Abstract
Background
During reverse total shoulder arthroplasty, the functionality of the subscapularis remains unknown. The purpose of this study was to determine the integrity and function of the repaired subscapularis after reverse total shoulder arthroplasty using ultrasound, electromyography (EMG), and nerve conduction studies (NCS) to assess postoperative tendon healing, muscle, and nerve function.
Materials and methods
Patients who underwent reverse total shoulder arthroplasty and repair with minimum 6-month follow-up were included in the study. Patient-reported outcome, physical examination, ultrasound examination of the subscapularis tendon, subscapularis EMG, and lower subscapular NCS were performed. In addition, contralateral subscapularis ultrasound, EMG, and lower subscapular nerve nerve NCS were performed to establish normative values (abnormal defined at >20% increased latency or >50% decreased amplitude). Phi coefficients of association and point biserial coefficients were used to correlate the ultrasound examination, EMG, and NCS results with the functional outcomes.
Results
A total of 20 patients were included. Four patients had abnormal but intact subscapularis tendons on ultrasound. Nine patients had abnormal lower subscapular NCS compared with the contralateral shoulder. All patients had normal subscapularis EMGs. No significant correlation was found between the ultrasound and NCS results. No significant correlations were found between the ultrasound or the NCS results and any of the independent outcome variables.
Conclusion
This study demonstrates that the subscapularis remains neurologically functional after reverse total shoulder arthroplasty, based on EMG and NCS findings. Although side-to-side differences in lower subscapular NCS were identified in 45% of the postoperative shoulders, these abnormalities did not correlate with functional outcomes.
Keywords: Reverse total shoulder arthroplasty, subscapularis, electromyography, nerve conduction study, patient-reported outcomes, shoulder arthroplasty
Methods of subscapularis detachment and repair include subscapularis tenotomy, subscapularis peel, and lesser tuberosity osteotomy.1,8,12, 13, 14,17,24,32 During reverse total shoulder arthroplasty (RTSA), the functionality of the subscapularis remains unknown; therefore, repair of this tendon remains controversial.
Many studies have demonstrated subscapularis dysfunction following a deltopectoral approach to the shoulder involving subscapularis takedown and repair.2,5,15,16,19, 20, 21,27, 28, 29, 30 Subscapularis dysfunction has been defined as decreased active internal rotation strength, positive subscapularis examination maneuvers (ie, lift-off, belly-press, and bear hug), and the inability to tuck in the shirt behind the back. This has been described in postoperative patients after anatomic TSA,2,5,15,16,19, 20, 21 Latarjet procedures, open Bankart repair,27 and open instability procedures.29,30
In addition, postoperative subscapularis dysfunction has been shown to have poor correlation with tendon healing on imaging studies.2,5,15,20 Thus, the dysfunction is not simply secondary to inadequate tendon or lesser tuberosity osteotomy bone healing. When compared with preoperative imaging, postoperative imaging studies have demonstrated significant increases in subscapularis fatty degeneration and atrophy.12,18,21,29,30,34 Hypotheses for these findings include direct damage to the subscapularis muscle and/or disruption of the vascular or nerve supply to the muscle during the glenoid exposure.2,12,26,29,30
The primary purpose of this study was to examine the innervation of the subscapularis after RTSA, using electromyography (EMG) and nerve conduction studies (NCS). In addition, we sought to correlate the results of the EMG and NCS to integrity of the subscapularis tendon on ultrasound examination, physical examination findings, and outcome scores. The hypothesis of this investigation is that subscapularis function will not be altered after RTSA.
Materials and methods
We evaluated patients who underwent RTSA performed by the senior author (MK), who is a fellowship-trained shoulder surgeon at a single tertiary referral center. A random sample of subjects who met inclusion criteria were recruited from a prospective database of patients who underwent shoulder arthroplasty.
Inclusion criteria
All patients aged 18 and older who underwent RTSA for the treatment of a massive rotator cuff tear, rotator cuff tear arthropathy, or severe glenohumeral joint arthritis with glenoid erosion were included in the study. Patients were contacted, the study procedures explained, and if the subject was agreeable to undergo needle EMG and needle NCS, they were enrolled in the investigation. All underwent primary RTSA using a subscapularis tenotomy with tendon-to-tendon suture repair. Patients were a minimum 6 months’ status after RTSA. The mean time from surgery to study examination was 12 months (range, 6-32 months).
Exclusion criteria
Patients with a history of ipsilateral or contralateral open shoulder surgery, revision shoulder arthroplasty, reverse shoulder arthroplasty for the treatment of proximal humerus fracture, postoperative shoulder infection, fracture, or dislocation were excluded from the study. Patients with a history of prior subscapularis repair were excluded. All operative reports were reviewed, and if the subscapularis was irreparable at the conclusion of the procedure, the patient was excluded from the study.
Study protocol
Patients who met inclusion criteria were contacted by telephone. Details of the study were explained over the telephone, and if the patient was interested in participating in the study, an appointment was scheduled. To cover travel expenses, financial compensation in the amount of $50 was given to all participants who presented to the appointment. Research consent forms were reviewed in detail on the date of examination. The details of the examination are outlined below in the section “Clinical assessment.”
In addition, medical records of the patients who were involved in the study were retrospectively reviewed. Demographic information, preoperative diagnosis/indication for surgery, preoperative range of motion and physical examination, intraoperative subscapularis management, and operative details including implant type and surgical technique were obtained from the medical record. Preoperative patient-reported outcome measures including American Shoulder and Elbow Surgeons (ASES) score and visual analog score (VAS) for pain documented in the prospective database were collected.
Surgical technique and postoperative protocol
All patients received a preoperative regional intrascalene nerve block, general anesthesia, and were placed in a beach chair position. A standard deltopectoral approach was used. After electrocautery ligation of the anterior humeral circumflex vessels, a subscapularis tenotomy was performed approximately 1 cm medial to its insertion on the lesser tuberosity. After tenotomy, the subscapularis tendon was mobilized and freed of surrounding adhesions by performing a 360° release.
