Periprosthetic fracture of total shoulder arthroplasty is a relatively rare complication.2,3,5 The Cofield classification has been proposed as a means to classify and guide treatment of periprosthetic humeral fractures following total shoulder arthroplasties.3,5 Wright and Cofield classified fractures on the basis of location with respect to the tip of the humeral prosthesis; type A extends proximally greater than one-third the length of the stem, type B remains centered at the tip of the stem, and type C fractures are located distal to the tip of the prosthesis (Fig. 1).3,5
Figure 1.

Cofield classification of periprosthetic humerus fractures.
The Cofield classification recommends nonoperative management for well-fixed type A fractures and type C fractures. For type B fractures that are well-aligned with a well-fixed humeral component, a nonoperative trial could be considered; however, all loose type B fractures should be revised to a long stem implant with iliac crest bone graft.3
However, this classification system is based on Level IV evidence, and has been questioned with respect to interobserver reliability and its ability to guide treatment. Andersen et al1 suggested that the cause (trauma vs. chronic) and presence of implant loosening is more relevant to treatment choice than location of the fracture. Acute traumatic fractures around a well-fixed implant could be treated with internal fixation, whereas chronic fractures around loose implants would require revision, regardless of fracture location.
We present a case of a traumatic displaced Cofield B fracture in a 72-year-old man who was treated nonoperatively and went on to union with a stable implant.
Case report
The patient is a 72-year-old male who presented for evaluation of glenohumeral arthritis. The patient had a history of well-controlled hypertension and hyperlipidemia, but was otherwise healthy. History, clinical examination, and radiographs were all consistent for left-sided glenohumeral arthritis. A left total shoulder arthroplasty and biceps tenodesis was performed through a deltopectoral approach. Implants used were a size 40 large perform glenoid, 50 × 19 eccentric humeral head, and a 5B uncemented humeral stem (Wright; Wright Medical's, Memphis, Tennessee, USA/Tornier-Ascend Flex). There were no intraoperative complications.
Postoperatively, the patient was immobilized in a sling for 4 weeks, avoiding any active range of motion or lifting. The patient was allowed to come out of the sling only for gentle passive range of motion, cryotherapy, and scapular muscle isometric exercises. At 6 weeks, physiotherapy was advanced gradually to restore active range of motion with gradual discontinuation of the shoulder sling. The patient continued to do well and physiotherapy was advanced to gradual strengthening.
At 5 months postoperation, the patient fell, landing on his operative side. He sustained an isolated Cofield B fracture to his left shoulder (Fig. 2). Although the distal portion of the stem was clearly loose and displaced, a trial of nonoperative management was undertaken given that the proximal portion of the implant appeared to be well fixed on the trauma radiograph. The patient was followed clinically and radiographically over the next 9 months (Figs. 3 and 4). Over this period of time, the patient demonstrated both clinical and radiographic signs of healing, and restarted physiotherapy once his shoulder began moving as a unit and there was visible callus on radiography.
Figure 2.
(A) Cofield B fracture of left shoulder, acute (anteroposterior view). (B) Cofield B fracture of left shoulder, acute (axillary view).
Figure 3.
(A) Cofield B fracture of left shoulder after 7 weeks (anteroposterior view). (B) Cofield B fracture of left shoulder after 7 weeks (axillary view).
Figure 4.
(A) Cofield B fracture of left shoulder after 9 months (anteroposterior view). (B) Cofield B fracture of left shoulder after 9 months (axillary view).
The patient went on to heal with nonoperative management, resulting in a stable, well-fixed implant radiographically. At the most recent clinical follow-up, the patient had complete resolution of pain, full rotator cuff strength, and range of motion comparable to his contralateral side.
Discussion
Our case illustrates how the Cofield classification may not be able to help guide treatment of periprosthetic fractures around newer, metaphyseal-fit humeral implants. With the Cofield classification, this patient with a displaced fracture near the prosthesis tip would be considered a B type requiring revision and bone grafting.3 However, it is important to note that the Cofield classification and treatment guidelines were made with different prostheses in mind; namely, the Cofield and Neer implants.3,5 It is certainly possible that with the Tornier implant used in our patient, where the strength of fixation comes from the metaphyseal fit, the location of the fracture relative to the prosthesis tip in the diaphysis may not be as relevant compared with older implants. In fact, this patient’s fracture may be behaving more like a Cofield C type injury, where the fracture line is clear of the implant’s primary point of fixation.
