Abstract
In Japan, cervical cancer incidence has increased since the late 1990s especially among young women, despite a decreasing trend in most developed countries. Here, we examined age, period and birth cohort trends in cervical cancer incidence rates from 1985 to 2012. Incidence rates were ascertained using three population-based cancer registries and analyzed using Joinpoint regression and age-period-cohort models. We compared the findings in Japan to trends among Japanese-Americans in the Surveillance, Epidemiology, and End Results Registries and among women in South Korea using the Korea Central Registry. Age-standardized incidence rates in Japan decreased by 1.7% per year (95% confidence interval –3.3%, 0.0%) until 1997 and thereafter increased by 2.6% per year (1.1%, 4.2%). Incidence rates increased among women under age 50, were stable among women aged 50–54, and decreased or remained stable among women aged 55 and over. The age-standardized incidence rate ratio by birth cohort showed a U-shaped pattern with the lowest rates in women born in the late 1930s and 1940s. In comparison, women born before 1920 and after 1970 had about double the incidence. Increasing risk in recent birth cohorts was not evident in Japanese-American or South Korean women. The trends in Japan may be attributable to increasing prevalence of human papillomavirus (HPV) infection among young women. Screening and vaccination have been shown to be highly effective and would help reverse these trends.
Keywords: cervical cancer, incidence trend, Japan
Introduction
Worldwide, an estimated 570, 000 uterine cervical cancer cases were diagnosed in 2018.1 In most high-income countries, the incidence of invasive cervical cancer has decreased significantly in recent decades because of successful cervical cancer screening.2 In Japan, however, cervical cancer incidence rates increased by about 2% per year between 1997 and 2012, driven primarily by increasing rates among women in their 20s to 40s.3
Several factors could explain the recent increase in cervical cancer in pre-menopausal women in Japan, including low levels of screening uptake, changes in sexual behavior leading to increased prevalence of human papillomavirus (HPV) infection, and suspension of active recommendation of HPV vaccination in June 2013.4, 5 Age-period-cohort (APC) models can be used to gain insight into how trends across ages, calendar periods, and birth cohorts contribute to the observed population-level trends. Health system-scale changes, such as updated screening guidelines and their effective implementation, for example, would typically have a period effect, while changes in sexual behavior and in prevalence of HPV are likely to be generational and would present as a birth cohort effect.
A study from the Osaka Cancer Registry comparing APC trends across many cancer sites showed that for cervical cancer, after many decades of decline, rates started to increase steeply among those born after the 1950s.6 Similar cohort effects have been observed internationally and have been explained by changes in gender roles and sexual practices after the Second World War.2 Given that the Japanese trends were observed in Osaka alone,6 we endeavored to determine whether this pattern was generalizable to other regions of Japan, and to determine the presence and/or strength of period effects.
Specifically, the objective of the current study was to conduct a detailed analysis of age, period, and birth cohort trends in invasive cervical cancer incidence in Japan. We also analyzed trends in cervical cancer incidence rates in Japanese women living in the USA (Japanese-American) and South Korean women for comparison to examine the effect of prevention and control programs and policies between populations with similar genetic risk and where high-quality health system and incidence data are available.
Methods
Data Sources
We obtained cancer incidence data from the high-quality, population-based cancer registries of three Japanese prefectures (Yamagata, Fukui, and Nagasaki) between 1985 and 2012.7 These registries are considered the best approximation to national rates prior to the establishment of the national cancer registry in 2016.8, 9 In an exploratory analysis, we examined the incidence rate of carcinoma in situ (CIS) of the cervix to assess evidence for increases in cervical cancer screening. We also explored subtype-specific (i.e., squamous cell carcinoma and adenocarcinoma) patterns in incidence to examine the effect of cytology screening. In this subtype-specific analysis we obtained histological subtype data from the Nagasaki prefecture only because it had high quality histological diagnoses (95% cases were histologically verified) and data availability.
To maximize the years for comparison with Japanese-Americans, we used invasive cervical cancer rates starting in 1990 (the first year for which detailed race data was available) until 2014, from nine Surveillance, Epidemiology, and End Results (SEER) registries: California, Connecticut, Atlanta, Hawaii, Iowa, Detroit, New Jersey, New Mexico, Utah, and Seattle.10 South Korean incidence rates were obtained from the Korean Central Cancer Registry between 1999 and 2014.
Disease definition
We used International Classification of Diseases, tenth revision (ICD-10) code to identify invasive uterine cervical cancer (C53) and CIS of the cervix (D06). Histological subtype was categorized according to morphology code of the ICD for Oncology, third edition (ICD-O-3) into squamous carcinoma (8050–8130) and adenocarcinoma (8140–8490).
