Abstract
Plasmablastic lymphoma (PBL) is a rare and aggressive B-cell Non-Hodgkin lymphoma (NHL) associated with immunocompromised states such as HIV. We present a case of PBL in an HIV patient presenting as spontaneous tumor lysis syndrome and discuss the clinical challenges hence encountered.
Keywords: plasmablastic lymphoma, spontaneous tumor lysis syndrome, hiv lymphoma
Introduction
Plasmablastic lymphoma (PBL) is a B-cell lymphoma that was previously considered a subtype of terminally differentiated diffuse large B-cell lymphoma with plasmablastic differentiation; currently, it is recognized as a distinct entity of B-cell lymphoma [1]. It is often associated with immunosuppressive conditions such as the human immunodeficiency virus (HIV). In HIV patients, 2.6% of Non-Hodgkin's lymphoma (NHL) is found to be due to PBL [2]. Usually, it presents as an advanced stage (stage III or IV). Spontaneous tumor lysis syndrome is a rare complication of this rare lymphoma [3]. The aggressive nature of this tumor requires prompt diagnosis and treatment. In this report, we discuss a case of a 49-year-old man presenting with spontaneous tumor lysis syndrome secondary to rapidly dividing PBL.
Case presentation
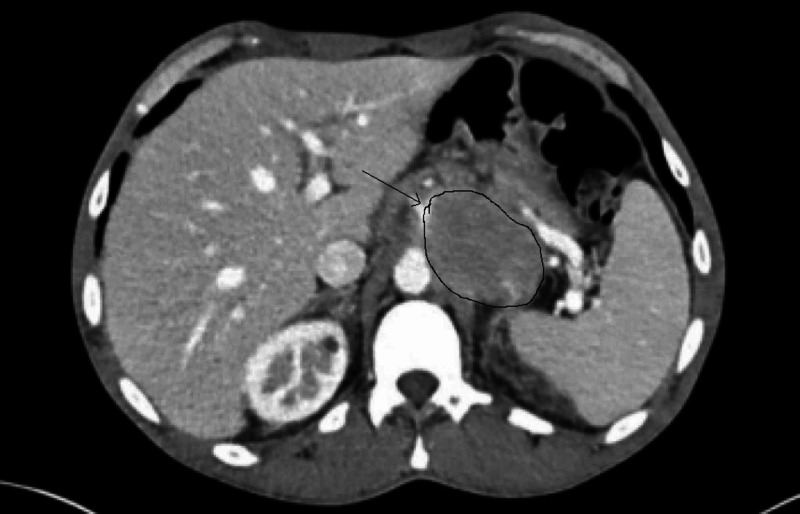
Our patient is a 49-year-old African-American man with the past medical history of gastritis and HIV since 2004; on anti-retroviral therapy (ART) of bictegravir, emtricitabine, and tenofovir alafenamide, he was compliant to his medications for the last seven years, and inconsistently before that, he had a CD4 count of 375 cells/Ul (normal: 500-1600 cells/Ul) and HIV viral load of 122 copies, the patient presented to the emergency department with the chief complaint of epigastric pain associated with persistent nausea, loss of appetite and episodic vomiting for few days. His symptoms had not resolved with proton pump inhibitors; he noticed weight loss and denied fever or night sweats. CT of the abdomen with contrast revealed 7.0*5.0*5.0 cm mildly enhancing soft tissue mass in the lesser sac invading the splenic artery (see Figure 1). CT-guided core needle biopsy of the mass was performed, and histopathological findings were consistent with the features of PBL (monomorphic diffuse lymphoid cells of plasmablastic morphology positive for CD38, CD138, MUM1 immunostains, and negative for CD20 and CD79a, Ki-67 was not checked). In situ hybridization for Epstein-Barr viral RNA (EBER) was negative. The patient improved clinically and was discharged to follow up as an outpatient with an oncologist for staging and treatment. On discharge, creatinine was 1.2 mg/dl, and hemoglobin was 11.6 gm/dl. Unfortunately, five days after discharge, the patient was rushed to the emergency department in a critical condition - he had profound hypotension (blood pressure: 71/55 mmHg) and acute kidney injury (creatinine: 4.11 mg/dl). He was admitted to the hospital where further workup revealed findings consistent with tumor lysis syndrome (uric acid: 14.1 mg/dl, phosphorous: 11.6 mg/dl, K: 6.2 mMol/L, calcium: 8.8 mg/dl, hemoglobin: 5.3 gm/dl, hematocrit: 15.4%, lactate dehydrogenase (LDH): 4704 U/Liter, haptoglobin: <30 mg/dl, corrected reticulocyte count: 1.8%, reticulocyte count: 69.300 cells/mm3), and negative coombs test. It was determined that the patient had Cairo-Bishop criteria (3/4 laboratory and grade II clinical). The patient received intravenous fluid resuscitation, dexamethasone 4 mg twice daily, rasburicase, allopurinol, and other supportive measures as a blood transfusion. The patient continued to have worsening kidney function and hyperkalemia resistant to medical therapy, so hemodialysis was to be done but was refused by the family who changed the code status to comfort measures. The patient expired one week after the presentation, and two weeks after the PBL diagnosis was made. Unfortunately, time did not allow for fluorescence in situ hybridization (FISH), comprehensive staging, or the initiation of chemotherapy.
