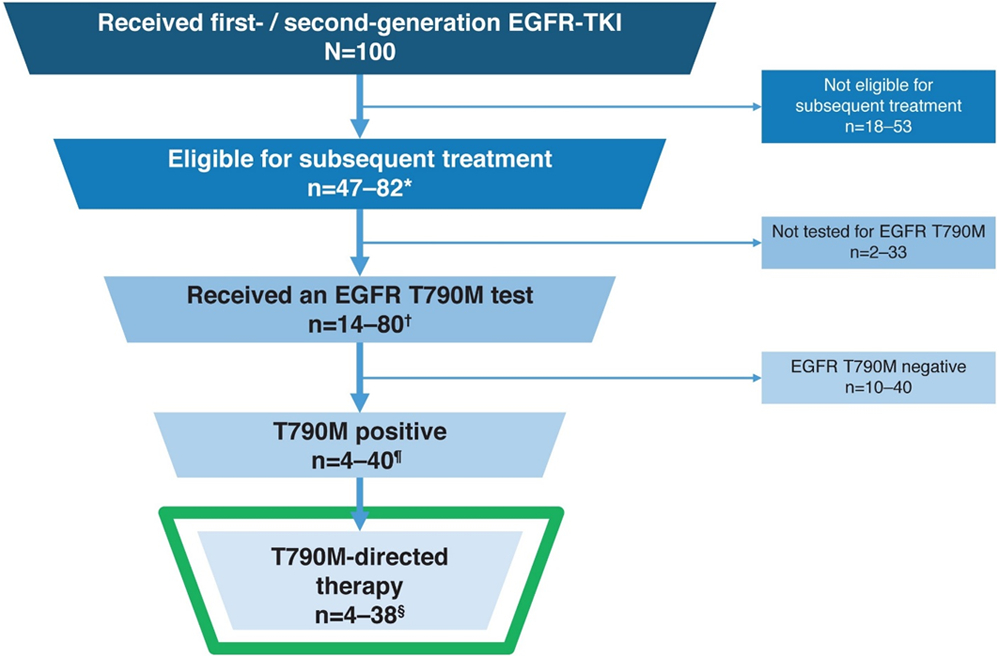
Approximation of proportion of patients with EGFRm positive advanced NSCLC treated with first- / second-generation EGFR-TKIs and who go on to receive osimertinib as second-line therapy [6-12,19,93,95,98,102,103,111,138-140,143-145]
*Based on the proportion of patients who discontinued first-line EGFR-TKIs in randomized-controlled trials and who received subsequent systemic treatment: 47–82%
†Based on real-world evidence studies indicating the proportion of patients who received an EGFR T790M test: FLATIRON (30%) and REMEDY (97% of patients with samples collected)
¶Based on a meta-analysis of literature indicating a prevalence of 50% (Wang et al. BMC 2018), and real-world evidence studies indicating a lower than expected T790M positive rate: FLATIRON (28%) and REMEDY (31%)
§Based on real-world evidence studies, the vast majority of patients who test positive for T790M receive osimertinib treatment: FLATIRON (96%) and REMEDY (90%)
FLATIRON: US Flatiron Electronic Health Record-derived database study
REMEDY: A prospective study of molecular testing status in the EGFR mutation positive NSCLC patients with disease progression during EGFR-TKI treatment
4. Third-generation EGFR-TKI therapy as first-line treatment
The selective nature of third-generation EGFR-TKIs, makes them an attractive therapeutic option in the first-line EGFRm positive NSCLC setting. The efficacy and safety of first-line osimertinib in advanced EGFRm positive NSCLC was recently assessed against the first-generation EGFR-TKIs erlotinib or gefitinib (standard EGFR-TKI) in the global Phase III FLAURA trial [19]. FLAURA achieved its primary endpoint, with osimertinib demonstrating PFS superiority over standard EGFR-TKI (median PFS, 18.9 versus 10.2 months, HR 0.46; 95% CI: 0.37–0.57, p < .001). The PFS benefit was consistent across all subgroups and was similar in patients with (HR 0.47) and without known CNS metastases (HR 0.46) at study entry. Response rates did not differ significantly between the treatment groups (80% with osimertinib and 76% with standard EGFR-TKI); however, the median duration of response was longer in patients treated with osimertinib (17.2 versus 8.5 months). At data cut-off, OS data were immature (25%) and were not statistically significant; however, they suggest a favorable trend for patients treated with osimertinib (HR 0.63, 0.45–0.88; p = .0068). PFS2 results are a good surrogate for OS and were non-calculable (NC) (95% CI: 23.7–NC) for osimertinib versus 20.0 months (95% CI: 18.2–NC), HR 0.58 (95% CI: 0.44–0.78; p = .0004) for standard EGFR-TKI [19]. Based on these findings, osimertinib is approved in the US, EU and Japan for the first-line treatment of patients with EGFRm (Ex19del / L858R) positive advanced NSCLC [105,106].
At present, the optimal therapeutic strategy for osimertinib and its positioning within the current sequential treatment paradigm is not definitive. When considering a first-line EGFR-TKI, several factors must be considered. Certainly, giving osimertinib in the first-line setting would provide every eligible patient with EGFRm positive NSCLC the chance to benefit from the improved efficacy (as shown in FLAURA) versus erlotinib or gefitinib, along with the known reduced risk of CNS progression associated with osimertinib treatment.
A second consideration would be that multiple exposures to systemic agents, as in the multiple EGFR-TKI sequencing strategy, can lead to increasing heterogeneity and genomic complexity in tumors, resulting in a poorer response with later lines of therapy [107]. Consequently, a patient may derive greater benefit from a more potent EGFR-TKI administered in the first-line setting rather than in the second-line or later.
A third consideration is the high risk of CNS metastases among patients with EGFRm positive NSCLC [108,109]. Thus, demonstrable efficacy and safety of EGFR-TKI therapy in patients with EGFRm positive NSCLC and CNS metastases is particularly relevant. Both AURA3 and FLAURA reported greater CNS efficacy with osimertinib versus platinum-pemetrexed and standard EGFR-TKI (gefitinib / erlotinib), respectively [17,18]. In FLAURA, CNS PFS in patients with measurable and / or non-measurable CNS lesions was not reached with osimertinib (95% CI: 16.5 months–NC) and was 13.9 months (95% CI: 8.3 months–NC) with standard EGFR-TKI (HR 0.48; 95% CI: 0.26–0.86; p = .014 [nominally statistically significant]). Moreover, CNS ORR were significantly higher in patients with one or more measurable CNS lesions (91% versus 68%; odds ratio, 4.6; 95% CI: 0.9–34.9; p = .066) and in patients with measurable and / or non-measurable CNS lesions (66% and 43%; odds ratio, 2.5; 95% CI: 1.2–5.2; p = .011) versus standard EGFR-TKI, and the probability of experiencing a CNS progression event was consistently lower with osimertinib [18].
Currently, no direct comparable data for second- versus third-generation EGFR-TKIs exists in the first-line setting. Moreover, OS data for both AURA3 and FLAURA are immature [19,79]. Interestingly, in the post-progression analysis of FLAURA, the PFS benefit with osimertinib versus standard EGFR-TKI was maintained throughout all time-to-event post-progression endpoints: PFS HR 0.46, time to first subsequent treatment HR 0.51, PFS2 HR 0.58, and time to second subsequent treatment 0.60 [110]. This step-wise increase of the statistically significant hazard ratios HRs provides confidence in the interim OS data. Nevertheless, the final OS results are eagerly awaited.
The position of osimertinib in the treatment pathway may also be dictated by the resistance mechanisms identified in the first-line setting and the available treatment options following progression. Very limited data are available for resistance mechanisms to osimertinib as first-line therapy but preliminary data from AURA (first-line cohort) and FLAURA suggest similar resistance mechanisms to those observed with osimertinib in the second-line T790M setting (Figure 3), and also to first- and second-generation EGFR-TKIs, with the exception of T790M mutation, of which there was no evidence [111,112]. Further research into resistance mechanisms to first-line osimertinib are ongoing with the ELIOS (NCT03239340) trial [113].