Also, this case lends credence to Andersen et al’s1 suggestion that the context of a patient’s periprosthetic fracture is more important than the location in the humerus. In this study, the authors reviewed a series of 36 periprosthetic fractures of the humerus and found that fractures could be subdivided into acute fractures occurring around stable implants, or fractures occurring as a result of progressive loosening and osteolysis. The authors found that the presence of loosening in conjunction with chronic bone changes was a better determinant of treatment than the location of the fracture in relation to the stem tip.1 In our case, this also held true—the patient had sustained an acute fracture around a well-fixed implant without signs of osteolysis and ultimately did not require revision.
We believe that the ability of this fracture to heal nonoperatively is related to the evolving design of the modern total shoulder arthroplasty and the strength of the metaphyseal fit.
Radiographic changes around humeral components in shoulder arthroplasty was investigated by Raiss et al.4 The uncemented stem studied by the authors was also a short-stem, metaphyseal fit implant designed for proximal bony ingrowth with a polished distal portion. At an average follow-up of 5.5 years, only 2 of 67 uncemented total shoulder arthroplasties were loose or at risk of loosening. One was judged to be “at risk” because of the presence of radiolucent lines; the other was due to traumatic fracture. Moreover, spot welds were present in 82.1% of total shoulders—further indication of strong fixation proximally, in the metaphyseal bone.
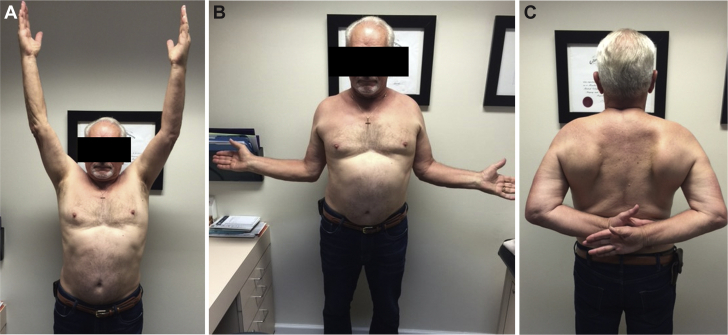
It is unclear what the result of this patient’s injury would have been had he been injured earlier in the postoperative course. Presumably, had his humerus fractured prior to any meaningful bony ingrowth, the stem would have loosened much more precipitously and may have been less likely to heal nonoperatively. Timing of periprosthetic fractures in relation to the index operation may be a consideration for any future classification systems and treatment guidelines. In our case, the metaphyseal fixation was strong enough to allow for a nonoperative management protocol similar to that of a native proximal humerus fracture. Revision surgery was avoided and the patient was able to attain an excellent outcome with full range of motion (Fig. 5) and rotator cuff strength.
Figure 5.
(A) Range of motion at final follow up. Forward elevation. (B) Range of motion at final follow up. External rotation. (C) Range of motion at final follow up. Internal rotation.
Conclusion
The Cofield classification may require some adaptation to reliably classify periprosthetic fractures of modern shoulder implants. Defining fractures in relation to the primary point of fixation, rather than the tip of the stem, could help group similar fractures together regardless of implant type. Also, taking into account whether a fracture is related to acute trauma or chronic osteolysis could further help guide treatment. Overall, our case suggests that the Cofield classification as written may not be able to guide the treatment of periprosthetic fractures in modern short-stem implants.
Disclaimer
Benjamin Y. Jong was a paid consultant for Tornier (Wright Medical).
The other authors, their immediate families, and any research foundations with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article.
Footnotes
Institutional review board approval was not required for this case report.
References
- 1.Andersen J.R., Williams C.D., Cain R., Mighell M., Frankle M. Surgically treated humeral shaft fractures following shoulder arthroplasty. J Bone Joint Surg Am. 2013;95:9–18. doi: 10.2106/JBJS.K.00863. [DOI] [PubMed] [Google Scholar]
- 2.Athwal G.S., Sperling J.W., Rispoli D.M., Cofield R.H. Periprosthetic humeral fractures during shoulder arthroplasty. J Bone Joint Surg Am. 2009;91:594–603. doi: 10.2106/JBJS.H.00439. [DOI] [PubMed] [Google Scholar]
- 3.Kumar S., Sperling J.W., Haidukewych G.H., Cofield R.H. Periprosthetic humeral fractures after shoulder arthroplasty. J Bone Joint Surg Am. 2004;86:680–689. doi: 10.2106/00004623-200404000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Raiss P., Edwards T.B., Deutsch A., Shah A., Bruckner T., Loew M. Radiographic changes around humeral components in shoulder arthroplasty. J Bone Joint Surg Am. 2014;96 doi: 10.2106/JBJS.M.00378. e54-1-9. [DOI] [PubMed] [Google Scholar]
- 5.Wright T.W., Cofield R.H. Humeral fractures after shoulder arthroplasty. J Bone Joint Surg Am. 1995;77:1340–1346. doi: 10.2106/00004623-199509000-00008. [DOI] [PubMed] [Google Scholar]