Figure 1. CT scan of the abdomen .
Contrast-enhanced CT abdomen demonstrating a mildly enhancing soft tissue mass in the lesser sac invading the splenic artery.
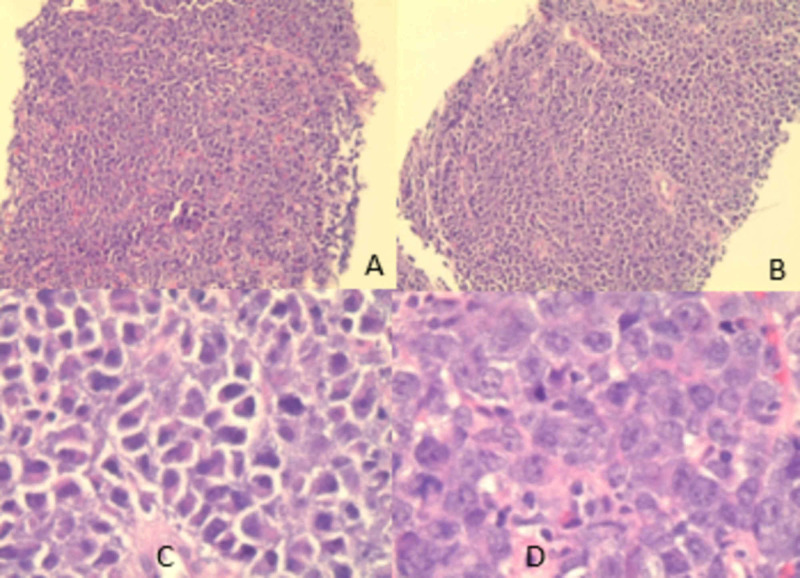
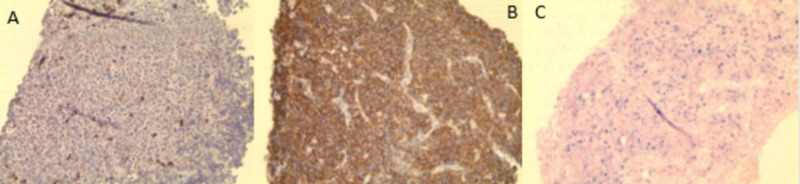
Immunohistochemistry lab reported the following results (Fugures 2-3): the neoplastic cells were positive for CD45, CD4, CD138 (syndecan-1), MUM-1, and c-myc; whereas cells were negative for cytokeratin AE1/AE3, CD3, CD5, CD79a, CD19, CD20, CD8, CD22, CD10, BCL-6, BCL-2, CD30, CD56, and ALK-1.
Figure 2. CT scan guided biopsy results.
Images A and B are low power view. Image C is a high power view showing plasmacytoid features with hexcentric nuclei and paranuclear hofs. Image D shows prominent nucleoli.
Figure 3. Immunohistochemistry lab results .
Image A shows cells which are negative for CD79a. Image B shows positive CD138 and image C shows negative Epstein-Barr virus (EBV) stain.
