Abstract
Quantification of cardiac troponin I (cTnI), a protein biomarker used for diagnosing myocardial infarction, has been achieved in native patient plasma based on an immunoaffinity enrichment strategy and isotope dilution (ID) liquid chromatography-tandem mass spectrometry (LC-MS/MS) method. The key steps in the workflow involved isolating cTnI from plasma using anti-cTnI antibody coupled to magnetic nanoparticles, followed by an enzymatic digestion with trypsin. Three tryptic peptides from cTnI were monitored and used for quantification by ID-LC-MS/MS via multiple reaction monitoring (MRM). Measurements were performed using a matrix-matched calibration system. NIST SRM 2921 Human Cardiac Troponin Complex acted as the calibrant and a full-length isotopically labeled protein analog of cTnI was used as an internal standard. The method was successfully demonstrated on five patient plasma samples, with cTnI concentrations measuring between 4.86 μg/L and 11.3 μg/L (signifying moderate myocardial infarctions). LC-MS/MS measurement precision was validated by three unique peptides from cTnI and two MRM transitions per peptide. Relative standard deviation (CV) from the five plasma samples was determined to be ≤14.3%. This study has demonstrated that quantification of cTnI in native plasma from myocardial infarction patients can be achieved based on an ID-LC-MS/MS method. The development of an ID-LC-MS/MS method for cTnI in plasma is a first step for future certification of matrix-based reference materials, which may be used to help harmonize discordant cTnI clinical assays.
Keywords: Protein biomarker, Cardiac troponin I, Mass spectrometry, Isotope dilution, Quantification, Multiple reaction monitoring
Introduction
Human cardiac troponin I (cTnI) is a well-established diagnostic biomarker of heart muscle damage. Upon cardiac muscle cell death, the troponin protein complex (consisting of T, C, and I subunits) is released from tissue into blood at levels that are far too low for direct measurement by current analytical techniques [1, 2]. At these concentrations – often near the low μg/L range – existing clinical assays rely on indirect, immunochemical-based measurements (i.e., ELISAs) using a capture and detection antibody conjugation system [2]. However, most commercial cTnI clinical immunoassays vary from each other by the types of antibodies, calibrators, and detection techniques used [3–6]. Therefore, quantification of this low-abundant protein biomarker is hampered by the use of non-standardized approaches among labs resulting in considerable measurement variability, ambiguous short- and long-term medical monitoring, and compromised treatment outcomes for patients [5, 7]. The lack of standardization for existing assays has been described as a major hurdle for the use of cTnI in primary care [8]. To address this issue, the certification of a matrix-based reference material has been proposed to ensure calibration traceability of cTnI measurements and sample comparability. However, a reference measurement method is needed first to assign true cTnI concentrations in blood [2, 9].
While it is critical for the clinical community to improve the comparability of cTnI clinical measurement results, (efforts are underway to harmonize clinical ELISA assays through the use of commutable reference materials, without a reference measurement procedure established [10, 11]) the ultimate goal for achieving absolute quantification of cTnI in blood remains. ID-LC-MS/MS techniques are considered the ‘gold-standard’ for absolute quantification of proteins, achieving the highest levels of accuracy and improved specificity, albeit at the cost of sensitivity when compared to immunoassays [12–14]. Direct measurements based on ID-LC-MS/MS are more accurate and specific because the method requires the use of an isotope-labeled protein internal standard analogous to the target protein, which can alleviate biases associated with sample processing and instrument variability [15–17]. Although a LC-MS/MS-based assay continues to be far from realization in routine clinical use, this technique should be considered when the highest degree of accuracy and specificity is required, such as in the development of certified reference materials that are fundamental to creating a traceability chain for clinical quantitative measurements [18, 19].
Since LC-MS/MS detection is not as sensitive as an ELISA, recent reports have demonstrated significant improvements in MS-based assays for achieving the necessary sensitivity to measure cTnI, enriched from a complex matrix. Picard et al. [20] demonstrated accurate quantification of troponin, purified from myocardial tissue, by measuring multiple tryptic peptides from cTnI via ID-LC-MS/MS. While in a similar study, Huillet et al. [21] used cTnI-spiked serum samples to validate an immunoenrichment and ID-LC-MS/MS detection strategy based on the use of an intact protein internal standard before performing relative cTnI quantification on patient serum samples. Previous studies also demonstrated the use of cation-exchange chromatography enrichment on depleted serum to measure a single tryptic cTnI peptide using a synthetic peptide internal standard quantification approach [22, 23]. Subsequent work from the same lab demonstrated the ability to quantify cTnI in digested human plasma from a single peptide using a unique enrichment strategy (SISCAPA) based on the use of anti-peptide antibodies [24]. That work also used spiked synthetic peptide internal standards for quantification. Furthermore, Zhao et al. [25] reported a MS-based assay to evaluate immunoassays using cTnI as a model protein. In that study, cTnI measurements were determined via a single, epitope peptide (with trypsin missed cleavage sites in its sequence) in patient plasma using albumin depletion and immunoaffinity enrichment prior to LC-MS/MS analysis.
Here, we present improvements toward the development of a specific and sensitive ID-LC-MS/MS method for quantification of cTnI in clinical samples obtained from myocardial infarction patients. This work is the first to establish quantification of native cTnI from patient plasma via ID-LC-MS/MS using a matrix-matched calibration system and a full-length isotopically labeled protein internal standard. Accuracy of the measurement procedure is demonstrated by way of quantitative agreement among three tryptic peptides unique to the cTnI molecule (and multiple fragmentation transitions per peptide). The use of an intact protein internal standard spiked into the unprocessed plasma is meant to alleviate biases that have been routinely observed during protein quantification studies, such as incomplete enzymatic digestion [26] or partial enrichment [27]. Immunoaffinity enrichment was achieved using in-house synthesized magnetic nanoparticles covalently bound to anti-cTnI monoclonal antibody. Nanoparticle conjugates have been shown to be superior to commercial microparticles for immunoaffinity enrichment schemes, owing to their increased surface-area-to-mass ratio [28]. The use of multiple reaction monitoring (MRM) on a triple quadrupole MS system allows simultaneous monitoring of native and isotope-labeled cTnI peptides, as well as a tryptic peptide from within the constant region of the anti-cTnI monoclonal antibody. Relative quantification of the anti-cTnI antibody simultaneously with immuno-extracted cTnI can ensure the robustness of the assay by monitoring the uniformity of the immunoenrichment procedure [28, 29]. Through the incremental improvements made to previously published efforts, this work highlights the expanding potential for developing an SI-traceable ID-LC-MS/MS-based method that can be used to harmonize high-throughput clinical cTnI ELISA assays.