Figure 3.
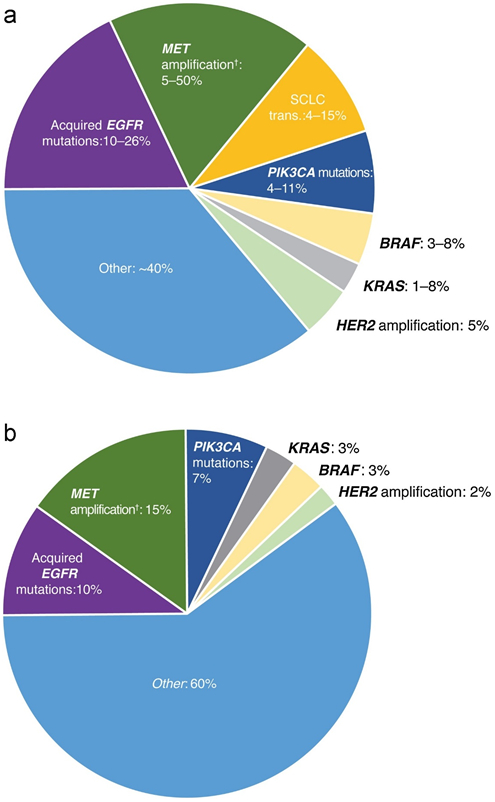
A. Resistance mechanisms post ≥second-line osimertinib* [146-152]
Composite pie chart (range of data values correspond to the size of each segment)
*Resistance mechanism reported may overlap with another
†Resistance mechanisms in plasma; frequency of MET amplification is expected to be higher in tissue
B. Plasma-based resistance mechanisms post first-line osimertinib (FLAURA)* [112]
*Resistance mechanism reported may overlap with another
†Resistance mechanisms in plasma; frequency of MET amplification is expected to be higher in tissue
Given the dearth of post-osimertinib treatment options, there is an argument that greater survival benefit may be derived from the sequential initiation of EGFR-TKIs rather than initiating osimertinib in the first-line setting. However, the PFS benefit with osimertinib demonstrated as preserved throughout time-to-event post-progression endpoints versus standard EGFR-TKIs in the first-line setting must be noted. Furthermore, the majority of patients with progression on standard EGFR-TKIs receive chemotherapy.
5. Emerging approaches to targeting EGFR-mutant NSCLC
It is critical that we explore novel approaches to overcome acquired resistance mechanisms to EGFR-TKIs and to determine the appropriate sequencing of therapy to prolong patient survival.
The acquired C797S mutation, which blocks the covalent binding of second- and third-generation EGFR-TKIs to the ATP-binding site, has been identified as a potential resistance mechanism for irreversible third-generation EGFR-TKIs. In the post-second-line T790M setting, data have indicated that the C797S mutation is acquired while retaining both EGFRm and T790M mutations [114-116]. The emergence of this tertiary acquired mutation that mediates resistance to all known third-generation EGFR-TKIs is driving the development of fourth-generation EGFR-TKIs. EAI001, EAI045, and JBJ-04–125-02 are allosteric EGFR inhibitors that target T790M and C797S EGFR mutants, and have demonstrated initial efficacy in the preclinical setting [117-119]. Importantly, C797S acquisition after first-line osimertinib only co-exists with EGFRm (since osimertinib prevents T790M resistance). Moreover, in-vitro modelling supports the potential use of osimertinib in combination with first-generation EGFR-TKIs to target EGFRm / C797S resistance when T790M and C797S mutations occur in trans allelic context [120].
As new resistance mechanisms to EGFR-TKIs come to light, it is becoming clear that the inhibition of EGFR alone may not be sufficient for sustained antitumor activity. Combined MET and EGFR inhibition to target EGFR-TKI acquired resistance driven by MET amplification is a compelling therapeutic approach, with Phase Ib studies in patients with EGFRm positive NSCLC and MET positive acquired resistance demonstrating promising safety, tolerability, and preliminary activity of osimertinib (TATTON, NCT02143466) [121] or gefitinib (NCT02374645) [122] in combination with the MET-TKI savolitinib. This approach provided the basis for the ongoing Phase II SAVANNAH (NCT03778229) trial, which will investigate the efficacy of osimertinib in combination with savolitinib in patients with EGFRm and MET positive NSCLC, following progression on osimertinib treatment. Indeed, a multi-drug, biomarker-directed Phase II platform trial (ORCHARD; NCT03944772) is evaluating resistance mechanisms and combination treatment options for patients with EGFRm positive NSCLC whose disease has progressed on first-line osimertinib therapy. Preliminary results from an ongoing Phase I trial (NCT02609776) investigating the EGFR-cMET bispecific antibody JNJ-372 demonstrated a manageable tolerability profile [123]. Recently, several case reports have shown that the administration of crizotinib (an ALK / ROS1 / MET inhibitor) in combination with osimertinib is effective and well tolerated in patients with EGFRm positive NSCLC with acquired T790M and MET amplification resistance mutations [124]. Other MET plus EGFR combinations have been investigated, but showed discordant results, so the benefit of this approach remains to be fully elucidated [125-129].
Other potential combinations that target resistance mechanisms that are currently under investigation include EGFR-TKIs in combination with MEK1 / 2 inhibition [121], antibodies against EGFR [130], antibodies against vascular endothelial growth factor (VEGF; NCT02803203) and VEGF receptor (NCT02789345), mTOR inhibition (NCT02503722) and immunotherapy. Preclinical models have demonstrated that activation of the EGFR pathway induces PD-L1 expression, enhancing susceptibility of the lung tumors to PD-1 blockade [131]. This suggested that the combination of PD-1 blockade with EGFR-TKIs might be a viable therapeutic approach to extend duration of treatment response and delay development of resistance in the EGFRm positive NSCLC setting [131]. However, the combination approach of EGFR-TKIs with immunotherapy (gefitinib + durvalumab [132], erlotinib + atezolizumab [133], pembrolizumab + gefitinib [134]) yielded an unexpectedly high incidence of grade 3 / 4 adverse events. Thus, further development of this approach is considered controversial. However, other immunotherapy approaches may provide benefit as salvage treatment strategies following progression on EGFR-TKIs. For instance, in the Phase III IMpower150 study, atezolizumab in combination with bevacizumab and chemotherapy has shown promising activity in patients with pretreated EGFRm positive NSCLC following progression on EGFR-TKIs [135]. This suggests that immunotherapy checkpoint inhibitors in combination with chemotherapy or other treatments may be a viable therapeutic approach following resistance to EGFR-TKIs. Furthermore, the ongoing KEYNOTE-789 trial (NCT03515837) is evaluating if chemotherapy combined with pembrolizumab improves outcomes in patients with EGFRm positive NSCLC and progression on an EGFR-TKI.
In addition to immunotherapy checkpoint inhibitors, other emerging immunotherapy strategies warrant investigation for treatment of resistance to EGFR-TKIs, such as combinations with anti-CD73 agents (for example oleclumab; NCT03381274).
The combination of EGFR-TKIs with chemotherapy is also under investigation for treatment-naïve patients where the availability of third-generation EGFR-TKIs is limited. The Phase III study NEJ009 evaluated the superiority of gefitinib in combination with pemetrexed-carboplatin versus gefitinib monotherapy in patients with newly-diagnosed EGFRm positive NSCLC. Although the combination did not demonstrate superiority in PFS2, the results reported a potential increase in long survivors [136]. Another Phase III study showed the addition of pemetrexed-carboplatin to gefitinib therapy significantly prolonged PFS and OS in patients with chemotherapy-naïve EGFRm positive NSCLC, albeit with increased toxicity [137].