Statistical analysis
We estimated age-standardized and age-specific incidence rates of cervical cancer in women aged 20 to 84 in Japan. Temporal trends in incidence were estimated using Joinpoint regression. Joinpoint employs piecewise loglinear regression to estimate annual percent changes and identify any year in which a significant change in trends occurs, which is called the Joinpoint. A 2-tailed p-value of less than 0.05 was considered significant. The analysis was performed using the Joinpoint Regression Program (version 4.5.0.1) from the National Cancer Institute (NCI).
To investigate the age, period, and birth cohort effects, we used the NCI APC analysis web tool.11 The relationship between ages, periods, and birth cohorts is perfectly collinear and therefore cannot be uniquely separated. Instead, APC models must be reparametrized in terms of estimable parameters. The APC model in this study uses the age-cohort longitudinal form which is commonly used in cancer surveillance research to evaluate etiologic changes across generations.12, 13
APC analyses require a Lexis diagram of incidence rates by calendar year and age. The Lexis diagram must be symmetrical, meaning that the age intervals are of the same length as the calendar year intervals. The incidence data was provided in five-year age groups so calendar time was aggregated to five-year intervals. For Japan, five intervals spanned between 1988 and 2012. For Japanese-Americans, four intervals spanned between 1993 and 2012. For South Korea, three intervals spanned between 1998 and 2012. Because data in South Korea was only available starting in 1999, we imputed age-specific rates for 1998 using the annual percent change of the first segment of the Joinpoint analysis.
Age-specific net annual percent changes in incidence rate are used to illustrate the consequence of trends in birth cohort rates.11 Period and cohort effects were presented as incidence rate ratios (IRRs) adjusted for the other two time scales. To facilitate data interpretation, the period spanning 1998–2002, which was the middle interval of the study period for Japan, was the reference value for period analyses where the birth years spanning 1936–1940, which had the lowest-risk among Japanese women, were reference values for birth cohort analyses.
Results
Age-standardized and age-specific trends
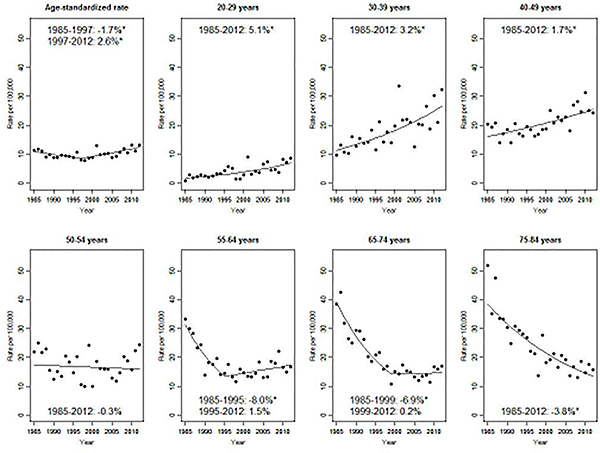
There were 6,760 invasive cervical cancer cases diagnosed in women aged 20–84 between 1985 and 2012 in the included Japanese registries. Overall, between 1997 and 2012, the age-standardized incidence rate increased significantly by 2.6% per year, after a significant decline of 1.7%/year between 1985 and 1997 (Figure 1). Age-specific incidence rate trends varied. Among women aged less than 50, there was a steady, significant increase in incidence rates between 1985 and 2012, with the highest annual percent change in the youngest women (5.1%/year for women aged 20–29, 3.2% for 30–39, 1.7% for 40–49). Incidence rates were stable for women aged 50–54. For women aged 55 and over, incidence rates decreased significantly in the 1980s and 1990s. Age-specific incidence rates in Figure 1 are stratified by age groups defined based on similar trends. Estimated annual percent change for each 5-year age-group can be found in Supplementary Table 1.