RTSA was performed using a 145° neck-shaft angle, on-lay humeral stem design (16 shoulders) (Aequalis Ascend Flex; Tornier/Wright Medical) or 155° neck-shaft angle, inlay humeral stem design (4 shoulders) (Aequalis Reverse II; Tornier/Wright Medical, Bloomington, MN, USA). All humeral components were press-fit in cases using the Ascend flex humeral stem and cemented in cases in which Aequalis Reverse II was used. All glenoid baseplates used the Aequalis Reverse II threaded post with 4 locking screws. Two of the glenospheres were 36 mm eccentric, and the remaining 18 were 36 mm centered. In addition, 4 subjects had increased lateralization of the glenoid using the bony increased offset reverse shoulder arthroplasty technique (3/4 36-mm centered glenosphere and 1/4 36-mm eccentric glenosphere).
After component implantation, the subscapularis was repaired via a direct tendon-to-tendon repair using multiple No. 2 braided, nonabsorbable sutures in an interrupted figure-of-eight fashion.
Postoperative rehabilitation
Patients were placed in abduction sling for 6 weeks. They were allowed full elbow, wrist, and finger range of motion immediately after surgery. They began passive and active assist supine forward elevation and scapular squeezes 1 week after surgery. Patients were restricted from performing extension and internal rotation for 12 weeks. Active range of motion in the frontal plane began as soon as they could demonstrate scapular control and activate the anterior deltoid. Sling was discontinued at 6 weeks, and they were allowed to perform full active range of motion at 12 weeks without restriction.
Clinical assessment
All patients returned for a single postoperative clinical assessment as a part of this study. The study visit included completion of questionnaires, physical examination, ultrasound examination, EMG, and NCS evaluation. The ultrasound, EMG, and NCS were performed by a single physiatrist (AP) with advanced training and extensive clinical experience in these techniques.
The questionnaires included demographic information, medical history, Single Assessment Numeric Evaluation (SANE) rating of the operative shoulder, Short Form 12, and the ASES shoulder assessment of bilateral shoulders. A complete physical examination of bilateral shoulders was performed. All shoulder examinations were performed by a trained member of the research team who was blinded to the results of the ultrasound, EMG, and NCS. The physical examination included active and passive range of motion measurements of the following: forward elevation, external rotation with the arm at the side, external rotation with the arm abducted 90°, and internal rotation with the arm abducted 90°. All range of motion measurements were made with a goniometer. Internal rotation behind the back was documented as the most cranial level the patient was able to bring his or her thumb, and was graded in the following sequential manner: to the hip, buttock, specific lumbar, or specific thoracic level. Subscapularis-specific strength tests, including the belly-press, lift-off, and bear hug examinations, were performed and recorded as positive or negative. Internal rotation strength was measured using a handheld dynamometer (Microfet, Hoggan Scientific, LLC, Salt Lake City, UT, USA). This was performed by asking the patient to internally rotate the shoulder with maximal strength while pressing his or her forearm against the dynamometer held in the examiner’s hand. The internal rotation was held for 3 seconds, and maximum pressure was recorded. After 30 seconds of rest, the patient was asked to repeat the strength testing. This was performed 3 times, and each maximum pressure was recorded.
The subscapularis tendon of bilateral shoulders was evaluated with ultrasound by a single physiatrist (AP) who has extensive clinical experience and training with shoulder ultrasound. Ultrasound examination of the subscapularis muscle was performed using a Sonosite M-Turbo U/S machine and a 15-6 MHz transducer (Fujifilm Sonosite, Inc., Bothell, WA, USA). The patient was in a seated position with the arm relaxed by the side and the dorsum of the hand resting on the ipsilateral thigh. The transducer is placed in a horizontal position anteriorly over the head of the humerus. The superficial deltoid muscle, the greater and lesser tuberosities, and biceps tendon were located. The arm is externally rotated to bring the subscapularis tendon into view as it inserts on the lesser tuberosity. The tendon was examined dynamically and against resistance. The transducer was then rotated to a vertical position to assess the transverse view of the tendon. The tendon was assessed for complete and partial tears. Findings were reported as normal, abnormal (partial-thickness tear/tendon thinning), or full-thickness tear.
EMG evaluation of the subscapularis muscle of bilateral shoulders was performed by a single physiatrist (AP) with a Natus/Nicolet EMG machine using Synergy software (Natus Neurology Inc., Middleton, WI, USA). EMG examination of the subscapularis muscle was performed using a technique described by Németh et al.22 With the patient seated and the shoulder in an abducted, externally rotated position with the hand behind the head, the subscapularis muscle was palpated posterior to the axillary line and anterior to the scapula. A monopolar EMG needle 50 mm in length was directed into the muscle, deep to the scapula. Single needle examination was performed using a disposable needle electrode. The presence of insertional/spontaneous activity (fibrillations and positive sharp waves) and fasciculations was assessed. The arm was then lowered to an abducted, internally rotated position with the dorsum of the hand against the lower lumbar region (as permitted by range of motion). Muscle recruitment was performed using the Gerber Lift-Off test or abducted internal rotation as allowed by range of motion. Motor unit action potentials were assessed for amplitude, duration, phasicity, and recruitment.