At the core of future research will be the need to determine the appropriate sequencing of treatments for patients with advanced EGFRm positive NSCLC along with standard EGFR-TKIs and, as the disease landscape evolves, with use of later-generation EGFR-TKIs in the first-line setting, such as osimertinib. This will ultimately require selected treatments for a given patient to be based on the results of comprehensive, sensitive molecular profiling throughout the treatment journey. In the first-line setting, this may include EGFR-TKIs administered either as monotherapy, or in combination with other mechanistic approaches, in order to improve benefit in molecularly identified high-risk patients or delaying resistance. Monitoring dynamic disease evolution using liquid biopsies is likely to play an important role in this setting, enabling clinicians to serially monitor changes in circulating tumor DNA, mutation burden, the appearance or disappearance of mutations during treatment, and as a predictor of response or progression. Finally, as our biological and translational understanding of acquired resistance continues to develop, this will inform specific treatments for subsequent lines of therapy in the second-line setting and beyond.
6. Conclusions
Advancements in the clinical development of EGFR-TKIs have led to evolving improvements in clinical outcomes for patients with EGFRm positive advanced NSCLC. In the first-line setting, osimertinib treatment provided superior efficacy to previous standard EGFR-TKI therapy with significantly longer PFS and improvements in time to treatment failure and time to second subsequent therapy or death. Furthermore, preclinical and clinical data indicate that osimertinib can cross the intact blood-brain barrier, and CNS efficacy has been demonstrated in patients with EGFRm positive advanced NSCLC. Acquired resistance in patients receiving first-line osimertinib is currently being investigated but, encouragingly, preliminary data show that no new unexpected mechanisms of resistance have been so far identified. Due to the observed multifactorial resistance mechanisms, including EGFR mutations, activation of bypass signaling pathways, phenotypic and histologic transformation, new therapeutic strategies are needed to tailor personalized treatment options. Several clinical development programs are ongoing to investigate the role of combination approaches with osimertinib. Further understanding of these potential combinations will provide critical information to inform future treatment decisions.
Supplementary Material
Acknowledgements
The authors would like to acknowledge Natasha Cary and Laura Crocker, of iMed Comms, Macclesfield, UK, an Ashfield Company, part of UDG Healthcare plc, for medical writing support that was funded by AstraZeneca in accordance with Good Publications Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Funding
This research was funded by AstraZeneca US. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Abbreviations:
- CI
confidence interval
- CNS
central nervous system
- ctDNA
circulating tumor DNA
- ddPCR
droplet digital polymerase chain reaction
- EGFR
epidermal growth factor receptor
- EGFRm
EGFR-TKI sensitizing mutation
- Ex19del
exon 19 deletion
- HR
hazard ratio
- mTOR
mammalian target of rapamycin
- NC
non-calculable
- NSCLC
non-small-cell lung cancer
- ORR
objective response rate
- OS
overall survival
- PFS
progression-free survival
- PPA
positive percentage agreement
- RCT
randomized control trial
- SoC
standard of care
- TKI
tyrosine kinase inhibitor
- VEGF
vascular endothelial growth factor
References
- [1].World Health Organization. Cancer - Key facts, 2018. Available at http://www.who.int/news-room/fact-sheets/detail/cancer accessed on 20 February.
- [2].Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre-Finn C, et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018;29:iv192–iv237. [DOI] [PubMed] [Google Scholar]
- [3].Kalemkerian GP, Narula N, Kennedy EB, Biermann WA, Donington J, Leighl NB, et al. Molecular Testing Guideline for the Selection of Patients With Lung Cancer for Treatment With Targeted Tyrosine Kinase Inhibitors: American Society of Clinical Oncology Endorsement of the College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology Clinical Practice Guideline Update. J Clin Oncol 2018;36:911–9. [DOI] [PubMed] [Google Scholar]
- [4].Zhang YL, Yuan JQ, Wang KF, Fu XH, Han XR, Threapleton D, et al. The prevalence of EGFR mutation in patients with non-small cell lung cancer: a systematic review and meta-analysis. Oncotarget 2016;7:78985–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Cho J, Chen L, Sangji N, Okabe T, Yonesaka K, Francis JM, et al. Cetuximab response of lung cancer-derived EGF receptor mutants is associated with asymmetric dimerization. Cancer Res 2013;73:6770–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Fukuoka M, Wu YL, Thongprasert S, Sunpaweravong P, Leong SS, Sriuranpong V, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol 2011;29:2866–74. [DOI] [PubMed] [Google Scholar]
- [7].Inoue A, Kobayashi K, Maemondo M, Sugawara S, Oizumi S, Isobe H, et al. Updated overall survival results from a randomized phase III trial comparing gefitinib with carboplatin-paclitaxel for chemo-naive non-small cell lung cancer with sensitive EGFR gene mutations (NEJ002). Ann Oncol 2013;24:54–9. [DOI] [PubMed] [Google Scholar]
- [8].Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121–8. [DOI] [PubMed] [Google Scholar]
- [9].Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, et al. Final overall survival results from a randomised, phase III study of erlotinib versus chemotherapy as first-line treatment of EGFR mutation-positive advanced non-small-cell lung cancer (OPTIMAL, CTONG-0802). Ann Oncol 2015;26:1877–83. [DOI] [PubMed] [Google Scholar]
- [10].Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239–46. [DOI] [PubMed] [Google Scholar]
- [11].Wu YL, Zhou C, Liam CK, Wu G, Liu X, Zhong Z, et al. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: analyses from the phase III, randomized, open-label, ENSURE study. Ann Oncol 2015;26:1883–9. [DOI] [PubMed] [Google Scholar]
- [12].Kato T, Yoshioka H, Okamoto I, Yokoyama A, Hida T, Seto T, et al. Afatinib versus cisplatin plus pemetrexed in Japanese patients with advanced non-small cell lung cancer harboring activating EGFR mutations: Subgroup analysis of LUX-Lung 3. Cancer Sci 2015;106:1202–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wu YL, Zhou C, Hu CP, Feng J, Lu S, Huang Y, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:213–22. [DOI] [PubMed] [Google Scholar]
- [14].Wu YL, Cheng Y, Zhou X, Lee KH, Nakagawa K, Niho S, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol 2017;18:1454–66. [DOI] [PubMed] [Google Scholar]
- [15].Cross DA, Ashton SE, Ghiorghiu S, Eberlein C, Nebhan CA, Spitzler PJ, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov 2014;4:1046–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mok TS, Wu YL, Ahn MJ, Garassino MC, Kim HR, Ramalingam SS, et al. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N Engl J Med 2017;376:629–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wu YL, Ahn MJ, Garassino MC, Han JY, Katakami N, Kim HR, et al. CNS Efficacy of Osimertinib in Patients With T790M-Positive Advanced Non-Small-Cell Lung Cancer: Data From a Randomized Phase III Trial (AURA3). J Clin Oncol 2018;36:2702–9. [DOI] [PubMed] [Google Scholar]
