Abstract
Background
Patient-reported outcome measures (PROMs) are being increasingly used in orthopedic surgery; however, there is significant variability and burden associated with their administration. The visual analog scale (VAS) for function, strength, and pain may represent a simple and efficient way to measure outcomes, specifically after rotator cuff repair (RCR) surgery.
Purpose
To define the efficiency and longitudinal psychometric properties of VAS instruments assessing function, strength, and pain after RCR.
Methods
Single-question VAS measures assessing function, strength, and pain as a percentage of normal were administered alongside legacy PROMs in patients undergoing RCR. VAS and PROMs were administered at preoperative, 6- and 12-month time points between June 2017 and April 2018. An electronic registry was used to examine time-to-completion data. PROM performance was assessed using Spearman correlation coefficients. Both absolute and relative floor and ceiling effects were examined. Effect size was measured at 6 and 12 months through the calculation of Cohen's d coefficient. Receiver-operating curves with area under the curve calculations were used to determine the ability of preoperative VAS scores in predicting minimally clinically important difference achievement on American Shoulder and Elbow Surgeons score (ASES).
Results
A total of 190 patients (55.6 ± 10.9 years, 66.9% male) met criteria. The 3 VAS PROMs required less time to complete than ASES (1.36 ± 1.12 vs. 5.17 ± 2.39) and Patient-Reported Outcome Measurement Information System (PROMIS) Upper Extremity v2.0 (UE) Computer Adaptive Test (1.72 ± 1.48). Compared with ASES, VAS function, strength, and pain demonstrated fair correlations preoperatively (r = 0.44-0.46) that improved to good at 6 months (r = 0.61-0.67) and further improved at 1 year (r = 0.62-0.78). The performance of VAS measures with other function PROMs was comparable with performance relative to ASES, with poor to very good correlations preoperatively (r = 0.21-0.62) that improved to good to excellent by 1 year (r = 0.62-0.94). A significant relative ceiling effect was demonstrated by PROMIS UE at 12 months (16.9%). Large effect sizes were demonstrated by the ASES, Single Assessment Numeric Evaluation, Constant, PROMIS UE, and VAS function and strength instruments (Cohen d ≥ 0.8).
Conclusion
Single-question VAS assessments for function, strength, and pain are an efficient means for assessing outcome in RCR surgery and may be particularly useful in the postoperative setting. VAS instruments collectively trended toward floor effects preoperatively, suggesting that legacy instruments may more appropriately establish preoperative baselines. However, in the postoperative setting, VAS instruments demonstrate good-to-excellent correlation, minimized time-to-completion, and no appreciable floor or ceiling effects.
Keywords: Visual analog scale, VAS, RCR, rotator cuff, sports medicine, outcome, patient-reported outcomes, PROM
Patient-reported outcome measures (PROMs) are commonly used to evaluate patient benefits after interventions for a variety of pathologies.2,28 The use of PROMs has demonstrated widespread utility across orthopedic surgery, ranging from helping orthopedists track patient progress more closely4 and evaluate interventional efficacy,9 to improving shared decision-making models23,24 and informing value-based care initiatives.6,21,25 However, these scores are not without important systems-based5 and instrument-based challenges. From a systems perspective, significant financial and administrative burden exists in implementing and maintaining a patient-reported outcome program.5,12 From an instrument perspective, each PROM must be examined for acceptable psychometric properties25 and validated in each population of interest.18
For rotator cuff disease and rotator cuff repair (RCR) surgery, numerous PROMs have been applied, each of which has demonstrated variable comprehensiveness and efficiency.19 PROM selection has also been demonstrated to vary widely across institutions, due in part to a lack of consensus regarding the optimal PROMs to administer for specific conditions.20,30 When considered together, limitations in PRO instruments, collection systems, and PRO reporting have highlighted the specific need for the use of efficient, easily adopted PROMs that minimize question burden to maximize patient compliance.2,7 Visual analog scale (VAS) measures are single-question tools that seek to rate a patient's outcome based on a particular construct, such as pain, satisfaction, function, and strength. Such measures are widely applied in orthopedic surgery and have been validated as a useful measure of patient outcome.1,8,10,15,16, 17, 18,34
The purpose of the current study is to define the pre- and postoperative psychometric properties and time requirements of the VAS in the assessment of function, strength, and pain in RCR. Our central hypothesis is 3-fold: (1) VAS function, strength, and pain will demonstrate comparable performance relative to legacy outcome measures; (2) the aforementioned VAS instruments will demonstrate acceptable floor and ceiling effects across preoperative and postoperative time points; and (3) VAS measures will demonstrate superior efficiency when compared with legacy outcome measures.