Finally, the NCS was performed to test the lower subscapular nerve, which innervates the lower subscapularis and teres major muscle.25 The teres major muscle is relatively superficial and makes surface electrode recording possible. Again, a Natus/Nicolet EMG machine using Synergy software (Natus Neurology, Inc.) was used for the NCS. Filter settings were set at 5 Hz to 5 kHz. A bipolar stimulator with a 3 cm fixed interelectrode distance. Metal disk electrodes were used for recording. The surface recording electrode was placed over the teres major muscle, which was palpated lateral to the lower third of the lateral scapular border. The reference electrode was placed over the lateral scapular spine. Stimulation was performed at Erb’s point just posterior to the sternocleidomastoid muscle in the supraclavicular fossa. The patient was in a seated position with the shoulder in a relaxed position, the elbow flexed, and the dorsum of the hand resting on the ipsilateral thigh. Stimulation duration was 0.1-0.5 ms, with mAmps from 50 to 100 as needed to obtain supramaximal compound muscle action potential. Compound muscle action potential latency and amplitude were recorded bilaterally for comparison. Side-to-side latency and amplitude differences of >50% were considered abnormal (Fig. 1).
Figure 1.
(A) Electromyography and nerve conduction study electrode placement and (B) ultrasound confirmation of proper subscapularis muscle and nerve testing with stimulation at Erb’s point.
Statistical analysis
Statistical correlations were calculated between the NCS results and each of the postoperative physical examination findings and patient-reported outcome measures (ASES score, SANE score, and pain VAS). Similarly, correlations were calculated between the ultrasound results with physical examination findings and questionnaire results. Finally, correlations between the NCS and ultrasound examination results were calculated. Phi coefficients of association (ϕ) were calculated to determine the degree of correlation when both variables were dichotomous. Point biserial coefficients of association (rpb) were calculated to determine the degree of correlation when 1 variable was continuous. Correlation coefficients are on a continuum of −1.0 to +1.0, with a −1.0 value representing a perfect inverse relationship, 0 indicating no correlation, and +1.0 suggesting a perfect positive relationship. Descriptive statistics were calculated including mean and standard deviations then were using parametric statistical analysis performed unpaired Student’s t-test was to compare the preoperative and postoperative shoulder function, patient-reported outcome measures, as well as NCS results between the operative and nonoperative shoulders. Significance was set at P < .05.
Results
Twenty patients were enrolled in the study and completed all components of the clinical assessment. This included 14 females and 6 males with an average age of 72.7 years. Surgery was performed on the dominant shoulder in 14 cases and on the nondominant shoulder in 6 cases. All 20 subjects were right-hand dominant. The average body mass index was 28.15 ± 5.98.
Physical examination
Preoperative range of motion values for active forward elevation, external rotation, and internal rotation behind the back were available for all patients. The comparison data between preoperative and postoperative range of motion values are included in Table I, Table II, Table III. Postoperative internal rotation behind the back ranged from level of the iliac crest to thoracic spine level 3-4 (9 subjects’ lumbar spine level 3-5, 9 subjects could reach thoracic level 3-10, 1 patient to the level of the iliac crest, and 1 could not reach behind the back and was only able to get to approximately the hip level). The average postoperative internal rotation strength with the arm at the side was 8.08 ± 3.88 kilogram-force (kg-f) and with the arm abducted to 90° was 7.37 ± 3.19 kg-f. Internal rotation strength was also tested on the contralateral nonoperative shoulder and was not found to be statistically significantly different (internal rotation strength with the arm at the side, 9.57 ± 4.06, P = .23; internal rotation with the arm at 90°, 8.19 ± 2.99, P = .41).
Table I.
Comparison between preoperative and postoperative physical examination active and passive shoulder range of motion
| Preoperative range of motion (°) | Postoperative range of motion (°) | P value | |
|---|---|---|---|
| Forward elevation, active | 78.89 ± 46.13 (range, 20-160) | 136.25 ± 29.60 (range, 50-170) | .008∗ |
| Forward elevation, passive | 148.8 ± 40.76 (range, 60-180) | 152.35 ± 14.97 (range, 55-180) | .93 |
| External rotation, active | 31.9 ± 25.42 (range, 0-60) | 34.76 ± 17.78 (range, 5-75) | .78 |
| External rotation, passive | 56.67 ± 23.45 | 42.14 ± 15.7 | .06 |
| External rotation at 90° of abduction active | 60.0 ± 15.6 | 48.57 ± 5.16 (range, 0-90) | .04∗ |
| External rotation at 90°, passive | na | 53.33 ± 23.09 | |
| Internal rotation at 90°, active | 35.71 ± 11.58 (range, 0-40) | 46.43 ± 20.13 (range, 25-85) | .07 |
| Internal rotation behind back (spine level) | L1-buttock | T3/4-iliac crest | |
| Belly press | 11/20 positive | 1/20 positive | |
| Lift-off | 5/20 negative | 11/20 positive | |
| Bear hug | 9/20 positive | 3/20 positive |
Indicates a statistically significant difference.
Table II.
Patient-reported outcome measure results preoperative and postoperative
| Preoperative | Postoperative | P value | |
|---|---|---|---|
| ASES | 38.8 ± 17.7 | 83.0 ± 12.6 | <.001∗ |
| SANE | 34.6 ± 26.9 | 77.4 ± 17.7 | <.001∗ |
| VAS | 6.4 ± 1.9 | 0.4 ± 1.0 | <.001∗ |
| SF-12 | |||
| Physical Score | 38.32 ± 12.19 | ||
| Mental Health Score | 57.44 ± 6.79 | ||
ASES, American Shoulder and Elbow Surgeons; SANE, Single Assessment Numeric Evaluation; VAS, visual analog score; SF-12, Short Form 12.
Indicates a statistically significant difference.
Table III.
Average internal rotation strength test as measured by a handheld dynamometer
| Operative shoulder (kg-f) | Nonoperative shoulder (kg-f) | P value | |
|---|---|---|---|
| IR strength at side | 8.079 ± 3.877 | 7.37 ± 3.19 | .23 |
| IR strength at 90° abduction | 9.57 ± 4.058 | 8.19 ± 2.986 | .41 |
IR, internal rotation; kg-f, kilogram-force.