- [18].Reungwetwattana T, Nakagawa K, Cho BC, Cobo M, Cho EK, Bertolini A, et al. CNS Response to Osimertinib Versus Standard Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Patients With Untreated EGFR-Mutated Advanced Non–Small-Cell Lung Cancer. J Clin Oncol 2018;36:3290–7. [DOI] [PubMed] [Google Scholar]
- [19].Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:113–25. [DOI] [PubMed] [Google Scholar]
- [20].Hanna N, Johnson D, Temin S, Baker S Jr., Brahmer J, Ellis PM, et al. Systemic Therapy for Stage IV Non-Small-Cell Lung Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol 2017;35:3484–515. [DOI] [PubMed] [Google Scholar]
- [21].Novello S, Barlesi F, Califano R, Cufer T, Ekman S, Levra MG, et al. Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27:v1–v27. [DOI] [PubMed] [Google Scholar]
- [22].Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 2011;3:75ra26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kobayashi S, Boggon TJ, Dayaram T, Janne PA, Kocher O, Meyerson M, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med 2005;352:786–92. [DOI] [PubMed] [Google Scholar]
- [24].Yap TA, Vidal L, Adam J, Stephens P, Spicer J, Shaw H, et al. Phase I trial of the irreversible EGFR and HER2 kinase inhibitor BIBW 2992 in patients with advanced solid tumors. J Clin Oncol 2010;28:3965–72. [DOI] [PubMed] [Google Scholar]
- [25].Engelman JA, Zejnullahu K, Gale CM, Lifshits E, Gonzales AJ, Shimamura T, et al. PF00299804, an irreversible pan-ERBB inhibitor, is effective in lung cancer models with EGFR and ERBB2 mutations that are resistant to gefitinib. Cancer Res 2007;67:11924–32. [DOI] [PubMed] [Google Scholar]
- [26].Li D, Ambrogio L, Shimamura T, Kubo S, Takahashi M, Chirieac LR, et al. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene 2008;27:4702–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Watanabe M, Oizumi S, Kiuchi S, Yamada N, Yokouchi H, Fukumoto S, et al. The Effectiveness of Afatinib in a Patient with Advanced Lung Adenocarcinoma Harboring Rare G719X and S768I Mutations. Intern Med 2018;57:993–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ke EE, Wu Y-L. Afatinib in the first-line treatment of epidermal-growth-factor-receptor mutation-positive non-small cell lung cancer: a review of the clinical evidence. Therapeutic advances in respiratory disease 2016;10:256–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Yang JC, Wu YL, Schuler M, Sebastian M, Popat S, Yamamoto N, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 2015;16:141–51. [DOI] [PubMed] [Google Scholar]
- [30].Mok TS CY, Zhou X, Lee KH, Nakagawa K, Niho S, Lee M, Linke R, Rosell R, Corral J, Migliorino MR, Pluzanski A, Sbar EI, Wang T, White JL, Wu YL. Improvement in Overall Survival in a Randomized Study That Compared Dacomitinib With Gefitinib in Patients With Advanced Non-Small-Cell Lung Cancer and EGFR-Activating Mutations. J Clin Oncol 2018;36:2244–50. [DOI] [PubMed] [Google Scholar]
- [31].Mok TS, Cheng Y, Zhou X, Lee KH, Nakagawa K, Niho S, et al. Improvement in Overall Survival in a Randomized Study That Compared Dacomitinib With Gefitinib in Patients With Advanced Non–Small-Cell Lung Cancer and EGFR-Activating Mutations. J Clin Oncol 2018;36:2244–50; Data Supplement. [DOI] [PubMed] [Google Scholar]
- [32].Sequist LV, Yang JC, Yamamoto N, O’Byrne K, Hirsh V, Mok T, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327–34. [DOI] [PubMed] [Google Scholar]
- [33].Park K, Tan EH, O’Byrne K, Zhang L, Boyer M, Mok T, et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol 2016;17:577–89. [DOI] [PubMed] [Google Scholar]
- [34].Miller VA, Hirsh V, Cadranel J, Chen YM, Park K, Kim SW, et al. Afatinib versus placebo for patients with advanced, metastatic non-small-cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX-Lung 1): a phase 2b/3 randomised trial. Lancet Oncol 2012;13:528–38. [DOI] [PubMed] [Google Scholar]
- [35].Katakami N, Atagi S, Goto K, Hida T, Horai T, Inoue A, et al. LUX-Lung 4: a phase II trial of afatinib in patients with advanced non-small-cell lung cancer who progressed during prior treatment with erlotinib, gefitinib, or both. J Clin Oncol 2013;31:3335–41. [DOI] [PubMed] [Google Scholar]
- [36].Ramalingam SS, Janne PA, Mok T, O’Byrne K, Boyer MJ, Von Pawel J, et al. Dacomitinib versus erlotinib in patients with advanced-stage, previously treated non-small-cell lung cancer (ARCHER 1009): a randomised, double-blind, phase 3 trial. Lancet Oncol 2014;15:1369–78. [DOI] [PubMed] [Google Scholar]
- [37].Oxnard GR, Arcila ME, Sima CS, Riely GJ, Chmielecki J, Kris MG, et al. Acquired resistance to EGFR tyrosine kinase inhibitors in EGFR-mutant lung cancer: distinct natural history of patients with tumors harboring the T790M mutation. Clin Cancer Res 2011;17:1616–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Yu HA, Arcila ME, Rekhtman N, Sima CS, Zakowski MF, Pao W, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res 2013;19:2240–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Arcila ME, Oxnard GR, Nafa K, Riely GJ, Solomon SB, Zakowski MF, et al. Rebiopsy of lung cancer patients with acquired resistance to EGFR inhibitors and enhanced detection of the T790M mutation using a locked nucleic acid-based assay. Clin Cancer Res 2011;17:1169–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Sun JM, Ahn MJ, Choi YL, Ahn JS, Park K. Clinical implications of T790M mutation in patients with acquired resistance to EGFR tyrosine kinase inhibitors. Lung Cancer 2013;82:294–8. [DOI] [PubMed] [Google Scholar]
- [41].Kuiper JL, Heideman DA, Thunnissen E, Paul MA, van Wijk AW, Postmus PE, et al. Incidence of T790M mutation in (sequential) rebiopsies in EGFR-mutated NSCLC-patients. Lung Cancer 2014;85:19–24. [DOI] [PubMed] [Google Scholar]
- [42].Li W, Ren S, Li J, Li A, Fan L, Li X, et al. T790M mutation is associated with better efficacy of treatment beyond progression with EGFR-TKI in advanced NSCLC patients. Lung Cancer 2014;84:295–300. [DOI] [PubMed] [Google Scholar]
- [43].Akamatsu H, Delmonte A, John T, Su W-C, Lee J-S, Chang G-C, et al. 421P EGFR mutation analysis for prospective patient (pt) selection in AURA3 Phase III trial of osimertinib vs platinum-pemetrexed (plt-pem) in pts with EGFR T790M positive advanced non-small cell lung cancer (NSCLC). Ann Oncol 2017;28:mdx671.010. [DOI] [PubMed] [Google Scholar]
- [44].Jenkins S, Chih-Hsin Yang J, Janne PA, Thress KS, Yu K, Hodge R, et al. EGFR mutation analysis for prospective patient selection in two Phase II registration studies of osimertinib. J Thorac Oncol 2017;12:1247–56. [DOI] [PubMed] [Google Scholar]
- [45].Stewart EL, Tan SZ, Liu G, Tsao MS. Known and putative mechanisms of resistance to EGFR targeted therapies in NSCLC patients with EGFR mutations-a review. Transl Lung Cancer Res 2015;4:67–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Morgillo F, Della Corte CM, Fasano M, Ciardiello F. Mechanisms of resistance to EGFR-targeted drugs: lung cancer. ESMO Open 2016;1:e000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Yang JC, Ahn MJ, Kim DW, Ramalingam SS, Sequist LV, Su WC, et al. Osimertinib in Pretreated T790M-Positive Advanced Non-Small-Cell Lung Cancer: AURA Study Phase II Extension Component. J Clin Oncol 2017;35:1288–96. [DOI] [PubMed] [Google Scholar]