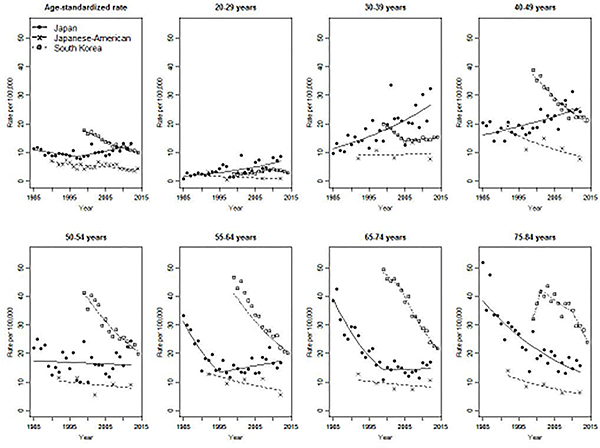
The age-standardized incidence rate of invasive cervical cancer among women living in Japan was nearly four times higher than for Japanese-American women (13.3 vs. 3.9/100,000 in 2012, Figure 2). Age-standardized incidence rates among Japanese-American women decreased steadily by 2%/year from 1990 to 2014. All age-specific incidence rates among Japanese-American women were stable or decreasing, and were lower than those of Japanese women living in Japan at all time points. Age-standardized incidence rates in South Korea were initially higher than the Japanese rates, but decreased by 3.7% per year from 1999 to 2014, crossing over the Japanese rate. Among women under 30, incidence rate in South Korea was lower than that in Japan for all observed calendar years. Among women aged 50 and over, however, incidence rates in South Korea exceeded Japanese incidence rates despite declining significantly (–4.5%/year among women aged 50–54, –5.6% for 55–64, –6.6% for 65–74 after 2004, and –8.1% for 75–84 after 2010).
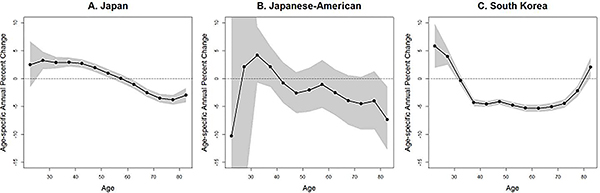
Consistent with Figures 1 and 2, age-specific annual percent changes (Figure 3) illustrate an increasing incidence trend among young women both in Japan and South Korea. The results from Japanese Americans showed wider confidence interval (CI) compare to those of Japanese and South Korean due to the small number of cases. In Japan, incidence rate increased significantly for women aged 25 to 49. In South Korea, incidence rate increased for women aged 20 to 29.
Cohort and period trends
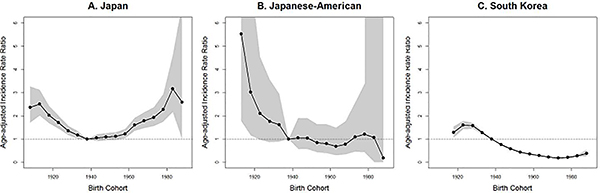
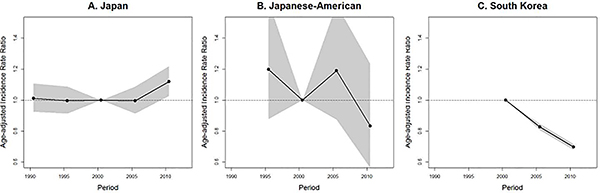
Age-standardized cohort IRRs are shown in Figure 4. In Japan, cervical cancer risk was lowest for women born between 1936 and 1955, with those born before 1920 and after 1970 having about double the risk. Among Japanese-Americans, risk decreased with each subsequent birth year from 1901 to 1940, but appears to have leveled off after 1940. In South Korea, cervical cancer risk was lower for all birth cohorts born after 1940, with the lowest risk occurring in the cohort born from 1971 to 1975.
The age-standardized period IRRs are shown in Figure 5. In Japan and among Japanese Americans, there was no significant period trend. There was, however, indication of an increase in risk in the most recent time period in Japan. In South Korea, the IRR was lower in the periods centered in 2005 and 2010 compared to the reference period, centered in 2000.
Histological subtypes and CIS
With respect to subtype specific trends in Nagasaki, the proportion of all cancers that were squamous cell carcinoma decreased (from 81% in 1988–1992 to 74% in 2008–2012, Supplementary Table 2) while that of adenocarcinoma increased (from 9% to 17%). There was a significant increase in incidence rate of adenocarcinoma for all ages combined (from 1.2/100,000 to 2.5/100,000, 3.2%/year, 95% CI: 1.3, 5.0), and no significant change in squamous cell carcinoma (10.3/100,000, –0.4%/year, 95% CI: –2.2, 1.5). Limiting to women aged 50 and over, however, there was a significant decrease in squamous cell carcinoma (from 25.0 to 15.2/100,000, –2.7%/year, 95% CI: –5.1, –0.2) and a non-significant increase in adenocarcinoma (from 2.7 to 3.9/100,000, 2.2%/year, 95% CI: –2.0, 6.6).