Methods
Study design and cohort establishment
The current study aggregated and analyzed PROM data collected by a prospectively maintained institutional registry (Outcome Based Electronic Research Database; Universal Research Solutions, Columbia, MO, USA). Data were compiled for all primary arthroscopic RCRs between June 2017 and April 2018. Inclusion criteria included full completion of preoperative and 6-month PRO data and primary RCR. Exclusion criteria included failure to complete preoperative PRO data and concomitant surgical procedures (ie, bicep tenodesis, distal clavicle excision, and capsular release). Patients with concomitant acromioplasty were included.32 Demographic data were collected inclusive of age, sex, and worker's compensation.
Patient-reported outcomes measures
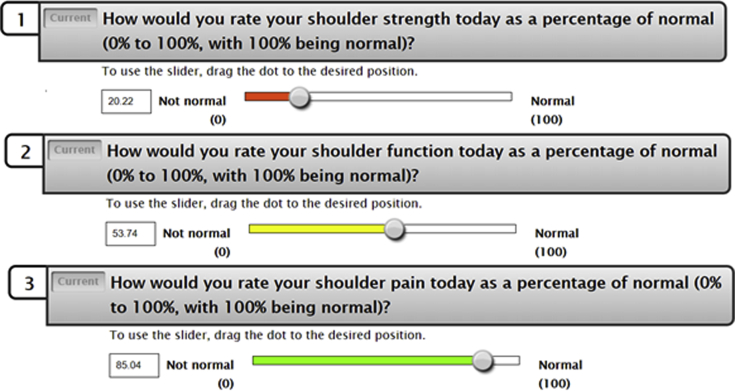
Legacy PROMs of interest examined in this study include the American Shoulder and Elbow Surgeons score (ASES), Single Assessment Numeric Evaluation (SANE), Quick Disabilities of the Arm, Shoulder, and Hand (qDASH), the Constant-Murley score, Short Form 12 (SF12) Physical Component Score (PCS), Veteran's Rand 12 (VR12) PCS, and the Patient-Reported Outcome Measurement Information System (PROMIS) Upper Extremity v2.0 (UE) Computer Adaptive Test (CAT). Three additional questionnaires were used to administer VAS measures, each of which assessed shoulder function, strength, and pain. Each PROM was administered on an iPad by a qualified research assistant alongside legacy instruments at each time point. The response was graded on a scale of 0-100, examining shoulder function, strength, or pain as a percent of normal similar to the SANE PROM (Fig. 1).
Figure 1.
Visual analog scale (VAS) instruments for strength, function, and pain. Pictured are examples of how each VAS instrument appears during computer adaptive testing, with a color scale correlating to the level of pain experienced helping the patients best estimate their strength, function, and pain, respectively.