Materials and methods
Materials
Standard Reference Material (SRM) 2921 – Human Cardiac Troponin Complex (cTn) – is a buffered solution of cTn isolated from human heart tissue, and was obtained from the National Institute of Standards and Technology (NIST) [5]. Monoclonal antibody against human cTnI (clone 19C7) was purchased from HyTest Ltd. (Turku, Finland). The synthetic, stable isotope labeled peptide DLPSPIE[13C5, 15N]R was purchased from Anaspec, Inc. (California, USA). The isotopically labeled cTnI protein internal standard, which has the same amino acid sequence as endogenous cTnI (see Electronic Supplementary Material (ESM) Fig. S1), but contains isotopically labeled K-[13C6, 15N4] and R-[13C6, 15N2] residues, was expressed using cell-free E. coli lysate and purified by Promise Advanced Proteomics (Grenoble, France). cTnI-negative (undetectable cTnI by ELISA) and cTnI-positive patient plasma (heparinized) samples were provided through the Department of Laboratory Medicine at the University of Washington in Seattle, USA. All cTnI samples were de-identified from routine clinical testing at the University of Washington, and approved for use through NIST’s Human Subjects Protection Office and the University of Maryland’s Human Subjects Protection Office prior to acceptance. A de-identified donor pool with Na+ heparin (anti-coagulant) collected from 50 apparently healthy individuals was also obtained through Golden West Biologicals, Inc. (California, USA). Aminopropyltriethoxysilane (APTES) was purchased from Gelest, Inc. (Pennsylvania, USA). RapiGest™ SF was purchased from Waters Corporation (Massachusetts, USA), and sequencing grade modified trypsin was purchased from Promega Corporation (Wisconsin, USA). High purity LC-MS grade water and acetonitrile containing formic acid were purchased from Honeywell-Burdick and Jackson (Michigan, USA). Protein LoBind centrifuge tubes were purchased from Eppendorf (New York, USA). All other chemicals were purchased from Sigma-Aldrich (Missouri, USA).
Immobilization of anti-cTnI monocloncal antibody onto synthesized magnetic nanoparticles
The synthesis for amine functionalized silica coated magnetic nanoparticles (≈ 85 nm diameters) has been previously described [28]. The amine functionalized nanoparticles were treated with 2% glutaraldehyde in phosphate buffer solution (PBS, pH 7.4), rotary mixed for 2 h, and washed three times with PBS. The nanoparticles were re-dispersed in PBS and anti-cTnI antibody (0.9 mg/mg nanoparticles). Sodium cyanoborohydride (final 5 mmol/L) was added (use caution and work in a fume hood when handling sodium cyanoborohydride), and the magnetic nanoparticles were rotary mixed for 24 h to 48 h. After immobilization, the antibody-magnetic nanoparticle conjugates were treated with ethanolamine in PBS (0.2 mmol/L, pH 7.4) for 1 h to deactivate unreacted aldehyde groups. Next, the antibody-nanoparticle conjugates were incubated in PBS containing 0.2% (w/v) bovine serum albumin (BSA) / 0.05% (v/v) Tween 20 for 16 h at room temperature to block any additional unreacted sites and limit non-specific binding. Finally, the antibody-nanoparticle conjugates were washed three times with PBS containing 0.1% (w/v) BSA / 0.05% (v/v) Tween 20 and reconstituted in PBS. Three batches of antibody-magnetic nanoparticle conjugates were prepared simultaneously and combined to form one lot of anti-cTnI antibody-nanoparticle conjugates for use in cTnI capture studies.
Calibrant and sample preparation for patient plasma sample analysis using an isotopically labeled protein internal standard
Human cTn from NIST SRM 2921 (31.2 ng/μL ±1.4 ng/μL) was used as a calibrant in this work [5]. SRM 2921 was thawed to room temperature prior to use, and stocks were prepared gravimetrically by diluting SRM 2921 in 0.1% BSA solution in PBS (BSA/PBS). Full-length isotopically labeled cTnI internal standard was also prepared in 0.1% BSA/PBS. Patient plasma samples were thawed and equilibrated to room temperature. Patient plasma samples were then centrifuged for 10 min at 5000 g to sediment any precipitated material. Matrix-matched calibrants were prepared gravimetrically by mixing pooled cTnI-negative (non-detectable by ELISA) patient plasma (0.9 mL) with various amounts of SRM 2921 (1.78, 2.48, 6.04, 12.1, 18.4, and 24.5 ng) and a constant amount of isotopically labeled cTnI internal standard (12.9 ng). Samples were prepared gravimetrically by mixing cTnI-positive patient plasma (0.9 mL) with the same isotopically labeled cTnI internal standard (12.9 ng). Both samples and calibrants were diluted 2-fold with PBS (final volume = 1.8 mL) to reduce the viscosity of the plasma. For immuno-capture of cTnI, 30 μL of anti-cTnI antibody-magnetic nanoparticle conjugates were added to the samples and calibrants and incubated at room temperature for 8 h with gentle rotary mixing. Following incubation, non-specific proteins were removed by washing the nanoparticles two times with tris buffer (20 mmol/L tris, 150 mmol/L sodium chloride) containing 0.05% Tween 20, and once with ammonium bicarbonate. The samples and calibrants were then resuspended in 50 mmol/L ammonium bicarbonate for digestion.