Discussion
Plasmablastic lymphoma is a rare B-cell NHL, which is characterized by its aggressive presentations in HIV patients. Historically, it is associated with HIV diagnosis; some cases were reported in transplant patients or steroid therapy for autoimmune diseases [4]. Many cases were reported in HIV negative patients. The majority of cases are EBV positive. Male:female ratio is around 3:1 [5]. The oral cavity represents the most common location followed by other locations such as the gastrointestinal tract, lymph nodes, visceral cranium, cervix, thorax, skin, and retroperitoneum [6-10]. The prognosis of patients with PBL is generally poor with a median overall survival of 6-19 months, and there are no clear cut differences between HIV-positive and HIV-negative patients based on a meta-analysis of 277 patients [11, 12]. It was reported in 122 cases review of literature that the median overall survival time for the whole group was 14 months, with a five-year survival rate of 31%. Variation mostly depends on stage, chemotherapy response, and antiretroviral therapy [13]. Prospective clinical trials for treatment options are lacking; therapy is considered based on available case reports and small retrospective case series.
Tumor lysis syndrome (TLS) can be the first manifestation of an underlying malignancy; it can rarely occur in rapidly dividing leukemia, lymphoma, or solid tumors. TLS is a potentially fatal oncologic emergency, and it is particularly important that clinicians recognize the laboratory (uric acid, potassium, calcium, and phosphorus) and clinical findings that suggest the diagnosis. TLS can lead to many complications, such as acute kidney injury, arrhythmias, seizures, and rarely liver damage.
TLS is more commonly seen after treatment of hematological malignancies such as acute leukemia and NHL; incidence is generally decreased with the use of preventive measures. Based on a model of risk stratification done by an international TLS expert panel who made recommendations for TLS prophylaxis in 2010, frequent laboratory monitoring, aggressive intravenous hydration and a single dose of rasburicase (0.1-0.2 mg/kg) were recommended for patients with high risk for TLS (depending on the type of tumor; "high risk" includes: leukocytosis of more than 100*10^9/L, stage III/IV tumors, doubling of LDH level, or renal dysfunction with elevated potassium, phosphorus, and uric acid), if clinically necessary, rasburicase can be repeated [14]. Tumor lysis syndrome is a serious complication of this rare subtype of B-cell NHL. In one retrospective observational study of ten years, two of six patients diagnosed with plasmablastic lymphoma presented with spontaneous tumor lysis syndrome. Unfortunately, all patients presenting with spontaneous tumor lysis syndrome expired before receiving chemotherapy. These patients had a mean CD4 count of <200/mm3 [3] compared to our patient's CD4 count of 375/mm3 (see Table 1).
Table 1. Comparison between our patient and other PBL known characteristics.
PBL - plasmablastic lymphoma; TLS - tumor lysis syndrome
| Characteristic | Our patient | Usual PBL patients [5] |
| Age | 49 years old | Around 40 years old |
| Sex | Male | Male < female |
| Median CD4 count | 356 cells/UI | 180 cells/UI |
| Location | Gastrointestinal tract (GIT) “Lesser Sac” | Oral cavity most common, other include GIT, lymph nodes, visceral cranium, cervix, thorax skin, eyelid, and retroperitoneum |
| Presentation with TLS | Yes | One study reported 33%, 2/6 (rarely stated in literature) [3] |
| Histopathology and immunohistochemistry | Positive for CD45, CD4, CD138, CD79a, CD19, and negative for CD20 | Usually CD20 negative (positive only in <2% of cases) and plasmacytic markers (CD38, CD79a, CD138, and/or MUM1, IRF4) positive |
| Epstein-Barr virus | Negative | Positive in > 80% |
This description of cases emphasizes that PBL can present as TLS, and we can notice that the likelihood of TLS rises with elevated LDH, knowing it is a rapidly replicating tumor with high tumor burden. More future studies are needed to establish specific pathophysiology with this presentation. Spontaneous tumor lysis syndrome occurs rarely but has fatal consequences. Clinicians should be wary about the intrinsic and extrinsic risk factors with special attention to malignancies with a high proliferation rate [15].