Statistical analysis
Time-to-completion metrics were calculated using data for available PROMs by compiling time and question completion data across preoperative, 6-month, and 1-year time points. Given non-normal distributions for ASES and VAS instruments on Shapiro-Wilk testing, psychometric analysis used Spearman correlation coefficients to examine the strength of association between VAS subscales and legacy function PROMs. Correlation coefficients were classified by the strength of association, with >0.8 equating to excellent, 0.71-0.8 equating to very good, 0.61-0.7 equating to good, 0.41-0.6 equating to fair, and 0.21-0.4 equating to poor.3,14 Absolute floor and ceiling effects were calculated by examining the percentage of respondents reporting achievement of the absolute lowest and highest scores. In the case that no one achieved absolute minimum or maximum score thresholds, relative floor and ceiling effects were calculated based on the minimum and maximum scores in the distribution. A percentage of ≥15% was designated as a significant floor or ceiling effect.3,29,31 Instrument responsiveness was evaluated at both 6- and 12-month time points through the calculation of Cohen's d, with an effect size (ES) of <0.20 corresponding to negligible, 0.20-0.50 to small, 0.50-0.80 to moderate, and >0.80 equating to a large ES.22,26,33
Results
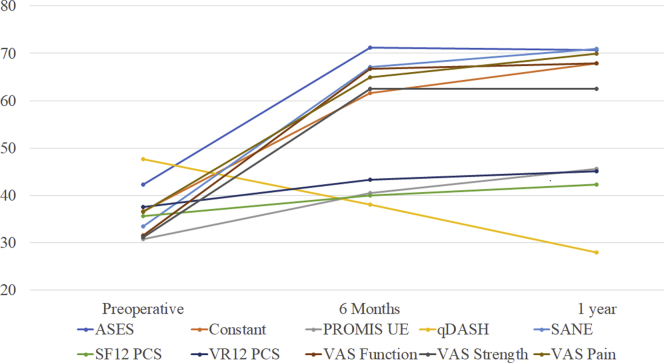
A total of 190 patients (55.6 ± 10.9 years, 66.9% male, 21.1% worker's compensation) met inclusion and exclusion criteria (Table I). The loss to follow-up from 6 months to 1 year was 48 patients (25.2%), with no significant differences in age, worker's compensation status, laterality or sex between responders, and those lost to follow-up (Table II). The average time to complete the 3 VAS metrics was 1.36 ± 1.12 minutes, whereas the average time to complete the ASES was 5.17 ± 2.39 minutes. The average completion time was 1.72 ± 1.48 for the PROMIS UE CAT, 0.75 ± 0.60 for SANE, and 1.87 ± 1.68 for the qDASH. For each PROM, significant improvements in PRO score occurred from preoperative to 6-month and 1-year values (Fig. 2).
Table I.
Patient demographics
| Age (yr) | 55.9 ± 10.9 |
| Worker's compensation | 40 (21.1) |
| Laterality | |
| Right | 125 (65.8) |
| Left | 65 (34.2) |
| Sex | |
| Male | 127 (66.9) |
| Female | 63 (33.1) |
Age reported as average ± standard deviation; all other demographic variables reported as count (percent).
Table II.
Comparison of patient demographics at 1 year
| Group 1 | Group 2 | P value | |
|---|---|---|---|
| Age (yr) | 56.2 ± 9.8 | 55.0 ± 11.0 | .51 |
| WC | 27 (19.0) | 13 (27.1) | .33 |
| Laterality | .40 | ||
| Right | 91 (64.1) | 34 (70.8) | |
| Left | 51 (35.9) | 14 (29.2) | |
| Sex | .30 | ||
| Male | 92 (64.8) | 35 (72.9) | |
| Female | 50 (35.2) | 13 (28.1) |
WC, worker's compensation.
Age reported as average ± standard deviation; all other demographic variables reported as count (percent).
Group 1: n = 142, representing patients who completed appropriate follow-up.
Group 2: n = 48, representing patients who failed to complete appropriate follow-up.
Figure 2.
Patient-reported outcome scores across time points. All PROMs were found to demonstrate significant improvement in PRO scores across follow-up time periods on 1-way ANOVA with a type 1 error rate of 5% (all P < .001). PROM, patient-reported outcome measure; ANOVA, analysis of variance; ASES, American Shoulder and Elbow Surgeons score; PROMIS, Patient-Reported Outcome Measurement Information System; UE, upper extremity; qDASH, Quick Disabilities of the Arm, Shoulder, and Hand; SANE, Single Assessment Numeric Evaluation; SF12 PCS, Short Form 12 Physical Component Score; VR12 PCS, Veterans Rand 12 Physical Component Score; VAS, visual analog scale.