Belly-press, bear hug, and lift-off tests
Preoperative examination of belly press and bear hug was available for all 20 patients. The lift-off test was documented as negative for 5, and 15 of 20 of the remaining patients could not place or could not tolerate the arm in this position to elicit this examination maneuver preoperatively. Of the 20 study subjects, 11 had a positive preoperative belly-press test and 9 had a positive bear hug test. Of the 8 subjects who could tolerate the lift-off examination, 5 were negative and 3 were positive preoperatively. On follow-up examination, 1 of 20 had a positive belly-press, 3 of 20 had a positive bear-hug, and 11 of 20 had a positive lift-off test postoperatively. The patient who had a positive belly-press test had an intact subscapularis on ultrasound, normal EMG results, and slightly delay NCS results compared with the contralateral side (operative side: latency 5.52 ms, amplitude 7 mV, duration 14.53 ms; contralateral shoulder: latency 6.77 ms, amplitude 4.3 mV, duration 12.03 ms). The 3 subjects with positive postoperative bear hug had an intact subscapularis on ultrasound as well as normal EMG results. Of the 11 subjects with positive lift-off, only 1 had a torn subscapularis on ultrasound, 2 had abnormal tendon classified as partial tear on ultrasound, and the others were all intact and additionally all had normal EMG results.
Postoperative questionnaires
The average postoperative ASES score was 83.02 ± 12.58, which was significantly improved from preoperative values (preoperative ASES, 38.81 ± 17.74), P < .001. The average postoperative visual analog scale was 0.7. The average postoperative SANE rating was 77.38 ± 17.65, which was significantly improved from preoperative values (preoperative SANE, 34.62 ± 26.88), P < .001. The average postoperative Short Form 12 Physical Composite Scale and Mental Health Composite Scale scores were 39.3 and 57.5, respectively.
Ultrasound
On the operative shoulder, ultrasound examination revealed 16 intact, normal-appearing subscapularis tendons. The subscapularis tendon of 4 shoulders was noted to be abnormal (3 partial-thickness tears and 1 full-thickness tear) (Fig. 2). The ultrasound examination of the contralateral, nonoperative shoulder demonstrated 18 normal tendons and 2 abnormal tendons (1 partial-thickness tear and 1 full-thickness tear).
Figure 2.
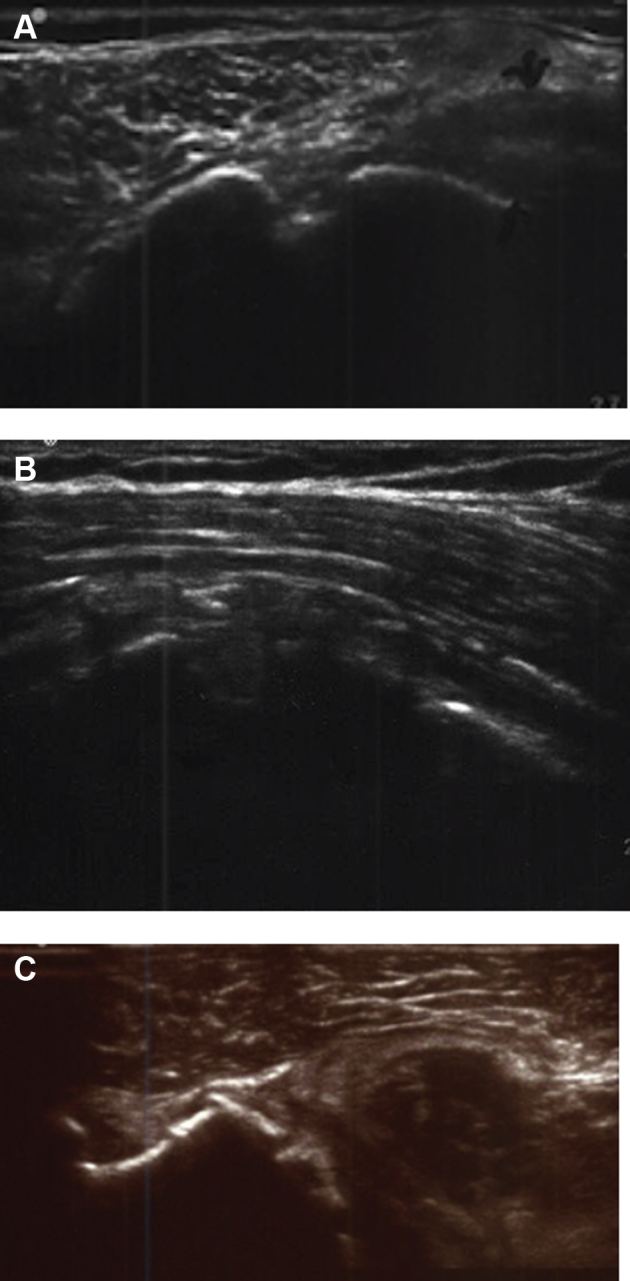
Ultrasound images demonstrating a (A) normal, (B) attenuated, and (C) full-thickness tear in postoperative RTSA shoulders.
EMG
All EMG results, of the operative and contralateral subscapularis muscles, were normal, with no signs of denervation.
Nerve conduction studies
On the operative shoulder, the average latency of the lower subscapularis NCS was 4.5 ms and the average amplitude was 1.4 mV. On the contralateral, nonoperative shoulder, the average latency was 4.2 ms and the average amplitude was 1.5 mV. As no “normative” values exist, the latency and amplitude values of the operative shoulder were compared with the contralateral, “control” shoulder. Abnormal latency was defined as a >20% increase compared with the contralateral shoulder. Abnormal amplitude was defined as a >50% decrease compared with the contralateral shoulder. Using these definitions, a total of 9 operative shoulders had either an abnormal amplitude or latency. Six operative shoulders demonstrated abnormal latency, 7 demonstrated abnormal amplitude, and 4 demonstrated abnormalities of both latency and amplitude. Of note, 2 contralateral, nonoperative shoulders demonstrated abnormal latency, and 3 demonstrated abnormal amplitude. No contralateral shoulders had both latency and amplitude abnormalities. There was no statistically significant difference (P < .05) between the operative and nonoperative subscapularis with regard to mean latency (operative shoulder mean, 4.33 ± 1.24 ms; contralateral shoulder mean, 4.31 ± 1.35 ms, P = .98), amplitude (operative shoulder mean, 1.41 ± 1.57 mV; contralateral shoulder, 1.48 ± 1.17 mV, P = .86), or duration (operative shoulder mean, 12.77 ± 8.25 ms; contralateral shoulder, 10.37 ± 3.87 ms, P = .27).