Trypsin digestion
Captured cTnI was digested in situ with the antibody-conjugated nanoparticles. The samples and calibrants were denatured in 0.1% (v/v) RapiGest™ SF surfactant in 50 mmol/L ammonium bicarbonate and boiled for 5 min. The released proteins were reduced with a final concentration of 10 mmol/L dithiothreitol at 60 °C while shaking for 45 min, and alkylated with 10 mmol/L iodoacetamide in the dark at room temperature for 45 min. Trypsin was added to the samples (1:2.5 w/w trypsin-to-protein ratio) and all samples were incubated for 16 h at 37 °C. The digests were acidified with a final concentration of 5% (v/v) formic acid for 45 min at 37 °C and then centrifuged at 4 °C to remove the RapiGest™ SF degradation products and magnetic nanoparticles. The digests were dried using a vacuum centrifuge. Samples were reconstituted with a final volume of 10 μL of isotopically labeled DLPSPIER peptide internal standard (0.0479 ng/μL) – a surrogate peptide for the anti-cTnI antibody used to determine the relative amount of antibody in each sample using a one-point calibration.
LC-MS/MS analysis
LC-MS/MS (dynamic MRM) analysis was performed on an Agilent 1200 LC system coupled in-line with an Agilent 6490A triple quadrupole MS. For peptide measurements, 8 μL of sample was injected via autosampler, and the LC separation was performed at a flow rate of 200 μL/min on a Zorbax (Agilent) SB-C18 reverse-phase analytical column (2.1 mm × 150 mm, 3.5 μm particles) at 35 °C using an increasing linear gradient of organic/aqueous solvent (acetonitrile/water containing 0.1% (v/v) formic acid) starting at 5% organic and increasing up to 70% (v/v) organic over 30 min, followed by a column wash and re-equilibration. The MS system operated in a positive-ion polarity. The following MS settings were used: fragmentor 380 V, cell accelerator 4 V, electron multiplier 800 V, capillary voltage 3500 V. A dynamic MRM scan type was used with a delta retention time of 1 min for (unlabeled and labeled) NITEIADLTQK, TLLLQIAK, DLPSPIER peptides and a delta retention time of 3 min for the (unlabeled and labeled) AYATEPHAK peptide, which had a wider dynamic MRM window due to its wider peak width and slightly less consistent retention time. Collision energies were optimized experimentally for each peptide transition by MRM monitoring and noting maximum signal intensity. In the final method, two fragmentation transitions were monitored for three cTnI peptides (NITEIADLTQK, TLLLQIAK, AYATEPHAK) and a single antibody peptide (DLPSPIER) for both the isotopically labeled and unlabeled peptides. All labeled peptides were quantified using identical source and fragmentation conditions as their unlabeled analogs.
Peak areas were integrated using MassHunter Qualitative Analysis B.06.00 software. The concentration of cTnI was determined by generating calibration curves using SRM 2921 as the calibrant and plotting peak area ratios of the unlabeled to labeled cTnI peptide versus known mass ratios of unlabeled to labeled cTnI peptide. Peptide concentrations were interpolated against the linear equation and multiplied by the known concentration of internal standard. The average concentration of all peptides detected using multiple transitions was used to determine cTnI concentrations, since peptide abundance is used as a surrogate for protein abundance.
Results and discussion
Immunoaffinity enrichment of cTnI
Given that cTnI is a low abundant plasma protein, an enrichment step prior to LC-MS/MS analysis was needed to achieve quantification in the low μg/L range [30]. Nanoparticles conjugated with anti-cTnI monoclonal antibody provided an effective way to enrich cTnI while simultaneously reducing sample complexity [15, 31, 32]. The high binding surface area-to-volume ratio of the magnetic nanoparticles [28, 33, 34] helps improve cTnI enrichment, especially when large sample volume (1 mL to 2 mL) processing is involved [35]. Immunodepletion was also avoided because cTnI has been reported to interact with abundant proteins retained on immunodepletion devices, which would result in low protein recovery [21, 22]. To this end, anti-cTnI monoclonal antibody was immobilized to silica coated magnetic nanoparticles via a glutaraldehyde coupling reaction for immunoaffinity enrichment of cTnI. Furthermore, immunoenrichment was performed at the protein level, rather than at the peptide level (SISCAPA), to ensure that multiple peptides from cTnI could be quantified concurrently from a single antibody enrichment and to avoid digesting large volumes of unprocessed plasma.
NIST SRM 2921 and post-spiked isotopically labeled cTnI peptides were used for immunoenrichment recovery studies. Several steps, including incubation time and the amount of antibody-nanoparticle conjugates added during the enrichment step, were optimized. Recovery of cTnI spiked into buffer at clinically-relevant concentrations was achieved with 93% efficiency when 30 μL of antibody-nanoparticle conjugates (≈ 2.5 μg antibody) were added to the sample and incubated for 8 h. In addition, limits of detection and quantification of the LC-MS/MS assay were determined by digesting SRM 2921 at various concentrations with a constant amount of antibody-magnetic nanoparticles conjugates. The absolute limit of detection and quantification were estimated to be 165 pg (S/N ≥ 3 for each MRM transition) and 325 pg cTnI (S/N ≥ 10 for each MRM transition), respectively. During method development, inter- and intra-assay reproducibility was also evaluated by analyzing three replicates of immunoenriched cTnI spiked at three levels representative of acute myocardial infarction (5, 10, 15 ng) on three different days, and was determined to be <10% (ESM Table S1).