- [48].Jin Y, Shao Y, Shi X, Lou G, Zhang Y, Wu X, et al. Mutational profiling of non-small-cell lung cancer patients resistant to first-generation EGFR tyrosine kinase inhibitors using next generation sequencing. Oncotarget 2016;7:61755–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Ji W, Choi C-M, Rho JK, Jang SJ, Park YS, Chun S-M, et al. Mechanisms of acquired resistance to EGFR-tyrosine kinase inhibitor in Korean patients with lung cancer. BMC Cancer 2013;13:606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Uramoto H, Yamada T, Yano S, Kondo N, Hasegawa S, Tanaka F. Prognostic value of acquired resistance-related molecules in Japanese patients with NSCLC treated with an EGFR-TKI. Anticancer Res 2012;32:3785–90. [PubMed] [Google Scholar]
- [51].Sos ML, Rode HB, Heynck S, Peifer M, Fischer F, Kluter S, et al. Chemogenomic profiling provides insights into the limited activity of irreversible EGFR Inhibitors in tumor cells expressing the T790M EGFR resistance mutation. Cancer Res 2010;70:868–74. [DOI] [PubMed] [Google Scholar]
- [52].Yun CH, Mengwasser KE, Toms AV, Woo MS, Greulich H, Wong KK, et al. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc Natl Acad Sci U S A 2008;105:2070–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Sequist LV, Martins RG, Spigel D, Grunberg SM, Spira A, Janne PA, et al. First-line gefitinib in patients with advanced non-small-cell lung cancer harboring somatic EGFR mutations. J Clin Oncol 2008;26:2442–9. [DOI] [PubMed] [Google Scholar]
- [54].Riely GJ, Yu HA, Arcila ME, Hellmann MD, Ladanyi M, Kris MG. Response to erlotinib and prognosis for patients with de novo epidermal growth factor receptor (EGFR) T790M mutations. J Clin Oncol 2013;31:Abstract 8018. [Google Scholar]
- [55].Li Y, Appius A, Pattipaka T, Feyereislova A, Cassidy A, Ganti AK. Real-world management of patients with epidermal growth factor receptor (EGFR) mutation-positive non-small-cell lung cancer in the USA. PLoS One 2019;14:e0209709–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Rosell R, Molina MA, Costa C, Simonetti S, Gimenez-Capitan A, Bertran-Alamillo J, et al. Pretreatment EGFR T790M mutation and BRCA1 mRNA expression in erlotinib-treated advanced non-small-cell lung cancer patients with EGFR mutations. Clin Cancer Res 2011;17:1160–8. [DOI] [PubMed] [Google Scholar]
- [57].Su KY, Chen HY, Li KC, Kuo ML, Yang JC, Chan WK, et al. Pretreatment epidermal growth factor receptor (EGFR) T790M mutation predicts shorter EGFR tyrosine kinase inhibitor response duration in patients with non-small-cell lung cancer. J Clin Oncol 2012;30:433–40. [DOI] [PubMed] [Google Scholar]
- [58].Maheswaran S, Sequist LV, Nagrath S, Ulkus L, Brannigan B, Collura CV, et al. Detection of mutations in EGFR in circulating lung-cancer cells. N Engl J Med 2008;359:366–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Lee Y, Lee GK, Lee YS, Zhang W, Hwang JA, Nam BH, et al. Clinical outcome according to the level of preexisting epidermal growth factor receptor T790M mutation in patients with lung cancer harboring sensitive epidermal growth factor receptor mutations. Cancer 2014;120:2090–8. [DOI] [PubMed] [Google Scholar]
- [60].Zhao J, Feng HH, Zhao JY, Liu LC, Xie FF, Xu Y, et al. A sensitive and practical method to detect the T790M mutation in the epidermal growth factor receptor. Oncol Lett 2016;11:2573–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Liu Y, Sun L, Xiong ZC, Sun X, Zhang SL, Ma JT, et al. Meta-analysis of the impact of de novo and acquired EGFR T790M mutations on the prognosis of patients with non-small cell lung cancer receiving EGFR-TKIs. Onco Targets Ther 2017;10:2267–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Oxnard GR, Miller VA, Robson ME, Azzoli CG, Pao W, Ladanyi M, et al. Brief report: Screening for germline EGFR T790M mutations through lung cancer genotyping. J Thorac Oncol 2012;7:1049–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Yu HA, Arcila ME, Harlan Fleischut M, Stadler Z, Ladanyi M, Berger MF, et al. Germline EGFR T790M mutation found in multiple members of a familial cohort. J Thorac Oncol 2014;9:554–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].ClinicalTrials.gov. INHERIT EGFR - Studying Germline EGFR Mutations (INHERIT), 2018. Available at https://clinicaltrials.gov/ct2/show/NCT01754025, accessed on 9 May 2019.
- [65].Takezawa K, Pirazzoli V, Arcila ME, Nebhan CA, Song X, de Stanchina E, et al. HER2 amplification: a potential mechanism of acquired resistance to EGFR inhibition in EGFR-mutant lung cancers that lack the second-site EGFRT790M mutation. Cancer Discov 2012;2:922–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 2007;316:1039–43. [DOI] [PubMed] [Google Scholar]
- [67].Poh ME, Liam CK, Rajadurai P, Chai CS. Epithelial-to-mesenchymal transition (EMT) causing acquired resistance to afatinib in a patient with epidermal growth factor receptor (EGFR)-mutant lung adenocarcinoma. J Thorac Dis 2018;10:E560–E3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Schrock Alexa B. VWZ, Hsieh Wen-Son, Madison Russell, Creelan Benjamin, Silberberg Jeffrey, Costin Dan, Bharne Anjali, Bonta Ioana, Bosemani Thangavijayan, Nikolinakos Petros, Ross Jeffrey S., Miller Vincent A., Ali Siraj M., Klempner Samuel J., Ou Sai-Hong Ignatius. Receptor Tyrosine Kinase Fusions and BRAF Kinase Fusions are Rare but Actionable Resistance Mechanisms to EGFR Tyrosine Kinase Inhibitors. Journal of Thoracic Oncology 2018;13:1312–23. [DOI] [PubMed] [Google Scholar]
- [69].Piotrowska Z, Isozaki H, Lennerz JK, Gainor JF, Lennes IT, Zhu VW, et al. Landscape of Acquired Resistance to Osimertinib in EGFR-Mutant NSCLC and Clinical Validation of Combined EGFR and RET Inhibition with Osimertinib and BLU-667 for Acquired RET Fusion. Cancer Discov 2018;8:1529–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Offin M, Somwar R, Rekhtman N, Benayed R, Chang JC, Plodkowski A, et al. Acquired ALK and RET Gene Fusions as Mechanisms of Resistance to Osimertinib in EGFR-Mutant Lung Cancers. JCO Precis Oncol 2018;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Yu HA, Suzawa K, Jordan E, Zehir A, Ni A, Kim R, et al. Concurrent Alterations in EGFR-Mutant Lung Cancers Associated with Resistance to EGFR Kinase Inhibitors and Characterization of MTOR as a Mediator of Resistance. Clin Cancer Res 2018;24:3108–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Walter AO, Sjin RT, Haringsma HJ, Ohashi K, Sun J, Lee K, et al. Discovery of a mutant-selective covalent inhibitor of EGFR that overcomes T790M-mediated resistance in NSCLC. Cancer Discov 2013;3:1404–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Sequist LV, Soria JC, Goldman JW, Wakelee HA, Gadgeel SM, Varga A, et al. Rociletinib in EGFR-mutated non-small-cell lung cancer. N Engl J Med 2015;372:1700–9. [DOI] [PubMed] [Google Scholar]
- [74].Lee K-O, Cha MY, Kim M, Song JY, Lee J-H, Kim YH, et al. Abstract LB-100: Discovery of HM61713 as an orally available and mutant EGFR selective inhibitor. Cancer Res 2014;74:LB–100. [Google Scholar]
- [75].Xu X, Mao L, Xu W, Tang W, Zhang X, Xi B, et al. AC0010, an Irreversible EGFR Inhibitor Selectively Targeting Mutated EGFR and Overcoming T790M-Induced Resistance in Animal Models and Lung Cancer Patients. Mol Cancer Ther 2016;15:2586–97. [DOI] [PubMed] [Google Scholar]
- [76].Lelais G, Epple R, Marsilje TH, Long YO, McNeill M, Chen B, et al. Discovery of (R,E)-N-(7-Chloro-1-(1-[4-(dimethylamino)but-2-enoyl]azepan-3-yl)-1H-benzo[d]imid azol-2-yl)-2-methylisonicotinamide (EGF816), a Novel, Potent, and WT Sparing Covalent Inhibitor of Oncogenic (L858R, ex19del) and Resistant (T790M) EGFR Mutants for the Treatment of EGFR Mutant Non-Small-Cell Lung Cancers. J Med Chem 2016;59:6671–89. [DOI] [PubMed] [Google Scholar]