Correlations analysis
The statistical correlations between ultrasound, NCS findings, and physical examination as well as questionnaire results are described in Table IV. Several statistically significant correlations were found. These included inverse correlations between rotator cuff status on ultrasound and ASES scores as well as SANE score. In addition, statistically significant positive correlations were found between abnormal NCS amplitudes and the lift-off test, as well as between abnormal NCS latency or amplitude and internal rotation strength with the arm at the side. No significant correlations were identified between ultrasound findings and NCS findings.
Table IV.
Statistical correlations between ultrasound, nerve conduction study findings and physical examination, questionnaire results
| Ultrasound | NCS latency | NCS amplitude | NCS latency and amplitude | NCS latency or amplitude | |
|---|---|---|---|---|---|
| Active FF (rpb) | (-) 0.36, P = .11 | 0.26, P = .28 | 0.13, P = .60 | 0.20, P = .41 | 0.20, P = .41 |
| Passive FF (rpb) | (-) 0.16, P = .48 | 0.18, P = .44 | 0.23, P = .34 | 0.22, P = .35 | 0.21, P = .37 |
| Active ER at 0° (rpb) | (-) 0.29, P = .20 | (-) 0.23, P = .33 | 0.03, P = .89 | (-) 0.21, P = .37 | (-) 0.01, P = .97 |
| Passive ER at 0° (rpb) | (-) 0.31, P = .18 | (-) 0.26, P = .27 | 0.04, P = .87 | (-) 0.30, P = .19 | 0.04, P = .85 |
| Active ER ROM at 90° (rpb) | (-) 0.38, P = .09 | (-) 0.32, P = .18 | (-) 0.08, P = .74 | (-) 0.13, P = .58 | (-) 0.26, P = .27 |
| Passive ER ROM at 90° (rpb) | (-) 0.34, P = .13 | (-) 0.34, P = .14 | (-) 0.11, P = .65 | (-) 0.16, P = .49 | (-) 0.28, P = .23 |
| Active IR at 90° (rpb) | 0.15, P = .53 | (-) 0.11, P = .64 | 0.36, P = .12 | 0.11, P = .64 | 0.15, P = .54 |
| Passive IR ROM at 90° (rpb) | 0.07, P = .25 | (-) 0.04, P = .85 | 0.33, P = .16 | 0.13, P = .60 | 0.17, P = .47 |
| IR strength at 0° (rpb) | 0.06, P = .80 | 0.16, P = .49 | 0.35, P = .13 | (-) 0.02, P = .92 | 0.51, P = .02∗ |
| IR strength at 90° (rpb) | 0.05, P = .82 | (-) 0.03, P = .91 | 0.17, P = .48 | (-) 0.15, P = .52 | 0.26, P =.13 |
| Belly-press test (f) | (-) 0.11, P = 1.00 | 0.15, P = 1.00 | 0.17, P = 1.00 | 0.11, P = 1.00 | 0.21, P = 1.00 |
| Lift-off test (f) | (-) 0.02, P = 1.00 | 0.07, P = 1.00 | 0.60, P = .02∗ | 0.30, P = .28 | 0.39, P = .17 |
| Bear hug test (f) | (-) 0.20, P = 1.00 | 0.28, P = .52 | 0.31, P = .28 | 0.21, P = .58 | 0.38, P = .22 |
| ASES (rpb) | (-) 0.53, P = .01∗ | 0.16, P = .51 | (-) 0.03, P = .90 | (-) 0.11, P = .65 | 0.20, P = .40 |
| SANE (rpb) | (-) 0.52, P = .01∗ | (-) 0.15, P = .54 | 0.11, P = .65 | (-) 0.05, P = .83 | 0.01, P = .97 |
| Pain VAS (rpb) | 0.31, P = .18 | 0.18, P = .45 | (-) 0.02, P = .92 | (-) 0.04, P =.87 | 0.17, P =.47 |
NCS, nerve conduction study; FF, forward flexion; ER, external rotation; ROM, range of motion; IR, internal rotation; ASES, American Shoulder and Elbow Surgeons; SANE, Single Assessment Numeric Evaluation; VAS, visual analog score.
Inverse correlations are identified by preceding (-). Phi (φ) coefficients of association were calculated to determine the correlation when both variables were dichotomous. This included the correlation between ultrasound and NCS status. For analysis in which the impairment or functional variable was continuous (passive ER ROM, IR strength, ASES score, and SANE score), a point biserial coefficient (rpb) was computed to evaluate the degree of correlation.
Indicates statistically significant results.