Recovery of cTnI was further evaluated by spiking SRM 2921 into unprocessed, normal human plasma, which mimicked the patient plasma matrix. Approximately 30% of cTnI was recovered when low amounts of SRM 2921 (i.e., 5, 10, 15 ng) were spiked into diluted human donor plasma, indicating that pathological levels of cTnI could be quantified from patient plasma samples using protein immunoaffinity enrichment and LC-MS/MS. Although large cTnI protein losses occurred during sample preparation, biases were alleviated when a full-length isotopically labeled cTnI protein internal standard was employed.
cTnI quantification using a full-length isotopically labeled protein internal standard
For quantification utilizing SRM 2921 and an isotopically labeled cTnI protein internal standard, three tryptic peptides – NITEIADLTQK, TLLLQIAK, and AYATEPHAK were used as surrogate peptides for cTnI. Monitoring several tryptic cTnI peptides via LC-MS/MS analysis can provide information on the stability or heterogeneity of cTnI [36]. TLLLQIAK and AYATEPHAK peptides were selected as surrogate peptides for cTnI because they had no known post-translational modifications, were located near the stable region of cTnI (ESM Fig. S1), and produced reasonable MRM signals. NITEIADLTQK was used as the third surrogate peptide for mostly similar reasons, although it lies just outside of the consensus stable region of cTnI. During proof-of-concept studies, slightly lower levels of NITEIADLTQK were observed when compared to TLLLQIAK and AYATEPHAK for endogenous cTnI in pooled patient plasma samples. Inter-peptide variability may be due to heterogeneity of the calibrator (SRM 2921) or enhanced degradation/truncation of the calibrator in matrix, when compared to the endogenous form [37, 38]. In this regard, selection of a natural, purified cTnI calibrant, even at the protein level, remains imperfect in comparison to endogenous cTnI found in patient plasma. However, accessibility to exact-matched, matrix-based calibrants is currently, perhaps, the greatest limiting factor for any protein quantification effort. Currently, SRM 2921 remains the best fit-for-purpose calibrant available.
As depicted in Fig. 1, ISADAMMQALLGAR and NIDALSGMEGR were also considered to act as surrogate peptides for cTnI, mainly because of their higher MRM responses compared to AYATEPHAK. H ow ever, ISADAMMQALLGAR and NIDALSGMEGR contained possible post-translational modification sites (i.e., methionine oxidation and serine phosphorylation). Furthermore, when spiked cTnI (i.e., SRM 2921) was recovered from normal patient plasma NIDALSGMEGR was not detected in any of the samples, which supports the likelihood that cTnI spiked into a plasma matrix results in protein truncation or modifications near the C-terminus (ESM Fig. S2). Table 1 summarizes the MRM transition list of the three cTnI tryptic peptides selected for cTnI quantification using the protein internal standard and a single antibody tryptic peptide that acted as a qualitative control to assess the anti-cTnI antibody-magnetic nanoparticle enrichment.
Fig. 1.
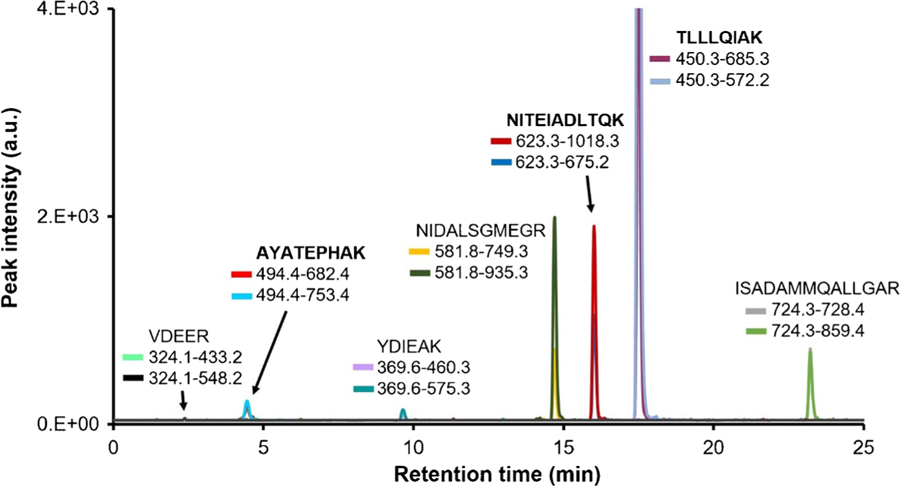
Extracted ion chromatogram of tryptic cTnI peptides screened for MRM analysis and assay development. Two transitions per cTnI peptide were analyzed. The bolded tryptic peptides NITEIADLTQK, TLLLQIAK, and AYATEPHAK produced the highest signal response and contained no known post-translational modifications. These peptides were ultimately selected to act as surrogate peptides for cTnI quantification by ID-LC-MS/MS, using a full-length isotopically labeled protein internal standard. ISADAMMQALLGAR and NIDALSGMEGR produced high signal responses, but were not chosen due to their possible post-translational modifications (i.e. methionine oxidation and serine phosphorylation). ISADAMoxMoxQALLGAR, NIDALSGMoxEGR, cPLELAGLGFAELQLcR were also screened by MRM analysis, but were not detected
Table 1.