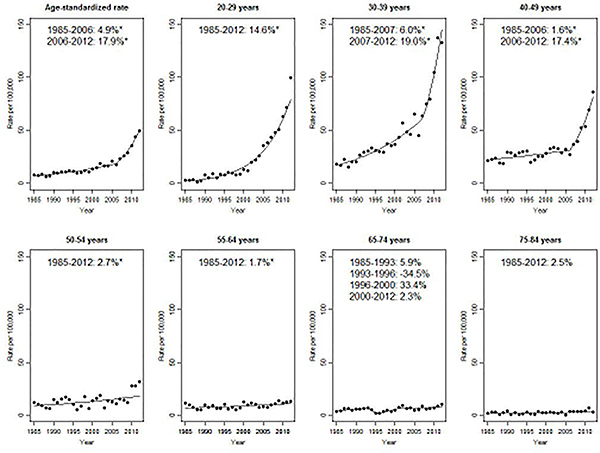
Finally, incidence rates of CIS of the cervix increased significantly over the study period, but particularly since 2006 (17.9%/year, 95% CI: 10.5, 25.8, Figure 6) and driven by patterns among women aged less than 50 (14.6%/year among women aged 20–29 after 1985, 19.0%/year for 30–39 after 2007, 17.4%/year for 40–49 after 2006). While incidence rates of CIS are low after age 50, they were nevertheless increasing among women aged 50–64.
Discussion
In this paper, we described the age, period, and birth cohort trends in cervical cancer incidence in Japan and compared them to trends among Japanese-American and South Korean women. We found that incidence rates in Japan have been rising since the late 1990s, particularly driven by a cohort effect of increasing risk in birth cohorts after 1960. In contrast, incidence rate was decreasing among Japanese-American women of all ages and in all but the youngest (<30 years) South Korean women.
Incidence trends
The increasing incidence rates of cervical cancer observed in Japan are inconsistent with decreasing trends in most high-income countries,2 but consistent with findings of other reports that have used high quality population-based data in Japan.3, 8 A recent summary of cancer mortality trends in Japan using data from the same three registries reported an increase in cervical cancer mortality rate starting in the 1990s.8 An APC analysis of trends of all cancers in Osaka reported that cervical cancer incidence rates followed a U-shaped cohort effect with the lowest risk among women born around 1950 and little or no period effect.6 U-shaped cohort effects have been observed in most European countries and China, but unlike in Japan, the overall incidence rates elsewhere have tended to decrease (with the exception of some Eastern European countries) attributable to stronger, decreasing period effects.2
In this study, the risk by birth year among Japanese-American and South Korean women decreased prior to the 1940s and the 1970s, respectively, and then leveled off, consistent with the decreasing or stable cohort trends in most Oceanic and Asian countries.2 A recent study from South Korea national cancer registry also reported increasing incidence rates of cervical cancer among women born after 197314 which was consistent with ours. The difference of cohort effect between Japanese, Japanese-American and South Korean women indicates that the drivers of cervical cancer incidence differ by country.
Cervical cancer screening and prevalence of HPV infection are two of the primary drivers of population-level trends in cervical cancer incidence. The influence of screening tends to appear as a period effect, while the changing prevalence of HPV tends to appear as a cohort effect.
Cervical cancer screening
The lack of an overall period effect (except for an uptick in the rate ratio in the latest period) in Japan suggests that cervical cancer screening is not having a measurable impact on reducing cervical cancer rates. An organized screening program for cervical cancer using Pap smear was initiated in Japan in 1983 and recommended for women aged 20 years and over every 2 years. Population-based screening is available at municipal level through the National Health Insurance System or the Social Insurance System for individuals younger than 75 years old, but there are no systematic call-and-recall systems and there is wide variation in screening activities within Japan. Nationally, screening uptake therefore remains low.15 According to the Comprehensive Survey of Living Conditions, there has been a recent increase in cervical screening from 25% coverage in 2007 to 34% coverage in 2016,5 but uptake is still well below the 50% national target for 2017,16 as well as the World Health Organization (WHO) recommendation of 80% for effectiveness.17 Screening uptake in the US and South Korea are significantly higher than in Japan. In a 2005 US survey, over 80% of women were guideline-concordant for cervical cancer screening.18 In South Korea, insurance records indicated an increase from 41% to 51% uptake between 2009 and 2014.19
In countries with effective screening programs, the proportion of cervical cancers that are squamous cell carcinomas decreases, with a concomitant increase in the proportion that are adenocarcinomas.20, 21 This is thought to occur because cytology screening is less effective at preventing adenocarcinomas, the precursors of which tend to arise in the endocervical canal, an area that is not well reached by the Pap test.22 The subtype-specific analyses we conducted using the Nagasaki data indicate a similar direction of shift, and the changes in the proportion by histological type were similar to those observed in the UK over the same time period. There, the proportion of cervical cancers that were adenocarcinomas increased from 11% in 1989 to 21% in 2009, while the proportion that were squamous cell carcinoma decreased from 71% in 1989 to 68% in 2009.23 There could be some effect of cytology screening in Japan over the observed period, however, the effects were not sufficiently large to appear as a period effect reduction in incidence rate of squamous cell carcinoma especially among younger women. Going forward, the use of HPV-DNA testing for screening will improve detection of both subtypes.24