- [77].Planken S, Behenna DC, Nair SK, Johnson TO, Nagata A, Almaden C, et al. Discovery of N-((3R,4R)-4-Fluoro-1-(6-((3-methoxy-1-methyl-1H-pyrazol-4-yl)amino)-9-methyl-9H-purin-2-yl)pyrrolidine-3-yl)acrylamide (PF-06747775) through Structure-Based Drug Design: A High Affinity Irreversible Inhibitor Targeting Oncogenic EGFR Mutants with Selectivity over Wild-Type EGFR. J Med Chem 2017;60:3002–19. [DOI] [PubMed] [Google Scholar]
- [78].Hong MH, Lee IY, Koh JS, Lee J, Suh B-C, Song H-J, et al. P3.02b-119 YH25448, a Highly Selective 3rd Generation EGFR TKI, Exhibits Superior Survival over Osimertinib in Animal Model with Brain Metastases from NSCLC: Topic: EGFR RES. J Thorac Oncol 2017;12:S1265–S6. [Google Scholar]
- [79].Mok TS, Wu Y-L, Ahn M-J, Garassino MC, Kim HR, Ramalingam SS, et al. Osimertinib or Platinum–Pemetrexed in EGFR T790M–Positive Lung Cancer. N Engl J Med 2017;376:629–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Goto Y, Nokihara H, Murakami H, Shimizu T, Seto T, Krivoshik AP, et al. ASP8273, a mutant-selective irreversible EGFR inhibitor in patients (pts) with NSCLC harboring EGFR activating mutations: Preliminary results of first-in-human phase I study in Japan. J Clin Oncol 2015;33:8014. [Google Scholar]
- [81].Goss G, Tsai C-M, Shepherd FA, Bazhenova L, Lee JS, Chang G-C, et al. Osimertinib for pretreated EGFR Thr790Met-positive advanced non-small-cell lung cancer (AURA2): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol 2016;17:1643–52. [DOI] [PubMed] [Google Scholar]
- [82].Goss G, Tsai CM, Shepherd FA, Ahn MJ, Bazhenova L, Crino L, et al. CNS response to osimertinib in patients with T790M-positive advanced NSCLC: pooled data from two phase II trials. Ann Oncol 2018;29:687–93. [DOI] [PubMed] [Google Scholar]
- [83].Wang Z, Chen R, Wang S, Zhong J, Wu M, Zhao J, et al. Quantification and dynamic monitoring of EGFR T790M in plasma cell-free DNA by digital PCR for prognosis of EGFR-TKI treatment in advanced NSCLC. PLoS One 2014;9:e110780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Zheng D, Ye X, Zhang MZ, Sun Y, Wang JY, Ni J, et al. Plasma EGFR T790M ctDNA status is associated with clinical outcome in advanced NSCLC patients with acquired EGFR-TKI resistance. Sci Rep 2016;6:20913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].NICE. Plasma EGFR mutation tests for adults with locally advanced or metastatic non-small-cell lung cancer. 2018.
- [86].Diaz LA Jr., Bardelli A. Liquid biopsies: genotyping circulating tumor DNA. J Clin Oncol 2014;32:579–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Sacher AG, Paweletz C, Dahlberg SE, Alden RS, O’Connell A, Feeney N, et al. Prospective Validation of Rapid Plasma Genotyping for the Detection of EGFR and KRAS Mutations in Advanced Lung Cancer. JAMA Oncol 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Kaburagi T, Kiyoshima M, Nawa T, Ichimura H, Saito T, Hayashihara K, et al. Acquired EGFR T790M Mutation After Relapse Following EGFR-TKI Therapy: A Population-based Multi-institutional Study. Anticancer Res 2018;38:3145–50. [DOI] [PubMed] [Google Scholar]
- [89].Jenkins S, Yang JC, Ramalingam SS, Yu K, Patel S, Weston S, et al. Plasma ctDNA Analysis for Detection of the EGFR T790M Mutation in Patients with Advanced Non-Small Cell Lung Cancer. J Thorac Oncol 2017;12:1061–70. [DOI] [PubMed] [Google Scholar]
- [90].Wu YL, Jenkins S, Ramalingam S, Han J-Y, Delmonte A, Hsia T-C, et al. MA08.03 Osimertinib vs Platinum-Pemetrexed for T790M-Mutation Positive Advanced NSCLC (AURA3): Plasma ctDNA Analysis. J Thorac Oncol 12:S386. [Google Scholar]
- [91].Oxnard GR, Thress KS, Alden RS, Lawrance R, Paweletz CP, Cantarini M, et al. Association Between Plasma Genotyping and Outcomes of Treatment With Osimertinib (AZD9291) in Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2016;34:3375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].NCCN. National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology: Non-Small Cell Lung Cancer (v7.2017), 2017. Available at https://www.nccn.org/professionals/physician_gls/PDF/nscl.pdf, accessed on 23 June 2017.
- [93].Seto T, Nogami N, Yamamoto N, Atagi S, Tashiro N, Yoshimura Y, et al. Real-World EGFR T790M Testing in Advanced Non-Small-Cell Lung Cancer: A Prospective Observational Study in Japan. Oncology and Therapy 2018;6:203–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Overman MJ, Modak J, Kopetz S, Murthy R, Yao JC, Hicks ME, et al. Use of research biopsies in clinical trials: are risks and benefits adequately discussed? J Clin Oncol 2013;31:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Nosaki K, Satouchi M, Kurata T, Yoshida T, Okamoto I, Katakami N, et al. Re-biopsy status among non-small cell lung cancer patients in Japan: A retrospective study. Lung Cancer 2016;101:1–8. [DOI] [PubMed] [Google Scholar]
- [96].Kawamura T, Kenmotsu H, Taira T, Omori S, Nakashima K, Wakuda K, et al. Rebiopsy for patients with non-small-cell lung cancer after epidermal growth factor receptor-tyrosine kinase inhibitor failure. Cancer Sci 2016;107:1001–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Stockley T, Souza CA, Cheema PK, Melosky B, Kamel-Reid S, Tsao MS, et al. Evidence-based best practices for EGFR T790M testing in lung cancer in Canada. 2018 2018;25:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Chiang A, Fernandes A, Pavilack M, Wu J, Laliberté F, Duh MS, et al. MA15.11 Real World Biomarker Testing and Treatment Patterns in Patients with Advanced NSCLC Receiving EGFR-TKIs. J Thorac Oncol 2018;13:S410–S1. [Google Scholar]
- [99].Roengvoraphoj M, Tsongalis GJ, Dragnev KH, Rigas JR. Epidermal growth factor receptor tyrosine kinase inhibitors as initial therapy for non-small cell lung cancer: focus on epidermal growth factor receptor mutation testing and mutation-positive patients. Cancer Treat Rev 2013;39:839–50. [DOI] [PubMed] [Google Scholar]
- [100].Cho JH, Sun J, Lee S, Ahn JS, Park K, Park KU, et al. OA10.05 An Open-Label, Multicenter, Phase II Single Arm Trial of Osimertinib in NSCLC Patients with Uncommon EGFR Mutation(KCSG-LU15–09). J Thorac Oncol 2018;13:S344. [Google Scholar]
- [101].Douillard JY, Ostoros G, Cobo M, Ciuleanu T, McCormack R, Webster A, et al. First-line gefitinib in Caucasian EGFR mutation-positive NSCLC patients: a phase-IV, open-label, single-arm study. Br J Cancer 2014;110:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Han JY, Park K, Kim SW, Lee DH, Kim HY, Kim HT, et al. First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol 2012;30:1122–8. [DOI] [PubMed] [Google Scholar]
- [103].Shi YK, Wang L, Han BH, Li W, Yu P, Liu YP, et al. First-line icotinib versus cisplatin/pemetrexed plus pemetrexed maintenance therapy for patients with advanced EGFR mutation-positive lung adenocarcinoma (CONVINCE): a phase 3, open-label, randomized study. Ann Oncol 2017;28:2443–50. [DOI] [PubMed] [Google Scholar]
- [104].Roeper J, Falk M, Tiemann M, Wesseler C, Wiest G, Sackmann S, et al. Risk of not receiving 2nd line therapy is high in EGFR mt+ pts: Real world data of certified lung cancer centers on treatment sequence in EGFR mt+ pts. J Clin Oncol 2018;36:e21220–e. [Google Scholar]
- [105].FDA. TAGRISSO (osimertinib) Supplement Approval for updates to the U.S. Prescribing Information, 2018. Available at https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2018/208065Orig1s008ltr.pdf, accessed on 26 April 2018.