MRM transitions of cTnI surrogate peptides used for quantification, and the MRM transitions for the antibody peptide (for identification and relative quantification). Isotopically labeled cTnI peptides are derived from a full-length isotopically labeled cTnI protein where arginine and lysine amino acids were labeled
| Peptide AA sequence | Precursor ion (m/z) | Product ion (m/z) | Collision energy (V) | Retention time (min) |
|---|---|---|---|---|
| AYATEPHAK | [494.4]2+ | [753.4]+ | 9 | 5.2 ± 3 |
| [682.4]+ | 9 | |||
| AYATEPHAK* | [498.4]2+ | [761.4]+ | 9 | |
| [690.4]+ | 9 | |||
| NITEIADLTQK | [623.3]2+ | [1018.3]+ | 10 | 16.2 ± 1 |
| [675.2]+ | 11 | |||
| NITEIADLTQK* | [627.3]2+ | [1026.5]+ | 10 | |
| [683.3]+ | 11 | |||
| TLLLQIAK | [450.3]2+ | [685.3]+ | 7 | 17.8 ± 1 |
| [572.2]+ | 8 | |||
| TLLLQIAK* | [454.3]2+ | [693.5]+ | 7 | |
| [580.4]+ | 8 | |||
| DLPSPIER (IgG) | [463.8]2+ | [514.2]+ | 17 | 13.7 ± 1 |
| [698.3]+ | 7 | |||
| DLPSPIE*R (IgG) | [466.8]2+ | [520.2]+ | 17 | |
| [704.2]+ | 7 |
The fragmentor voltage was set at 380 V. Labeled residues (indicated with an asterisk) contained 13 C and 15N isotopes
cTnI quantification in human plasma
A full-length isotopically labeled cTnI protein internal standard was used to measure native cTnI in cTnI-positive patient plasma samples. cTnI concentrations were determined in each of the five individual plasma samples obtained from myocardial infarction patients using the ID-LC-MS/MS measurement assay. Calibration was employed by spiking SRM 2921 and a full-length isotopically labeled protein internal standard into cTnI-negative (based on ELISA) patient plasma (ESM Fig. S3). Native cTnI and the internal standard were captured by immunoaffinity enrichment and subsequently analyzed by LC-MS/MS. Figure 2 shows representative extracted MRM chromatograms of the tryptic peptides used to quantify cTnI concentration in an individual myocardial infarction patient plasma sample. As shown in Fig. 3, the method response was linear between 1.98 μg/L and 27.2 μg/L with R2 > 0.994 for all monitored transitions. Using a matrix-matched calibration, surrogate peptide concentrations from a total of six transitions were calculated, and an average response was determined for the cTnI concentration of each individual patient plasma sample. Overall, cTnI concentrations ranged between 4.86 μg/L and 11.3 μg/L with CV’s ≤ 14.3% for the five patient samples, demonstrated in Table 2.
Fig. 2.
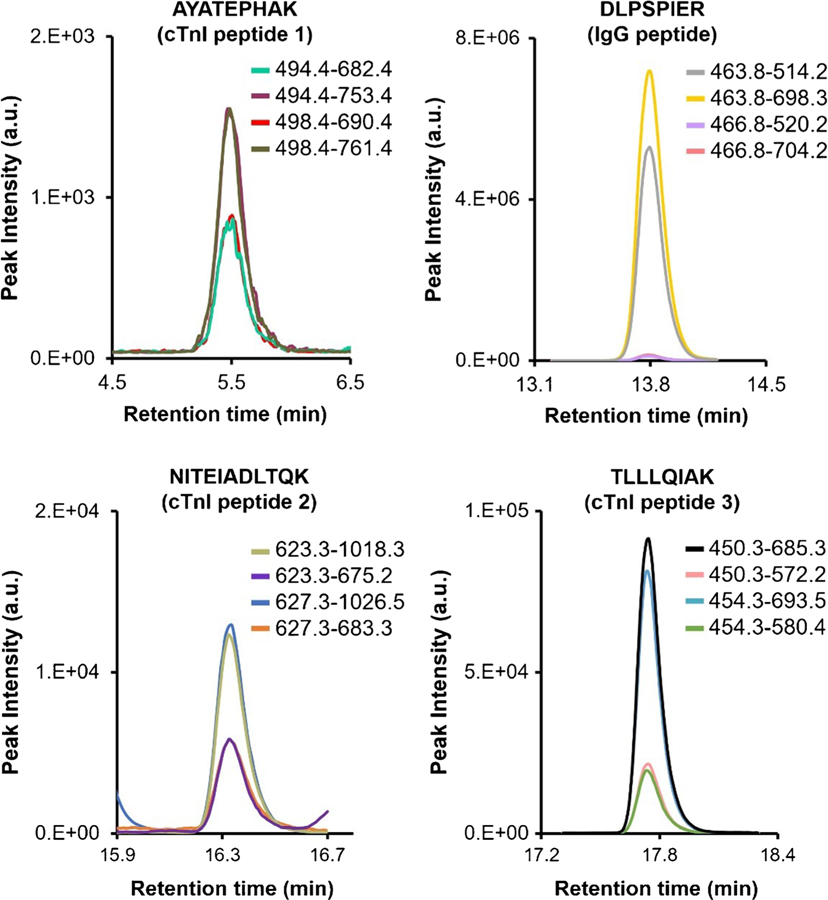
Representative MRM chromatograms of cTnI enriched from an individual myocardial infarction patient plasma sample. A single peptide from the anti-cTnI monoclonal antibody was also monitored to determine the relative amount of antibody in each immunoaffinity-purified patient plasma sample
Fig. 3.
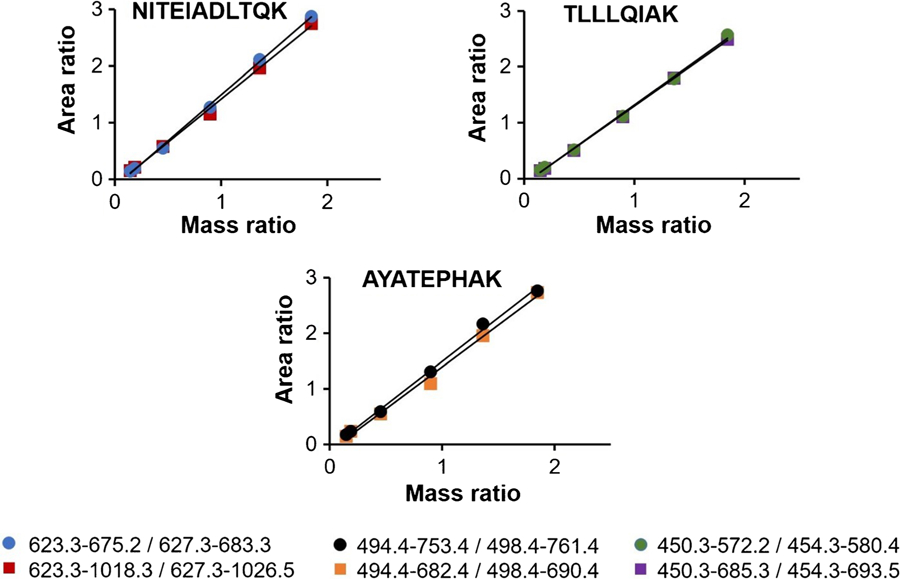
Calibration curves for monitored cTnI peptide transitions as a plot of unlabeled: labeled integrated peak area ratios versus unlabeled: labeled measured mass ratios. R2 estimates were >0.994 for all monitored transitions. Note: calibrants were matrix-matched and underwent all sample processing steps
Table 2.