The increasing incidence of CIS from the mid-2000s we observed may be caused by a change in registration for CIS of the cervix. Cervical intraepithelial neoplasia grade 3 (CIN3) has been registered as CIS since ICD-O-3 was introduced in Japan in 2002.25 Increased uptake of screening could also partly explained the increase of CIS, however the simultaneous increasing trend of invasive cervical cancer, and mortality from cervical cancer over the same period8 suggest instead that risk of cervical cancer is increasing, likely owing to increases in HPV infection.26
HPV infection
The strong birth cohort trends in Japan support the suggestion that changing prevalence of HPV infection is contributing to cervical cancer incidence rates. Recently, HPV prevalence in Japan was estimated to be 10.2%,27 comparable to the estimated global prevalence of 11.7%.28 It is however, the historic HPV prevalence rates and trends which would have influenced the trends we have reported, but historically, HPV prevalence has not been documented. A hospital-based study conducted between 1999 and 2007 reported that the age-specific HPV prevalence rate among cytologically normal women peaked at 36% in women aged 15–19, followed by a gradual decline to 11% in women aged 40–54.4 The higher prevalence of HPV infection in younger women (recent birth cohorts) is consistent with increasing incidence rates of invasive cervical cancer and CIS observed in this study.
The trends we reported are not attributable to HPV vaccination. In Japan, the bivalent and quadrivalent HPV vaccines were only licensed in 2009 and 2011 respectively, and they were added to the national immunization program (funded and recommended proactively through individualized invitation letters) for girls aged 12–16 in April 2013. The suspension of active recommendation of the vaccine since June 2013 by the Japanese Ministry of Health, Labour, and Welfare led a decline in vaccination rate approximately of 70% per year in 2012 down to 1.1% for girls aged 12 and to 3.9% for girls aged 13 in 2013, and 0.3% in 2016.29, 30 We therefore would expect a higher risk of HPV infection among girls who have passed through adolescence during the last five years.29 The US licensed the HPV vaccine for use in 2006. In South Korea the vaccine has been available since 2007 and has become part of the National Immunization Program since 2016.31, 32
In addition to vaccination, HPV prevalence is associated with age of sexual debut, number of sexual partners, and sexual practices. The proportion of girls aged 17–18 in Japan who had ever had sexual intercourse increased from less than 30% in 1987 to 47% in 2008, a change representative of broader shifts in attitudes toward sexuality among Japanese teenagers.33 These cultural shifts are likely to explain, at least in part, the increasing risk of cervical cancer in later birth cohorts. In the US and South Korea, similar changes in sexual practices are likely to have occurred,14 but may have been counter-acted by increasing participation rates of screening.
Cigarette smoking
Cigarette smoking is an established risk factor for cervical cancer among HPV-positive women.34 In Japan, smoking rates among women have been decreasing slightly in the last 10 years especially among young women from their twenties to forties.35 However, smoking has only been estimated to account for 2% of all cervical cancers,36 so is unlikely to affect the incidence trend.
Limitations
Three limitations of this work are worthy of mention. First, in the absence of a national, population-based cancer registry, we used three jurisdictional registries representing a population of just under 2 million women to study cervical cancer incidence patterns in Japan. A previous study has confirmed the representativeness and comparability of incidence data from the three prefectures included in the study (Yamagata, Fukui and Nagasaki) and one more (Miyagi) between 1985 and 2004.9 Although updated data were unavailable from Miyagi prefecture due to issues related to data transfer, high correlation coefficients were confirmed between the incidence trends in three and four prefectures between 1993 and 2007.8 The representativeness and comparability of these data sources for our use is, however, unknown until it can be compared to the national registry rates.
Second, cancers coded as ‘uterus, not otherwise specified (NOS)’ (ICD-10, C55) were not included in the analyses and are composed of both corpus and cervical cancers. In the three prefectural registries we included, among cancers coded as uterine cancer (either C53 (uterine cervix), C54 (uterine corpus) or C55), the proportion coded as C55 decreased from 14% in 1985 to 2% in 2012. In a sensitivity analysis, we added the NOS cancers to our analyses and found little effect on overall or age-specific results (data not shown). We therefore considered the effect of uterine cancer, NOS to be negligible in this study.