- [106].EMA. TAGRISSO (osimertinib) Summary of Product Characteristics, 2018. Available at https://www.ema.europa.eu/documents/product-information/tagrisso-epar-product-information_en.pdf, accessed on 22 February 2019.
- [107].Dagogo-Jack I, Shaw AT. Tumour heterogeneity and resistance to cancer therapies. Nat Rev Clin Oncol 2018;15:81–94. [DOI] [PubMed] [Google Scholar]
- [108].Iuchi T, Shingyoji M, Sakaida T, Hatano K, Nagano O, Itakura M, et al. Phase II trial of gefitinib alone without radiation therapy for Japanese patients with brain metastases from EGFR-mutant lung adenocarcinoma. Lung Cancer 2013;82:282–7. [DOI] [PubMed] [Google Scholar]
- [109].Han G, Bi J, Tan W, Wei X, Wang X, Ying X, et al. A retrospective analysis in patients with EGFR-mutant lung adenocarcinoma: is EGFR mutation associated with a higher incidence of brain metastasis? Oncotarget 2016;7:56998–7010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Planchard D, Boyer M, Lee JS, Dechaphunkul A, Cheema P, Takahashi T, et al. 128O Osimertinib vs standard of care (SoC) EGFR-TKI as first-line therapy in patients (pts) with untreated EGFRm advanced NSCLC: FLAURA post-progression outcomes. J Thorac Oncol 2018;13:S72–S3. [Google Scholar]
- [111].Ramalingam SS, Yang JC, Lee CK, Kurata T, Kim DW, John T, et al. Osimertinib As First-Line Treatment of EGFR Mutation-Positive Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2018;36:841–9. [DOI] [PubMed] [Google Scholar]
- [112].Ramalingam SS, Cheng Y, Zhou C, Ohe Y, Imamura F, Cho BC, et al. Mechanisms of acquired resistance to first-line osimertinib: Preliminary data from the phase III FLAURA study Oral presentation LBA50 at European Society for Medical Oncology (ESMO), Munich, Germany, 19–23 October 2018. [Google Scholar]
- [113].Piotrowska Zofia JC, Cripps Diana, Miranda Miguel F. P1.01–80 - ELIOS: A Multicenter, Open-Label, Molecular Profiling Study of Patients with EGFRm and NSCLC Treated with Osimertinib. WCLC; 2018. [Google Scholar]
- [114].Yu HA, Tian SK, Drilon AE, Borsu L, Riely GJ, Arcila ME, et al. Acquired Resistance of EGFR-Mutant Lung Cancer to a T790M-Specific EGFR Inhibitor: Emergence of a Third Mutation (C797S) in the EGFR Tyrosine Kinase DomainAcquired Resistance of EGFR-Mutant Lung Cancer to an EGFR InhibitorLetters. JAMA Oncology 2015;1:982–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Wang S, Tsui ST, Liu C, Song Y, Liu D. EGFR C797S mutation mediates resistance to third-generation inhibitors in T790M-positive non-small cell lung cancer. J Hematol Oncol 2016;9:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Thress KS, Paweletz CP, Felip E, Cho BC, Stetson D, Dougherty B, et al. Acquired EGFR C797S mutation mediates resistance to AZD9291 in non-small cell lung cancer harboring EGFR T790M. Nat Med 2015;21:560–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Wang S, Song Y, Liu D. EAI045: The fourth-generation EGFR inhibitor overcoming T790M and C797S resistance. Cancer Lett 2017;385:51–4. [DOI] [PubMed] [Google Scholar]
- [118].Jia Y, Yun CH, Park E, Ercan D, Manuia M, Juarez J, et al. Overcoming EGFR(T790M) and EGFR(C797S) resistance with mutant-selective allosteric inhibitors. Nature 2016;534:129–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].To C, Jang J, Chen T, Park E, Mushajiang M, De Clercq DJH, et al. Single and dual targeting of mutant EGFR with an allosteric inhibitor. 2019;CD-18–0903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Niederst MJ, Hu H, Mulvey HE, Lockerman EL, Garcia AR, Piotrowska Z, et al. The Allelic Context of the C797S Mutation Acquired upon Treatment with Third-Generation EGFR Inhibitors Impacts Sensitivity to Subsequent Treatment Strategies. Clin Cancer Res 2015;21:3924–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Ahn M, Han J, Sequist L, Cho BC, Lee JS, Kim S, et al. OA 09.03 TATTON Ph Ib Expansion Cohort: Osimertinib plus Savolitinib for Pts with EGFR-Mutant MET-Amplified NSCLC after Progression on Prior EGFR-TKI. J Thorac Oncol 2017;12:S1768. [Google Scholar]
- [122].Yang J, Fang J, Shu Y, Chang J, Chen G, He J, et al. OA 09.06 A Phase Ib Trial of Savolitinib plus Gefitinib for Chinese Patients with EGFR-Mutant MET-Amplified Advanced NSCLC. J Thorac Oncol 2017;12:S1769. [Google Scholar]
- [123].Haura EB, Cho BC, Lee JS, Han J-Y, Lee KH, Sanborn RE, et al. JNJ-61186372 (JNJ-372), an EGFR-cMet bispecific antibody, in EGFR-driven advanced non-small cell lung cancer (NSCLC). Journal of Clinical Oncology 2019;37:9009-. [Google Scholar]
- [124].York ER, Varella-Garcia M, Bang TJ, Aisner DL, Camidge DR. Tolerable and Effective Combination of Full-Dose Crizotinib and Osimertinib Targeting MET Amplification Sequentially Emerging after T790M Positivity in EGFR-Mutant Non-Small Cell Lung Cancer. J Thorac Oncol 2017;12:e85–e8. [DOI] [PubMed] [Google Scholar]
- [125].Scagliotti G, von Pawel J, Novello S, Ramlau R, Favaretto A, Barlesi F, et al. Phase III Multinational, Randomized, Double-Blind, Placebo-Controlled Study of Tivantinib (ARQ 197) Plus Erlotinib Versus Erlotinib Alone in Previously Treated Patients With Locally Advanced or Metastatic Nonsquamous Non-Small-Cell Lung Cancer. J Clin Oncol 2015;33:2667–74. [DOI] [PubMed] [Google Scholar]
- [126].Spigel DR, Ervin TJ, Ramlau RA, Daniel DB, Goldschmidt JH Jr., Blumenschein GR Jr., et al. Randomized phase II trial of Onartuzumab in combination with erlotinib in patients with advanced non-small-cell lung cancer. J Clin Oncol 2013;31:4105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Tarhini AA. Tremelimumab: a review of development to date in solid tumors. Immunotherapy 2013;5:215–29. [DOI] [PubMed] [Google Scholar]
- [128].Ou SI, Govindan R, Eaton KD, Otterson GA, Gutierrez ME, Mita AC, et al. Phase I Results from a Study of Crizotinib in Combination with Erlotinib in Patients with Advanced Nonsquamous Non-Small Cell Lung Cancer. J Thorac Oncol 2017;12:145–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Yoshioka H, Azuma K, Yamamoto N, Takahashi T, Nishio M, Katakami N, et al. A randomized, double-blind, placebo-controlled, phase III trial of erlotinib with or without a c-Met inhibitor tivantinib (ARQ 197) in Asian patients with previously treated stage IIIB/IV nonsquamous nonsmall-cell lung cancer harboring wild-type epidermal growth factor receptor (ATTENTION study). Ann Oncol 2015;26:2066–72. [DOI] [PubMed] [Google Scholar]