Conclusions
Plasmablastic lymphoma is a rare and aggressive HIV associated lymphoma. Spontaneous TLS can be a serious manifestation of PBL; as it is characterized by being a high-grade malignancy, and commonly presents as stage III/IV. We suggest that the clinicians should screen for the laboratory evidence of TLS when PBL is suspected and use guidelines for treatment as it could be a fatal emergency.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained by all participants in this study
References
- 1.Plasmablastic lymphoma versus diffuse large B cell lymphoma with plasmablastic differentiation: proposal for a novel diagnostic scoring system. Boy S, Heerden M, Pool R, Willem P, Slavik T. J Hematop. 2015;8:3–11. [Google Scholar]
- 2.A unique case of testicular plasmablastic lymphoma in a patient with human immunodeficiency virus. Moazez C, Amar S. Cureus. 2019;11:0. doi: 10.7759/cureus.4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Plasmablastic lymphoma in a community-based cancer center: a 10-year analysis of this rare disease in a underserved urban area. Jorge VM, Tiu A, Gupta S, Dourado C, Varadi G. Blood. 2019;134:5361. [Google Scholar]
- 4.Post transplant plasmablastic ‐transplant plasmablastic lymphoma of the skin. Nicol I, Boye T, Carsuzaa F, et al. Aust J Dermatol. 2003;149:889–891. doi: 10.1046/j.1365-2133.2003.05544.x. [DOI] [PubMed] [Google Scholar]
- 5.Broudy VC, Harrington RD. Williams Hematology. 9th edition. USA: McGraw-Hill Education; 2015. Hematologic manifestations of acquired immunodeficiency syndrome. [Google Scholar]
- 6.Plasmablastic lymphoma of visceral cranium, cervix and thorax in an HIV-negative woman. Masgala A, Christopoulos C, Giannakou N, Boukis H, Papadaki T, Anevlavis E. Ann Hematology. 2007;86:615–618. doi: 10.1007/s00277-007-0280-z. [DOI] [PubMed] [Google Scholar]
- 7.Epstein Barr virus and herpes virus 8 associated primary cutaneous plasmablastic lymphoma in the setting of renal transplantation. Verma S, Nuovo GJ, Porcu P, Baiocchi RA, Crowson AN, Magro CM. J Cutan Pathol. 2005;32:35–39. doi: 10.1111/j.0303-6987.2005.00258.x. [DOI] [PubMed] [Google Scholar]
- 8.Plasmablastic lymphoma of the stomach. A case report. Pruneri G, Graziadei G, Ermellino L, Baldini L, Neri A, Buffa R. http://www.haematologica.org/content/83/1/87. Hematologica. 1998;83:87–89. [PubMed] [Google Scholar]
- 9.Extra-oral plasmablastic lymphoma: report of a case and review of literature. Tavora F, Gonzalez-Cuyar LF, Sun CC, Burke A, Zhao XF. Hum Pathol. 2006;37:1233–1236. doi: 10.1016/j.humpath.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 10.Plasmablastic lymphoma of the retroperitoneum in an HIV-negative patient. Takahashi Y, Saiga I, Fukushima J, et al. Pathol Int. 2009;59:868–873. doi: 10.1111/j.1440-1827.2009.02457.x. [DOI] [PubMed] [Google Scholar]
- 11.Clinical and pathological differences between human immunodeficiency virus-positive and human immunodeficiency virus-negative patients with plasmablastic lymphoma. Castillo JJ, Winer ES, Stachurski D, et al. Leuk Lymphoma. 2010;51:2047–2053. doi: 10.3109/10428194.2010.516040. [DOI] [PubMed] [Google Scholar]
- 12.Clinicopathologic comparison of plasmablastic lymphoma in HIV-positive, immunocompetent, and posttransplant patients. Single-center series of 25 cases and meta-analysis of 277 reported cases. Morscio J, Dierickx D, Nijs J, et al. Am J Surg Pathol. 2014;38:875–886. doi: 10.1097/PAS.0000000000000234. [DOI] [PubMed] [Google Scholar]
- 13.Prognostic factors in chemotherapy-treated patients with HIV-associated plasmablastic lymphoma. Castillo JJ, Winer ES, Stachurski D, et al. Oncologist. 2010;15:293–299. doi: 10.1634/theoncologist.2009-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Recommendations for the evaluation of risk and prophylaxis of tumour lysis syndrome (TLS) in adults and children with malignant diseases: an expert TLS panel consensus. Cairo MS, Coiffier B, Reiter A, Younes A, TLS Expert Panel. Br J Haematol. 2010;149:578–586. doi: 10.1111/j.1365-2141.2010.08143.x. [DOI] [PubMed] [Google Scholar]
- 15.Spontaneous tumor lysis syndrome in diffuse large b-cell lymphoma: early diagnosis and management. Gangireddy M, Shrimanker I, Nookala V K, et al. Cureus. 2019;11:0. doi: 10.7759/cureus.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]