cTnI levels determined in five individual myocardial infarction patient plasma samples
| cTnI peptide | Patient sample 1 | Patient sample 2 | Patient sample 3 | Patient sample 4 | Patient sample 5 |
|---|---|---|---|---|---|
| AYATEPHAK [1] | 5.74 | 11.0 | 7.84 | 5.37 | 5.19 |
| AYATEPHAK [2] | 5.32 | 9.72 | 7.06 | 5.55 | 5.03 |
| NITEIADLTQK [1] | 4.78 | 10.4 | 6.59 | 5.10 | 4.13 |
| NITEIADLTQK [2] | 5.17 | 10.4 | 6.51 | 5.22 | 3.86 |
| TLLLQIAK [1] | 6.23 | 13.2 | 8.08 | 6.35 | 5.43 |
| TLLLQIAK [2] | 6.31 | 12.8 | 8.21 | 6.39 | 5.49 |
| Mean (μg/L) | 5.59 | 11.3 | 7.38 | 5.66 | 4.86 |
| Std. dev. | 0.610 | 1.41 | 0.759 | 0.567 | 0.695 |
| % CV | 10.9 | 12.5 | 10.3 | 10.0 | 14.3 |
As expected, the intra-peptide variability was determined to be lower than the inter-peptide variability. The precision within each individual peptide measurement (between MRM transitions) was <9%. As seen in Table 2, the TLLLQIAK and AYATEPHAK peptide measurements were highly consistent with each other. The data suggests that the NITEIADLTQK peptide, while not behaving exactly like the surrogate peptides within the stable region of cTnI, does provide consistent results for matrix-based measurements of cTnI. Therefore, NITEIADLTQK may still be considered as an important verification peptide for use in cTnI LC-MS/MS-based measurements. Quantifying three cTnI tryptic peptides simultaneously and independently by MRM analysis helped provide confidence in the accuracy of the ID-LC-MS/MS measurement results. The value in monitoring more than one tryptic peptide surrogate when quantifying proteins in matrix-based assays is clear when examining specific results from this work. For instance, TLLLQIAK and AYATEPHAK peptides show better correlation to each other than either peptide does to NITEIADLTQK for patient samples 3 and 5; whereas, AYATEPHAK and NITEIADLTQK are more strongly correlated in patient samples 2 and 4 than either peptide is to TLLLQIAK. In all five patient samples, however, TLLLQIAK is least correlated with NITEIADLTQK as compared to other pairings. But within the broader perspective, all three peptides are demonstrated to correlate reasonably well when considering the measurement challenge.
This method has been demonstrated on a relatively small number of clinical samples; however, the results seem to suggest that in vivo processing of individual patient plasma may have significant effects on downstream quantitative assessment of cTnI concentration, depending specifically on the peptide targets chosen. This should not come as a surprise considering the uniqueness of individual phenotypes, as well as the known heterogeneity of cTnI [39]. It is also important to consider C- and N-terminal degradation and other protein modifications that may occur asymmetrically because of the heterogeneity of disease state among a subset of individuals. Some reports, which suggest that no two patients’ plasma are likely to contain the same cTnI isoform composition, give further justification to the measurement of multiple peptide surrogates of cTnI [40]. Considering that modifications to cTnI are likely to occur both in the myocardial tissue and within blood, that individuals experience variations in clearance and degradation rates, and that this poorly defined measurand releases into the bloodstream at exceedingly low concentrations among a large dynamic range of plasma proteins, cTnI quantification should rightfully be considered as among the most difficult and complex measurement challenges facing the clinical laboratory.
Overall, this work improves upon previously reported matrix-based measurements of cTnI by LC-MS/MS in three important ways: 1) the use of intact proteins as the calibrators and internal standards decreases biases associated with immunoaffinity enrichment of cTnI from matrix, 2) agreement of three independent cTnI peptide measurements provides higher confidence in quantitative trueness, and 3) quantitative analysis is based on the direct measurement of the native protein itself, not a synthetic or spiked analog. Accuracy of ID techniques relies on a calibration system, in this case NIST SRM 2921, which consists of the native cTn complex (cTnI, cTnT, cTnC) isolated from human cardiac tissue. This biologically-source material is observed to have “a substantial degree of heterogeneity”, and there is evidence suggesting that SRM 2921 exists as a mixture of many possible cTnI complexes – i.e. ternary, binary, free, truncated, phosphorylated, acetylated, etc., similar to the heterogeneity commonly associated with cTn purified from plasma [5]. Although no material, including SRM 2921, can be expected to be an exact-matched analog representation of any individual’s heterogeneous and unique cTn profile, this material is the best fit-for-purpose choice for a calibrator currently available. The nature of the internal standard is somewhat less important. Whether the internal standard exactly represents the samples’ unique cTn profile is not as vital under the workflow of a double ID experiment where the ratio of peak area ratios normalizes out the contribution from the internal standard, thus rendering the exact internal standard concentration insignificant.