Third, in the international comparisons of incidence of cervical cancer, there may be some bias in comparisons among older women due to differential levels of hysterectomy by country, which have not been taken into account.37 Not accounting for hysterectomies leads to an overestimate in the at-risk population and a subsequent underestimate in risk of cervical cancer.38 Hysterectomy rate in Japan is lower than that in the US among older women (approximately 10% of women aged between 40 and 60 between 2001 and 2002 in Japan and nearly 20% of women aged between 50 and 60 between 2000 and 2010 in the US).38, 39 However, the increased incidence trend among young Japanese women observed in this study sould not be significantly impacted by hysterectomies.
Conclusions
Despite these limitations, this large, detailed analysis of cervical cancer incidence trends in Japan indicated increasing cervical cancer risk among the recent birth cohorts. This pattern was not obvious in Japanese-American or South Korean women. Based on our analyses, we hypothesize that the trends in Japan can be attributed to increasing HPV infection prevalence unopposed by comprehensive screening and, going forward, HPV vaccination. Recently, Sauvaget et al. outlined many of the system- and policy-level barriers to improved cervical cancer control in Japan. These included a lack of incentives for prevention activities among insurers, the absence of a unified national screening program or surveillance system, wide variation in municipal implementation of preventive services and a now 5-year gap in promotion of the HPV vaccine.15 If these barriers remain, incidence of invasive cervical cancer is likely to increase.
For most cancers in most high-income countries, we have fortunately observed a trajectory of progress over the last several decades, as science uncovers ways to efficiently prevent cervical cancer by well-organized cancer screening and high-coverage HPV vaccination.40 As an exception to this, the increasing risk of cervical cancer among young women is an urgent concern. In May 2018, the WHO called for all countries to take action to help end the suffering by cervical cancer.41, 42 Screening and vaccination have been shown to be highly effective and, if duly strengthened, would help reverse the distressing cervical cancer trends in Japan.
Supplementary Material
Novelty and Impact.
Cervical cancer incidence has increased in Japan, despite having decreased in most developed countries. We examined cervical cancer incidence trends by age, period and cohort and found a U-shaped incidence rate ratio by birth year. Women born after 1970 had double the risk compared to those born in the 1940s. Trends may be attributable to increasing prevalence of human papillomavirus among young women, suggesting an urgent need for targeted, effective cancer control programs in Japan.
Acknowledgements
The Radiation Effects Research Foundation (RERF), Hiroshima and Nagasaki, Japan is a public interest foundation funded by the Japanese Ministry of Health, Labour and Welfare (MHLW) and the US Department of Energy (DOE). This publication is based on RERF Research Protocol S2-17. The views of the authors do not necessarily reflect those of the two governments.
The authors sincerely thank the staff at the population-based registries of Yamagata, Fukui and Nagasaki.
Abbreviations used
- HPV
human papillomavirus
- APC
age-period-cohort
- CIS
carcinoma in situ
- SEER
Surveillance, Epidemiology, and End Results
- ICD-10
International Classification of Diseases, tenth revision
- ICD-O-3
International Classification of Diseases for Oncology, third edition
- NCI
National Cancer Institute
- IRR
incidence rate ratio
- CI
confidence interval
- WHO
World Health Organization
- CIN3
cervical intraepithelial neoplasia grade 3
- NOS
not otherwise specified
References
- 1.International Agency for Research on Cancer. GLOBOCAN2018. Available from: http://gco.iarc.fr/today/fact-sheets-cancers. Last accessed 16 October 2018.