Across the included time points, the VAS function measure demonstrated a wide range of correlation coefficients with legacy function measures (r = 0.26-0.94). Preoperative performance ranged from poor to excellent (r = 0.26-0.84), with 4 of 8 legacy measures demonstrating fair correlations (ASES, Constant, PROMIS UE, qDASH). Correlation coefficients for the VAS function increased from preoperative to 6-month time points (r = 0.55-0.85), and further from the 6-month to 1-year time point (r = 0.74-0.94) for all legacy PROMs. Correlative strengths for these measures increased from poor to excellent preoperatively (r = 0.26-0.84), to very good to excellent by 1 year (r = 0.74-0.94). Correlation of the VAS function with the VAS strength and VAS pain measures ranged from fair to excellent (r = 0.55-0.92), with the weakest correlations at 6 months (r = 0.55-0.78) and strongest correlations at 1 year relative to both VAS instruments (r = 0.83-0.92) (Table III).
Table III.
Correlation of the VAS function compared with legacy instrument
| PROMs | Preoperative | 6 mo | 1 yr |
|---|---|---|---|
| ASES | 0.46 | 0.67 | 0.78 |
| Constant | 0.55 | 0.78 | 0.91 |
| PROMIS UE | 0.51 | 0.73 | 0.83 |
| qDASH | −0.48 | −0.80 | −0.81 |
| SANE | 0.74 | 0.85 | 0.94 |
| SF12 PCS | 0.26 | 0.64 | 0.75 |
| VR12 PCS | 0.31 | 0.65 | 0.74 |
| VAS strength | 0.84 | 0.78 | 0.92 |
| VAS pain | 0.59 | 0.55 | 0.83 |
VAS, visual analog scale; PROM, patient-reported outcome measure; ASES, American Shoulder and Elbow Surgeons score; PROMIS, Patient-Reported Outcome Measurement Information System; UE, upper extremity; qDASH, Quick Disabilities of the Arm, Shoulder, and Hand; SANE, Single Assessment Numeric Evaluation; SF12 PCS, Short Form 12 Physical Component Score; VR12 PCS, Veterans Rand 12 Physical Component Score.
All values significant at α < 0.01.
Preoperatively, the performance of the VAS strength PROM ranged from poor to fair (r = 0.23-0.49) for all legacy function PROMs except SANE (r = 0.70). Correlations improved at 6 months for all legacy measures, demonstrating correlative strengths ranging from fair to very good (r = 0.55-0.79). Correlation coefficients continued to improve out until 1 year, at which point correlations with all legacy PROMs were very good to excellent (r = 0.71-0.92). In a fashion similar to the VAS function instrument, correlations between VAS strength and VAS pain were the weakest at 6 months (r = 0.48) and strongest at 1 year (r = 0.73) (Table IV). The preoperative performance of VAS pain ranged from very poor to fair relative to legacy function PROMs (r = 0.19-0.59), improving to fair to good at 6 months (r = 0.48-0.61), and improving further to fair to excellent at 1 year (r = 0.57-0.82). Across each time point, the strongest correlations with the VAS pain were exhibited by SANE (r = 0.59-0.82) (Table V).
Table IV.
Performance of the VAS strength relative to legacy instruments
| PROMs | Preoperative | 6 mo | 1 yr |
|---|---|---|---|
| ASES | 0.44 | 0.66 | 0.76 |
| Constant | 0.46 | 0.70 | 0.79 |
| PROMIS UE | 0.49 | 0.64 | 0.84 |
| qDASH | −0.48 | −0.76 | −0.78 |
| SANE | 0.70 | 0.79 | 0.89 |
| SF12 PCS | 0.23 | 0.56 | 0.71 |
| VR12 PCS | 0.27 | 0.55 | 0.72 |
| VAS function | 0.84 | 0.78 | 0.92 |
| VAS pain | 0.55 | 0.48 | 0.73 |
VAS, visual analog scale; PROM, patient-reported outcome measure; ASES, American Shoulder and Elbow Surgeons score; PROMIS, Patient-Reported Outcome Measurement Information System; UE, upper extremity; qDASH, Quick Disabilities of the Arm, Shoulder, and Hand; SANE, Single Assessment Numeric Evaluation; SF12 PCS, Short Form 12 Physical Component Score; VR12 PCS, Veterans Rand 12 Physical Component Score.