- [130].Janjigian YY, Smit EF, Groen HJ, Horn L, Gettinger S, Camidge DR, et al. Dual inhibition of EGFR with afatinib and cetuximab in kinase inhibitor-resistant EGFR-mutant lung cancer with and without T790M mutations. Cancer Discov 2014;4:1036–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Akbay EA, Koyama S, Carretero J, Altabef A, Tchaicha JH, Christensen CL, et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov 2013;3:1355–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Gibbons D, Chow L, Kim D, et al. Efficacy, safety and tolerability of MEDI4736 (durvalumab [D]), a human IgG1 antiprogrammed cell death-ligand-1 (PD-L1) antibody, combined with gefitinib (G): a phase I expansion in TKI-naive patients (pts) with EGFR mutant NSCLC. J Thorac Oncol 2016;11:S79. [Google Scholar]
- [133].Ma BBY, Rudin CM, Cervantes A, Dowlati A, Costa D, Schmid P, et al. 441O Preliminary safety and clinical activity of erlotinib plus atezolizumab from a Phase Ib study in advanced NSCLC. Ann Oncol 2016;27:mdw594.005. [Google Scholar]
- [134].Yang JC, Gadgeel SM, Sequist LV, Wu CL, Papadimitrakopoulou VA, Su WC, et al. Pembrolizumab in Combination With Erlotinib or Gefitinib as First-Line Therapy for Advanced NSCLC With Sensitizing EGFR Mutation. J Thorac Oncol 2019;14:553–9. [DOI] [PubMed] [Google Scholar]
- [135].Reck M, Jotte R, Mok TSK, Lim DW-T, Cappuzzo F, Orlandi F, et al. 104O IMpower150: An exploratory analysis of efficacy outcomes in patients with EGFR mutations. Ann Oncol 2019;30:mdz063.02. [Google Scholar]
- [136].Nakamura A, Inoue A, Morita S, Hosomi Y, Kato T, Fukuhara T, et al. Phase III study comparing gefitinib monotherapy (G) to combination therapy with gefitinib, carboplatin, and pemetrexed (GCP) for untreated patients (pts) with advanced non-small cell lung cancer (NSCLC) with EGFR mutations (NEJ009). ASCO. [Google Scholar]
- [137].Noronha V, Patil VM, Joshi A, Menon N, Chougule A, Mahajan A, et al. Gefitinib Versus Gefitinib Plus Pemetrexed and Carboplatin Chemotherapy in EGFR-Mutated Lung Cancer. J Clin Oncol 2019;Jco1901154. [DOI] [PubMed] [Google Scholar]
- [138].Wu Y-L, Zhou C, Hu C-P, Feng J, Lu S, Huang Y, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:213–22. [DOI] [PubMed] [Google Scholar]
- [139].Paz-Ares L, Tan EH, O’Byrne K, Zhang L, Hirsh V, Boyer M, et al. Afatinib versus gefitinib in patients with EGFR mutation-positive advanced non-small-cell lung cancer: overall survival data from the phase IIb LUX-Lung 7 trial. Ann Oncol 2017;28:270–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Wu Y-L, Cheng Y, Zhou X, Lee KH, Nakagawa K, Niho S, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol 2017;18:1454–66. [DOI] [PubMed] [Google Scholar]
- [141].Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735–42. [DOI] [PubMed] [Google Scholar]
- [142].Sequist LV, Wu Y, Schuler M, Kato T, Yang JC, Tanaka H, et al. PS02.20 Subsequent Therapies Post-Afatinib Among Patients with EGFR Mutation-Positive (EGFRm+) NSCLC in LUX-Lung 3, 6 and 7: Topic: Medical Oncology. J Thorac Oncol 2017;12:S1572. [Google Scholar]
- [143].Yang JJ, Zhou Q, Yan HH, Zhang XC, Chen HJ, Tu HY, et al. A phase III randomised controlled trial of erlotinib vs gefitinib in advanced non-small cell lung cancer with EGFR mutations. Br J Cancer 2017;116:568–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Wang Z-F, Ren S-X, Li W, Gao G-H. Frequency of the acquired resistant mutation T790 M in non-small cell lung cancer patients with active exon 19Del and exon 21 L858R: a systematic review and meta-analysis. BMC Cancer 2018;18:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Gray JE, Thakrar B, Sun P, Maclachlan S, Chehab N, Potter D. 509P Treatment (tx) patterns in patients (pts) with lung cancer starting 1st or 2nd generation (1G/2G) EGFR-TKI: A US insurance claims database analysis. Ann Oncol 2018;29:mdy425.020. [Google Scholar]
- [146].Le X, Puri S, Negrao MV, Nilsson M, Robichaux JP, Boyle TA, et al. Landscape of EGFR -dependent and -independent resistance mechanisms to osimertinib and continuation therapy post-progression in EGFR-mutant NSCLC. Clin Cancer Res 2018;24:6195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Lin C-C. Outcomes in patients with non-small-cell lung cancer and acquired Thr790Met mutation treated with osimertinib: a genomic study. Lancet Respir Med 2018;6:107–16. [DOI] [PubMed] [Google Scholar]
- [148].Oxnard GR, Hu Y, Mileham KF, Husain H, Costa DB, Tracy P, et al. Assessment of Resistance Mechanisms and Clinical Implications in Patients With EGFR T790M-Positive Lung Cancer and Acquired Resistance to Osimertinib. JAMA Oncol 2018;4:1527–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Piotrowska Z, Thress KS, Mooradian M, Heist RS, Azzoli CG, Temel JS, et al. MET amplification (amp) as a resistance mechanism to osimertinib. J Clin Oncol 2017;35:Abstract 9020. [Google Scholar]
- [150].Yang Z, Yang N, Ou Q, Xiang Y, Jiang T, Wu X, et al. Investigating Novel Resistance Mechanisms to Third-Generation EGFR Tyrosine Kinase Inhibitor Osimertinib in Non-Small Cell Lung Cancer Patients. Clin Cancer Res 2018;24:3097–107. [DOI] [PubMed] [Google Scholar]
- [151].Zhou C, Hu M, Zhu X, Sun Y, Lu X, Wang J, et al. OA10.07 Resistance Mechanisms of Osimertinib in Chinese Non-Small Cell Lung Cancer patients: Analysis from AURA17 Trial. J Thorac Oncol 2018;13:S345. [Google Scholar]
- [152].Papadimitrakopoulou VA, Wu YL, Han JY, Ahn MJ, Ramalingam SS, John T, et al. LBA51 Analysis of resistance mechanisms to osimertinib in patients with EGFR T790M advanced NSCLC from the AURA3 study. Ann Oncol 2018;29:mdy424.064–mdy424.064. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