Conclusion
This present work establishes that absolute quantification of cTnI can be achieved in patient plasma by ID-LC-MS/MS using a matrix-matched calibrant and a full-length stable-isotope protein internal standard. Overall, this assay is capable of measuring cTnI in patient plasma down to 2 μg/L with high precision using three surrogate cTnI peptides – a marked improvement over existing cTnI MS-based assays reported in the literature. Finally, this work represents a significant step towards quantification of cTnI in plasma/serum-based reference materials and towards establishing calibration traceability for clinical assays.
Supplementary Material
Acknowledgements
The authors thank Dr. Andrew Hoofnagle from the University of Washington for providing clinical samples and assistance, and Dr. Eric Kilpatrick from the National Institute of Standards and Technology for his logistical help receiving the patient plasma samples and for his scientific discussions. We also acknowledge the support of the Professional Research Experience Program (PREP) through the University of Maryland, College Park and the National Institute of Standards and Technology.
Footnotes
Publisher's Disclaimer: Disclaimer
Certain commercial equipment, instruments, and materials are identified in this paper to specify the experimental procedures and analytical methods adequately. In no case does such identification imply recommendation or endorsement by the National Institute of Standards and Technology, nor does it imply that the materials or equipment are necessarily the best available for the purpose.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s00216-018-0960-7) contains supplementary material, which is available to authorized users.
Compliance with ethical standards
Patient plasma samples were obtained as de-identified, residual clinical samples from the University of Washington. All work with human derived samples was reviewed and approved by the NIST Human Subjects Protection Office.
Conflict of interest The authors declare that they have no conflicts of interest.
References
- 1.Bunk DM, Dalluge JJ, Welch MJ. Heterogeneity in human cardiac troponin I standards. Anal Biochem. 2000;284(2):191–200. [DOI] [PubMed] [Google Scholar]
- 2.Panteghini M, Bunk DM, Christenson RH, Katrukha A, Porter RA, Schimmel H, et al. Standardization of troponin I measurements: an update. Clin Chem Lab Med. 2008;46(11):1501–6. [DOI] [PubMed] [Google Scholar]
- 3.Panteghini M Assay-related issues in the measurement of cardiac troponins. Clin Chim Acta. 2009;402(1–2):88–93. [DOI] [PubMed] [Google Scholar]
- 4.Panteghini M, Pagani F, Yeo KTJ, Apple FS, Christenson RH, Dati F, et al. Evaluation of imprecision for cardiac troponin assays at low-range concentrations. Clin Chem. 2004;50(2):327–32. [DOI] [PubMed] [Google Scholar]
- 5.Bunk DM, Welch MJ. Characterization of a new certified reference material for human cardiac troponin I. Clin Chem. 2006;52(2): 212–9. [DOI] [PubMed] [Google Scholar]
- 6.Tate JR, Bunk DM, Christenson RH, Katrukha A, Noble JE, Porter RA, et al. Standardisation of cardiac troponin I measurement: past and present. Pathology. 2010;42(5):402–8. [DOI] [PubMed] [Google Scholar]
- 7.Christenson RH, Duh SH, Apple FS, Bodor GS, Bunk DM, Dalluge J, et al. Standardization of cardiac troponin I assays: round robin of ten candidate reference materials. Clin Chem. 2001;47(3): 431–7. [PubMed] [Google Scholar]
- 8.Wu AHB, Christenson RH. Analytical and assay issues for use of cardiac troponin testing for risk stratification in primary care. Clin Biochem. 2013;46(12):969–78. [DOI] [PubMed] [Google Scholar]
- 9.Noble JE, Bunk DM, Christenson RH, Cole KD, He HJ, Katrukha AG, et al. Development of a candidate secondary reference procedure (immunoassay based measurement procedure of higher metrological order) for cardiac troponin I: I. antibody characterization and preliminary validation. Clin Chem Lab Med. 2010;48(11): 1603–10. [DOI] [PubMed] [Google Scholar]
- 10.Christenson RH, Duh SH, Apple FS, Bodor GS, Bunk DM, Panteghini M, et al. Toward standardization of cardiac troponin I measurements part II: assessing commutability of candidate reference materials and harmonization of cardiac troponin I assays. Clin Chem. 2006;52(9):1685–92. [DOI] [PubMed] [Google Scholar]
- 11.Tate JR, Bunk DM, Christenson RH, Barth JH, Katrukha A, Noble JE, et al. Evaluation of standardization capability of current cardiac troponin I assays by a correlation study: results of an IFCC pilot project. Clin Chem Lab Med. 2015;53(5):677–90. [DOI] [PubMed] [Google Scholar]
- 12.Villanueva J, Carrascal M, Abian J. Isotope dilution mass spectrometry for absolute quantification in proteomics: concepts and strategies. J Proteome. 2014;96:184–99. [DOI] [PubMed] [Google Scholar]
- 13.Hoofnagle AN, Roth MY. Improving the measurement of serum thyroglobulin with mass spectrometry. J Clin Endocrinol Metab. 2013;98(4):1343–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lange V, Picotti P, Domon B, Aebersold R. Selected reaction monitoring for quantitative proteomics: a tutorial. Mol Syst Biol. 2008;4:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schneck NA, Lowenthal M, Phinney K, Lee SB. Current trends in magnetic particle enrichment for mass spectrometry-based analysis of cardiovascular protein biomarkers. Nanomedicine. 2015;10(3): 433–46. [DOI] [PubMed] [Google Scholar]
- 16.Kilpatrick EL, Bunk DM. Reference measurement procedure development for C-reactive protein in human serum. Anal Chem. 2009;81(20):8610–6. [DOI] [PubMed] [Google Scholar]