Discussion
The findings of this investigation demonstrate that there is normal subscapularis muscle function on needle EMG assessment after RTSA. Ultrasound of the subscapularis did demonstrate tendon abnormalities as well as changes in latency, amplitude, and duration for the lower subscapular nerve. These changes did not seem to impact muscle function in the early postoperative period as seen on EMG. Although the subscapularis is often repaired when possible during RTSA, some unresolved issues persist regarding the utility of the repair. First, does subscapularis repair improve stability of the shoulder? Several previous studies have demonstrated significantly decreased postoperative dislocation rates with subscapularis repair after RTSA.9,11,31 Edwards et al9 prospectively followed 138 patients after RTSA. The subscapularis was repaired in 62 patients and was irreparable in 76. At an average follow-up of 36 months, 7 patients (9.2%) in the group without subscapularis repair had sustained a dislocation, whereas none in the subscapularis repair group had dislocated. Similarly, Trappey et al31 prospectively followed 284 patients after RTSA. The subscapularis was repaired in 161 and was irreparable in 123. At an average follow-up of 24 months, there were 14 dislocations (11.4%) in the group without repair and 1 dislocation (0.6%) in the repair group. In addition, they found no statistical difference in the dislocation rates between primary and revision RTSA, without respect to subscapularis repair. The biomechanical study by Oh et al23 adds further support to the notion that subscapularis repair increases postoperative RTSA stability, by demonstrating that an intact subscapularis increases anterior stability at all neck-shaft angles and all internal and external rotational positions. On the contrary, similarly designed studies have demonstrated no significant differences in instability rates with or without subscapularis repair.6,33 Our findings suggest that despite the change in the line of pull of the muscle tendon unit, the subscapularis muscle still functions in this position and contributes to shoulder function.
Second, does subscapularis repair improve the function of RTSA? Several studies have directly compared shoulder range of motion with respect to subscapularis repair.4,6,10,11,33 Wall et al33 demonstrated that subscapularis repair resulted in greater improvement in active internal rotation. Boulahia et al,4 however, demonstrated decreased active external rotation with subscapularis repair. Franceschetti et al10 compared patients with and without subscapularis repair who underwent RTSA with a 145° neck-shaft angle humeral prosthesis in addition to bony increased offset glenoid component. The authors reported no differences in VAS, Constant score, forward elevation, external rotation at both 0° and 90° of abduction, or internal rotation postoperatively between groups.
Finally, and most relevant to this study—does the subscapularis muscle function normally after repair? Many studies have demonstrated clinical subscapularis dysfunction after subscapularis repair.2,5,15,16,19, 20, 21,26,27,29,30 The majority of these studies have included patients who have undergone anatomic TSA. The study by Miller et al21 in 2003 was one of the first to address this issue. They reported on 41 patients after anatomic TSA who underwent subscapularis tenotomy with tendon-to-tendon repair. Postoperatively, 67% of their patients had a positive belly-press test, 67% had a positive lift-off test, and 68% had a functionally abnormal subscapularis, as defined by the inability to tuck in the back of the shirt. After this finding, several more studies identified postoperative anatomic TSA patients with abnormal subscapularis function and additionally performed imaging studies to evaluate healing of the subscapularis repair.2,12,26 Qureshi et al26 in 2008 reported on 30 patients who underwent anatomic TSA with lesser tuberosity osteotomy. They had a 100% lesser tuberosity healing rate based on x-ray. However, 40% of patients still had a positive belly press, and 17% had difficulty tucking in their shirt in the back. Despite these findings, our study provides evidence that the subscapularis muscle tendon unit is still functioning after tendon healing in the early postoperative period. Similarly, this investigation also found that 5% of patient had a positive belly press and 15% positive bear hug at early postoperative follow-up.
In addition, several studies have demonstrated fatty infiltration of the subscapularis after the anterior approach to the shoulder.7,12,18,29,30,34 Gerber et al12 in 2005 studied 39 patients who underwent anatomic TSA with lesser tuberosity osteotomy. There was a 100% osteotomy healing rate based on computed tomography scan; however, similarly, 11% of patients still had a positive belly press, and 25% had a positive lift-off test. They additionally demonstrated a significant increase in subscapularis fatty infiltration on the postoperative vs. the preoperative computed tomography scan. Scheibel et al30 in 2006 reported similar findings after open shoulder stabilization procedures. In addition, they compared the postoperative examination and imaging findings with a group who underwent arthroscopic stabilization procedures. This study included 10 patients who underwent an open procedure, involving subscapularis tenotomy, and 12 patients who underwent arthroscopic shoulder stabilization. Postoperatively, 70% of patients who had an open procedure had a positive belly-off examination, whereas none in the arthroscopic group had an abnormal examination. In addition, on postoperative magnetic resonance imaging, the open group had significantly more subscapularis fatty infiltration than the arthroscopic group. Many authors have speculated as to the cause of these findings, and the majority have guessed that this is most likely due to denervation or direct damage to the subscapularis muscle during the approach to the shoulder. The outcome of the EMG/NCS that we found provides evidence against this theory leaving a yet unknown explanation for the continued progression of fatty infiltration despite intact muscle function and nerve conduction.
A recent study by Armstrong et al3 reported on the results of EMG evaluation of the subscapularis in postoperative anatomic TSA patients. They included 30 patients who underwent anatomic TSA with subscapularis tenotomy, at least 1 year from surgery. In addition, they correlated these findings with ultrasound and physical examination results. EMG evaluation showed that no patients had signs of active denervation of the subscapularis. However, there was evidence of chronic denervation and reinnervation changes in the subscapularis in 9 patients. In contrast, the EMG findings in the current study found no EMG muscle abnormalities and had normal muscle EMG function.
To our knowledge, this was the first study examining the subscapularis of postoperative RTSA patients with EMG and NCS. In addition, this is the first study correlating physical examination to integrity of the subscapularis tendon in postoperative RTSA patients. In our study, 4 patients had abnormal subscapularis tendons on ultrasound. One was completely ruptured, whereas 3 were attenuated. Similar to previous studies on anatomic total shoulder, there was poor correlation between subscapularis tendon healing and physical examination results, including belly-press, lift-off, bear hug, active internal rotation, and internal rotation strength. However, we did find a statistically significant inverse relationship between tendon healing and ASES and SANE rating scores.
The NCS technique we used specifically studied the lower subscapularis nerve, and the contralateral shoulder was used as a control. This imperfect control group is one weakness of this study. A total of 9 shoulder demonstrated abnormal values of latency or amplitude compared with the contralateral shoulder. On correlation of the NCS with physical examination findings, 2 unexpected significant correlations were identified. First, abnormal NCS amplitudes positively correlated with normal lift-off examination. Second, abnormal NCS amplitudes or latencies positively correlated with increased internal rotation strength with the arm at the side.