- 2.Vaccarella S, Lortet-Tieulent J, Plummer M, Franceschi S, Bray F. Worldwide trends in cervical cancer incidence: impact of screening against changes in disease risk factors. Eur J Cancer 2013;49:3262–73. [DOI] [PubMed] [Google Scholar]
- 3.Ito Y Japanese Association of Cancer Registries Monograph Supplement No. 2 2016:85–97. [Google Scholar]
- 4.Onuki M, Matsumoto K, Satoh T, Oki A, Okada S, Minaguchi T, Ochi H, Nakao S, Someya K, Yamada N. Human papillomavirus infections among Japanese women: age‐related prevalence and type‐specific risk for cervical cancer. Cancer science 2009;100:1312–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cancer Information Service, National Cancer Center, Japan . Cancer screening rate. Available from: https://ganjoho.jp/reg_stat/statistics/dl_screening/index.html#a16. Last accessed 20 May 2018. [Google Scholar]
- 6.Ito Y, Ioka A, Nakayama T, Tsukuma H, Nakamura T. Comparison of trends in cancer incidence and mortality in Osaka, Japan, using an age-period-cohort model. Asian Pacific journal of cancer prevention : APJCP 2011;12:879–88. [PubMed] [Google Scholar]
- 7.Katanoda K, Sobue T, Tanaka H, I Miyashiro, eds. JACR Monograph Supplement No. 2 Tokyo: Japanese Association of Cancer Registries, 2016. [Google Scholar]
- 8.Katanoda K, Hori M, Matsuda T, Shibata A, Nishino Y, Hattori M, Soda M, Ioka A, Sobue T, Nishimoto H. An updated report on the trends in cancer incidence and mortality in Japan, 1958–2013. Japanese journal of clinical oncology 2015;45:390–401. [DOI] [PubMed] [Google Scholar]
- 9.Katanoda K, Ajiki W, Matsuda T, Nishino Y, Shibata A, Fujita M, Tsukuma H, Ioka A, Soda M, Sobue T. Trend analysis of cancer incidence in Japan using data from selected population-based cancer registries. Cancer science 2012;103:360–8. [DOI] [PubMed] [Google Scholar]
- 10.Surveillance, Epidemiology, and End Results Program. SEER*Stat Database: Incidence - SEER 9, plus remainder of CA and NJ, Nov 2016 Sub (1990–2014) detailed API plus White Non-Hispanic - pops projected from populations. Available from: https://seer.cancer.gov/. Released May 2017, based on the November 2016 submission.
- 11.Rosenberg PS, Check DP, Anderson WF. A web tool for age–period–cohort analysis of cancer incidence and mortality rates. Cancer Epidemiology and Prevention Biomarkers 2014;23:2296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenberg PS, Anderson WF. Proportional hazards models and age–period–cohort analysis of cancer rates. Statistics in medicine 2010;29:1228–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chernyavskiy P, Little MP, Rosenberg PS. A unified approach for assessing heterogeneity in age–period–cohort model parameters using random effects. Statistical methods in medical research 2017:0962280217713033. [DOI] [PubMed] [Google Scholar]
- 14.Moon EK, Oh CM, Won YJ, Lee JK, Jung KW, Cho H, Jun JK, Lim MC, Ki M. Trends and Age-Period-Cohort Effects on the Incidence and Mortality Rate of Cervical Cancer in Korea. Cancer research and treatment : official journal of Korean Cancer Association 2017;49:526–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sauvaget C, Nishino Y, Konno R, Tase T, Morimoto T, Hisamichi S. Challenges in breast and cervical cancer control in Japan. The Lancet Oncology 2016;17:e305–e12. [DOI] [PubMed] [Google Scholar]
- 16.Ministry of Health Labour and Welfare. Cancer Control Promotion Basic Plan. Available from: http://www.mhlw.go.jp/file/06-Seisakujouhou-10900000-Kenkoukyoku/gan_keikaku02.pdf. Last accessed 21 May 2018.
- 17.World Health Organization. Cancer control: Knowledge into action: WHO guide for effective programmes: Early detection WHO guide for effective programmes, vol. 2018, 2007. [PubMed] [Google Scholar]
- 18.Nelson W, Moser RP, Gaffey A, Waldron W. Adherence to cervical cancer screening guidelines for US women aged 25–64: data from the 2005 Health Information National Trends Survey (HINTS). Journal of Women’s Health 2009;18:1759–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shim S-H, Kim H, Sohn I-S, Hwang H-S, Kwon H-S, Lee SJ, Lee JY, Kim S-N, Lee K, Chang S. Nationwide cervical cancer screening in Korea: data from the National Health Insurance Service Cancer Screening Program and National Cancer Screening Program, 2009–2014. Journal of gynecologic oncology 2017;28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mathew A, George PS. Trends in incidence and mortality rates of squamous cell carcinoma and adenocarcinoma of cervix–worldwide. Asian Pacific journal of cancer prevention : APJCP 2009;10:645–50. [PubMed] [Google Scholar]
- 21.Lönnberg S, Hansen BT, Haldorsen T, Campbell S, Schee K, Nygård M. Cervical cancer prevented by screening: long‐term incidence trends by morphology in Norway. International journal of cancer 2015;137:1758–64. [DOI] [PubMed] [Google Scholar]