- 17.Meng ZJ, Veenstra TD. Targeted mass spectrometry approaches for protein biomarker verification. J Proteome. 2011;74(12):2650–9. [DOI] [PubMed] [Google Scholar]
- 18.Bunk DM. Reference materials and reference measurement procedures: an overview from a national metrology institute. Clin Biochem Rev. 2007;28(4):131–7. [PMC free article] [PubMed] [Google Scholar]
- 19.Panteghini M Standardization of cardiac troponin I measurements: the way forward? Clin Chem. 2005;51(9):1594–7. [DOI] [PubMed] [Google Scholar]
- 20.Picard G, Lebert D, Louwagie M, Adrait A, Huillet C, Vandenesch F, et al. PSAQ (TM) standards for accurate MS-based quantification of proteins: from the concept to biomedical applications. J Mass Spectrom. 2012;47(10):1353–63. [DOI] [PubMed] [Google Scholar]
- 21.Huillet C, Adrait A, Lebert D, Picard G, Trauchessec M, Louwagie M, et al. Accurate quantification of cardiovascular biomarkers in serum using protein standard absolute quantification (PSAQ (TM)) and selected reaction monitoring. Mol Cell Proteomics. 2012;11(2):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keshishian H, Addona T, Burgess M, Mani DR, Shi X, Kuhn E, et al. Quantification of cardiovascular biomarkers in patient plasma by targeted mass spectrometry and stable isotope dilution. Mol Cell Proteomics. 2009;8(10):2339–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keshishian H, Addona T, Burgess M, Kuhn E, Carr SA. Quantitative, multiplexed assays for low abundance proteins in plasma by targeted mass spectrometry and stable isotope dilution. Mol Cell Proteomics. 2007;6(12):2212–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuhn E, Addona T, Keshishian H, Burgess M, Mani DR, Lee RT, et al. Developing multiplexed assays for troponin I and interleukin-33 in plasma by peptide immunoaffinity enrichment and targeted mass spectrometry. Clin Chem. 2009;55(6):1108–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao C, Trudeau B, Xie H, Prostko J, Fishpaugh J, Ramsay C. Epitope mapping and targeted quantitation of the cardiac biomarker troponin by SID-MRM mass spectrometry. Proteomics. 2014;14(11):1311–21. [DOI] [PubMed] [Google Scholar]
- 26.Lowenthal MS, Liang YX, Phinney KW, Stein SE. Quantitative bottom-up proteomics depends on digestion conditions. Anal Chem. 2014;86(1):551–8. [DOI] [PubMed] [Google Scholar]
- 27.Lowenthal MS, Gasca-Aragon H, Schiel JE, Dodder NG, Bunk DM. A quantitative LC-MS/MS method for comparative analysis of capture-antibody affinity toward protein antigens. J Chromatogr B-Analyt Technol Biomed Life Sci. 2011;879(26):2726–32. [DOI] [PubMed] [Google Scholar]
- 28.Schneck NA, Phinney KW, Lee SB, Lowenthal MS. Quantification of antibody coupled to magnetic particles by targeted mass spectrometry. Anal Bioanal Chem. 2016;408(29):8325–32. [DOI] [PubMed] [Google Scholar]
- 29.Rogstad SM, Sorkina T, Sorkin A, Wu CC. Improved precision of proteomic measurements in immunoprecipitation based purifications using relative quantitation. Anal Chem. 2013;85(9):4301–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agewall S, Giannitsis E, Jernberg T, Katus H. Troponin elevation in coronary vs. non-coronary disease. Eur Heart J. 2011;32(4): 404–11B. [DOI] [PubMed] [Google Scholar]
- 31.Berna MJ, Zhen YJ, Watson DE, Hale JE, Ackermann BL. Strategic use of immunoprecipitation and LC/MS/MS for trace-level protein quantification: myosin light chain 1, a biomarker of cardiac necrosis. Anal Chem. 2007;79(11):4199–205. [DOI] [PubMed] [Google Scholar]
- 32.Mani V, Chikkaveeraiah BV, Rusling JF. Magnetic particles in ultrasensitive biomarker protein measurements for cancer detection and monitoring. Expert Opin Med Diagn. 2011;5(5):381–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peter JF, Otto AM. Magnetic particles as powerful purification tool for high sensitive mass spectrometric screening procedures. Proteomics. 2010;10(4):628–33. [DOI] [PubMed] [Google Scholar]
- 34.Safarik I, Safarikova M. Magnetic techniques for the isolation and purification of proteins and peptides. Biomagn Res Technol. 2004;2(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whiteaker JR, Zhao L, Zhang HY, Feng LC, Piening BD, Anderson L, et al. Antibody-based enrichment of peptides for mass-spectrometry-based quantification on magnetic beads of serum biomarkers. Anal Biochem. 2007;362(1):44–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beasley-Green A, Burris NM, Bunk DM, Phinney KW. Multiplexed LC-MS/MS assay for urine albumin. J Proteome Res. 2014;13(9):3930–9. [DOI] [PubMed] [Google Scholar]
- 37.He HJ, Lowenthal MS, Cole KD, Bunk D, Wang LL. An immunoprecipitation coupled with fluorescent western blot analysis for the characterization of a model secondary serum cardiac troponin I reference material. Clin Chim Acta. 2011;412(1–2):107–11. [DOI] [PubMed] [Google Scholar]
- 38.Apple FS. COUNTERPOINT - Standardization of cardiac troponin I assays will not occur in my lifetime. Clin Chem. 2012;58(1):169–71. [DOI] [PubMed] [Google Scholar]
- 39.Katrukha AG, Bereznikova AV, Filatov VL, Esakova TV, Kolosova OV, Pettersson K, et al. Degradation of cardiac troponin I: implication for reliable immunodetection. Clin Chem. 1998;44(12):2433–40. [PubMed] [Google Scholar]
- 40.Labugger R, Organ L, Collier C, Atar D, Van Eyk JE. Extensive troponin I and T modification detected in serum from patients with acute myocardial infarction. Circulation. 2000;102(11):1221–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