There are several limitations of this study. The NCS did not have a true control, as we used the contralateral shoulder as a control. We do not have baseline preoperative nerve conduction or EMG studies for comparison for changes in subscapularis nerve and muscle function. The sample size was small, limited mostly due to subjects not wanting to undergo an uncomfortable elective test not related to their treatment. This can impart some selection bias to our results. In addition, subgroup analysis of the influence of neck-shaft angle, eccentricity, or lateralization (use of bony increased offset reverse shoulder arthroplasty) was not able to be performed due to the small sample size in each group. Also, given the small sample size, we do not know if our findings are underpowered to demonstrate a significant difference between groups. Only the lower subscapular nerve was evaluated as the upper subscapular nerve is not easily accessible percutaneously and has these techniques have not been described to our knowledge, therefore we do not know what effects or damage occur to this nerve.
Conclusions
Despite what has been historically reported regarding abnormalities of the subscapularis after anatomic TSA, the findings of this study demonstrate that there is normal muscle function on needle EMG assessment after RTSA. Our findings also demonstrate tendon abnormalities on ultrasound as well as changes in latency, amplitude, and duration for the lower subscapular nerve. These results may explain why, despite an intact tendon repair, muscle atrophy is still seen. What is not known is if these changes are a result of soft tissue dissection during surgery or from changes experienced by the repaired subscapularis given the change in humeral position (lateralization and distalization). The significance of these findings indicates that in the setting of RTSA, the subscapularis muscle tendon unit is functional. Future studies are needed to determine how much the change in force vector with distalization and lateralization of the humerus influences shoulder range of motion.
Disclaimer
The other authors, their immediate families, and any research foundations with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article.
Footnotes
Approval was received from the University of Texas Southwestern Institutional Review Board (STU 062015-075).
References
- 1.Ahmad C., Wing D., Gardner T., Levine W., Bigliani L. Biomechanical evaluation of subscapularis repair used during shoulder arthroplasty. J Shoulder Elbow Surg. 2007;16:59S–64S. doi: 10.1016/j.jse.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong A., Lashgari C., Teefey S., Menendez J., Yamaguchi K. Ultrasound evaluation and clinical correlation of subscapularis repair after total shoulder arthroplasty. J Shoulder Elbow Surg. 2006;15:541–548. doi: 10.1016/j.jse.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong A., Southam J., Home A., Hollenbeak C., Flemming D., Kothari M. Subscapularis function after total shoulder arthroplasty: electromyography, ultrasound, and clinical correlation. J Shoulder Elbow Surg. 2016;25:1674–1680. doi: 10.1016/j.jse.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 4.Boulahia A., Edwards T.B., Walch G., Baratta R.V. Early results of a reverse design prosthesis in the treatment of arthritis of the shoulder in elderly patients with a large rotator cuff tear. Orthopedics. 2002;25:129–133. doi: 10.3928/0147-7447-20020201-16. [DOI] [PubMed] [Google Scholar]
- 5.Buckley T., Miller R., Nicandri G., Lewis R., Voloshin I. Analysis of subscapularis integrity and function after lesser tuberosity osteotomy versus subscapularis tenotomy in total shoulder arthroplasty using ultrasound and validated clinical outcome measures. J Shoulder Elbow Surg. 2014;23:1309–1317. doi: 10.1016/j.jse.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 6.Clark J., Ritchie J., Song F., Kissenberth M., Tolan S., Hart N. Complication rates, dislocation, pain, and postoperative range of motion after reverse shoulder arthroplasty in patients with and without repair of the subscapularis. J Shoulder Elbow Surg. 2012;21:36–41. doi: 10.1016/j.jse.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 7.De Wilde L., Coninck T., Neve F., Berghs B. Subscapularis release in shoulder replacement determines structural muscular changes. Clin Orthop Relat Res. 2012;470:2193–2201. doi: 10.1007/s11999-012-2291-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Defranco M.J., Higgins L.D., Warner J.J. Subscapularis management in open shoulder surgery. J Am Acad Orthop Surg. 2010;18:707–717. doi: 10.5435/00124635-201012000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Edwards T., Williams M., Labriola J., Elkousy H., Gartsman G., O’Connor D. Subscapularis insufficiency and the risk of shoulder dislocation after reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18:892–896. doi: 10.1016/j.jse.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 10.Franceschetti E., de Sanctis E.G., Ranieri R., Palumbo A., Paciotti M., Franceschi F. The role of the subscapularis tendon in a lateralized reverse total shoulder arthroplasty: repair versus nonrepair. Int Orthop. 2019;43:2579–2586. doi: 10.1007/s00264-018-4275-2. [DOI] [PubMed] [Google Scholar]
- 11.Friedman R.J., Flurin P.H., Wright T.W., Zuckerman J.D., Roche C.P. Comparison of reverse total shoulder arthroplasty outcomes with and without subscapularis repair. J Shoulder Elbow Surg. 2017;26:662–668. doi: 10.1016/j.jse.2016.09.027. [DOI] [PubMed] [Google Scholar]
- 12.Gerber C., Yian E., Pfirrmann C., Zumstein M., Werner C. Subscapularis muscle function and structure after total shoulder replacement with lesser tuberosity osteotomy and repair. J Bone Joint Surg Am. 2005;87:1739–1745. doi: 10.2106/JBJS.D.02788. [DOI] [PubMed] [Google Scholar]