- 22.Pimenta JM, Galindo C, Jenkins D, Taylor SM. Estimate of the and potential impact of prophylactic human papillomavirus vaccination. BMC cancer 2013;13:553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trent Cancer Registry, National Cancer Intelligence Network, eds. Profile of cervical cancer in England: Incidence, mortality and survival Sheffield: Trent Cancer Registry, NHS Cancer Screening Programmes, 201215p. [Google Scholar]
- 24.Andersson S, Mints M, Wilander E. Results of cytology and high-risk human papillomavirus testing in females with cervical adenocarcinoma in situ. Oncol Lett 2013;6:215–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization. International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3) Geneva: World Health Organization, 2000. [Google Scholar]
- 26.Peto J, Gilham C, Deacon J, Taylor C, Evans C, Binns W, Haywood M, Elanko N, Coleman D, Yule R, Desai M. Cervical HPV infection and neoplasia in a large population-based prospective study: the Manchester cohort. British journal of cancer 2004;91:942–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miura S, Matsumoto K, Oki A, Satoh T, Tsunoda H, Yasugi T, Taketani Y, Yoshikawa H. Do we need a different strategy for HPV screening and vaccination in East Asia? Int J Cancer 2006;119:2713–5. [DOI] [PubMed] [Google Scholar]
- 28.Bruni L, Diaz M, Castellsagué M, Ferrer E, Bosch FX, de Sanjosé S. Cervical human papillomavirus prevalence in 5 continents: meta-analysis of 1 million women with normal cytological findings. Journal of Infectious Diseases 2010;202:1789–99. [DOI] [PubMed] [Google Scholar]
- 29.Tanaka Y, Ueda Y, Egawa-Takata T, Yagi A, Yoshino K, Kimura T. Outcomes for girls without HPV vaccination in Japan. The lancet oncology 2016;17:868–9. [DOI] [PubMed] [Google Scholar]
- 30.Ministry of Health, Labour and Welfare. Information on regular vaccination. Available from https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/kenkou_iryou/kenkou/kekkaku-kansenshou/yobou-sesshu/index.html. Last accessed 1 Nov 2018,
- 31.Kang HS, Moneyham L. Attitudes toward and intention to receive the human papilloma virus (HPV) vaccination and intention to use condoms among female Korean college students. Vaccine 2010;28:811–6. [DOI] [PubMed] [Google Scholar]
- 32.Korea Centers for Disease Control and Prevention. National Immunization Program & International Cooperation. Available from: https://www.ghsagenda.org/docs/default-source/default-document-library/seventieth-world-health-assembly/national-immunization-program-international-cooperation---kcdc.pdf. Last accessed 21 May 2018.
- 33.Saotome T The reality of sexuality for teenage girls in Japan. JMAJ 2010;53:279–84. [Google Scholar]
- 34.Plummer M, Herrero R, Franceschi S, Meijer CJ, Snijders P, Bosch FX, de Sanjosé S, Muñoz N. Smoking and cervical cancer: pooled analysis of the IARC multi-centric case–control study. Cancer Causes & Control 2003;14:805–14. [DOI] [PubMed] [Google Scholar]
- 35.Ministry of Health, Labour and Welfare. Current smoking information. Available from: http://www.health-net.or.jp/tobacco/product/pd100000.html. Last accessed 26 July 2018.
- 36.Danaei G, Vander Hoorn S, Lopez AD, Murray CJL, Ezzati M. Causes of cancer in the world: comparative risk assessment of nine behavioural and environmental risk factors. The Lancet 2005;366:1784–93. [DOI] [PubMed] [Google Scholar]
- 37.Subramanian S, Sauvaget C. Cervical cancer in the US and Japan: We need better implementation of the evidence-base along the continuum of care. Journal of Cancer Policy 2018;15:29–31. [Google Scholar]
- 38.Rositch AF, Nowak RG, Gravitt PE. Increased age and race-specific incidence of cervical cancer after correction for hysterectomy prevalence in the United States from 2000 to 2009. Cancer 2014;120:2032–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anderson D, Yoshizawa T, Gollschewski S, Atogami F, Courtney M. Menopause in Australia and Japan: effects of country of residence on menopausal status and menopausal symptoms. Climacteric 2004;7:165–74. [DOI] [PubMed] [Google Scholar]
- 40.Hall MT, Simms KT, Lew JB, Smith MA, Brotherton JM, Saville M, Frazer IH, Canfell K. The projected timeframe until cervical cancer elimination in Australia: a modelling study. Lancet Public Health 2018. [DOI] [PubMed] [Google Scholar]
- 41.World Health Organization. WHO Director-General calls for all countries to take action to help end the suffering caused by cervical cancer. Available from: http://www.who.int/reproductivehealth/call-to-action-elimination-cervical-cancer/en/. Last accessed 7 July 2018.
- 42.World Health Organization eds. Comprehensive cervical cancer prevention and control - a healthier future for girls and women Geneva:WHO press, 2013. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.