- 13.Giuseffi S.A., Wongtriratanachai P., Omae H., Cil A., Zobitz M.E., An K.N. Biomechanical comparison of lesser tuberosity osteotomy versus subscapularis tenotomy in total shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21:1087–1095. doi: 10.1016/j.jse.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Heckman D.S., Hoover S.A., Weinhold P.S., Spang J.T., RA C. Repair of lesser tuberosity osteotomy for shoulder arthroplasty: biomechanical evaluation of the backpack and dual row techniques. J Shoulder Elbow Surg. 2011;20:491–498. doi: 10.1016/j.jse.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Jackson J., Cil A., Smith J., Steinmann S. Integrity and function of the subscapularis after total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19:1085–1090. doi: 10.1016/j.jse.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Jandhyala S., Unnithan A., Hughes S., Hong T. Subscapularis tenotomy versus lesser tuberosity osteotomy during total shoulder replacement: a comparison of patient outcomes. J Shoulder Elbow Surg. 2011;20:1102–1107. doi: 10.1016/j.jse.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 17.Lapner P., Sabri E., Rakhra K., Bell K., Athwal G. Comparison of lesser tuberosity osteotomy to subscapularis peel in shoulder arthroplasty. J Bone Joint Surg Am. 2012;94:2239–2246. doi: 10.2106/JBJS.K.01365. [DOI] [PubMed] [Google Scholar]
- 18.Lapner P., Sabri E., Rakhra K., Bell K., Athwal G. Healing rates and subscapularis fatty infiltration after lesser tuberosity osteotomy versus subscapularis peel for exposure during shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22:396–402. doi: 10.1016/j.jse.2012.05.031. [DOI] [PubMed] [Google Scholar]
- 19.Lapner P., Wood K., Zhang T., Athwal G. The return of subscapularis strength after shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24:223–228. doi: 10.1016/j.jse.2014.06.042. [DOI] [PubMed] [Google Scholar]
- 20.Liem D., Kleeschulte K., Dedy N., Sculte T., Steinbeck J., Marquardt B. Subscapularis function after transosseous repair in shoulder arthroplasty: transosseous subscapularis repair in shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21:1322–1327. doi: 10.1016/j.jse.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 21.Miller S., Hazrati Y., Klepps S., Chiang A., Flatow E. Loss of subscapularis function after total shoulder replacement: a seldom recognized problem. J Shoulder Elbow Surg. 2003;12:29–34. doi: 10.1067/mse.2003.128195. [DOI] [PubMed] [Google Scholar]
- 22.Németh G., Kronberg M., Broström L.A. Electromyogram (EMG) recordings from the subscapularis muscle: description of a technique. J Orthop Res. 1990;8:151–153. doi: 10.1002/jor.1100080120. [DOI] [PubMed] [Google Scholar]
- 23.Oh J., Shin S., McGarry M., Scott J., Heckmann N., Lee T. Biomechanical effects of humeral neck-shaft angle and subscapularis integrity in reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23:1091–1098. doi: 10.1016/j.jse.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 24.Ponce B.A., Ahluwalia R.S., Mazzocca A.D., Gobezie R.G., Warner J.J., Millett P.J. Biomechanical and clinical evaluation of a novel lesser tuberosity repair technique in total shoulder arthroplasty. J Bone Joint Surg Am. 2005;87:1–8. doi: 10.2106/JBJS.E.00441. [DOI] [PubMed] [Google Scholar]
- 25.Prakash K.M., Fook-Chong S.M.C., Leoh T.H., Dan Y.F., Nurjannah S., Tan Y.E. The lower subscapular nerve conduction studies and utilisation in brachial plexopathy evaluation. J Neurol Sci. 2006;247:77–80. doi: 10.1016/j.jns.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 26.Qureshi S., Hsiao A., Klug R., Lee E., Braman J., Flatow E. Subscapularis function after total shoulder replacement: results with lesser tuberosity osteotomy. J Shoulder Elbow Surg. 2008;17:68–72. doi: 10.1016/j.jse.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 27.Sachs R., Williams B., Stone M., Paxton L., Kuney M. Open Bankart repair: correlation of results with postoperative subscapularis function. Am J Sports Med. 2005;33:1458–1462. doi: 10.1177/0363546505275350. [DOI] [PubMed] [Google Scholar]
- 28.Scheibel M., Habermeyer P. Subscapularis dysfunction following anterior surgical approaches to the shoulder. J Shoulder Elbow Surg. 2008;17:671–683. doi: 10.1016/j.jse.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 29.Scheibel M., Nikulka C., Dick A., Schroeder R., Popp A., Haas N. Structural integrity and clinical function of the subscapularis musculotendinous unit after arthroscopic and open shoulder stabilization. Am J Sports Med. 2007;35:1153–1161. doi: 10.1177/0363546507299446. [DOI] [PubMed] [Google Scholar]
- 30.Scheibel M., Tsynman A., Magosch P., Schroeder R., Habermeyer P. Postoperative subscapularis muscle insufficiency after primary and revision open shoulder stabilization. Am J Sports Med. 2006;34:1586–1593. doi: 10.1177/0363546506288852. [DOI] [PubMed] [Google Scholar]
- 31.Trappey G.J., O'Connor D.P., Edwards T.B. What are the instability and infection rates after reverse shoulder arthroplasty? Clin Orthop Relat Res. 2011;469:2505–2511. doi: 10.1007/s11999-010-1686-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van den Berghe G.R., Nguyen B., Patil S., D'Lima D.D., Mahar A., Pedowitz R. A biomechanical evaluation of three surgical techniques for subscapularis repair. J Shoulder Elbow Surg. 2008;17:156–161. doi: 10.1016/j.jse.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 33.Wall B., Nove-Josserand L., O’Connor D., Edwards T., Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am. 2007;89:1476–1785. doi: 10.2106/JBJS.F.00666. [DOI] [PubMed] [Google Scholar]
- 34.Zumstein M.A., Pinedo M., Old J., Boileau P. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2011;20:146–157. doi: 10.1016/j.jse.2010.08.001. [DOI] [PubMed] [Google Scholar]