All values significant at α < 0.01.
Table V.
Performance of the VAS pain relative to legacy instruments
| PROMs | Preoperative | 6 mo | 1 yr |
|---|---|---|---|
| ASES | 0.46 | 0.61 | 0.62 |
| Constant | 0.47 | 0.56 | 0.80 |
| PROMIS UE | 0.39 | 0.56 | 0.64 |
| qDASH | −0.42 | −0.48 | −0.63 |
| SANE | 0.59 | 0.61 | 0.82 |
| SF12 PCS | 0.19 | 0.61 | 0.77 |
| VR12 PCS | 0.25 | 0.61 | 0.81 |
| VAS function | 0.59 | 0.55 | 0.83 |
| VAS strength | 0.55 | 0.48 | 0.73 |
VAS, visual analog scale; PROM, patient-reported outcome measure; ASES, American Shoulder and Elbow Surgeons score; PROMIS, Patient-Reported Outcome Measurement Information System; UE, upper extremity; qDASH, Quick Disabilities of the Arm, Shoulder, and Hand; SANE, Single Assessment Numeric Evaluation; SF12 PCS, Short Form 12 Physical Component Score; VR12 PCS, Veterans Rand 12 Physical Component Score.
All values significant at α < 0.01.
Analysis for floor and ceiling effects demonstrated no significant floor or ceiling affects for VAS instruments across preoperative, 6- and 12-month time points, although VAS function (13.2%), VAS strength (11.0%), and VAS pain (11.6%) trended toward significant absolute floor effects preoperatively. The PROMIS UE CAT demonstrated a significant relative ceiling effect 12 months postoperatively (16.9%), and ASES trended toward a relative ceiling effect at 12 months (11.3%). The SF12 PCS trended toward a relative ceiling effect at 6 months (11%) and 12 months (13.4%), as did the VR12 PCS at 6 months (11%) and 12 months (14.1%) (Table VI).
Table VI.
Absolute and relative floor and ceiling effects
| Instrument | Preoperative, n (%) |
6 mo, n (%) |
12 mo, n (%) |
|||
|---|---|---|---|---|---|---|
| Floor | Ceiling | Floor | Ceiling | Floor | Ceiling | |
| VAS function | 25 (13.2) | 3 (1.4) | 8 (4.2) | 3 (1.6) | 8 (5.6) | 12 (8.5) |
| VAS strength | 21 (11.1) | 2 (1.1) | 3 (1.6) | 5 (4.4) | 4 (2.8) | 12 (8.5) |
| VAS pain | 22 (11.6) | 1 (0.5) | 7 (3.7) | 18 (9.5) | 8 (5.6) | 4 (2.8) |
| qDASH | 1 (0.5) | 2 (1.1) | 3 (1.6) | 2 (1.1) | 10 (7.0) | 5 (3.5) |
| ASES | 1 (0.5) | 2 (1.1) | 5 (2.6) | 5 (2.6) | 4 (2.8) | 16 (11.3) |
| SANE | 19 (10) | 2 (1.1) | 8 (4.2) | 1 (0.5) | 8 (5.6) | 1 (2.9) |
| SF12 PCS | 2 (1.1) | 2 (1.1) | 5 (2.6) | 21 (11) | 1 (2.1) | 19 (13.4) |
| VR12 PCS | 3 (1.6) | 2 (1.1) | 10 (5.3) | 21 (11) | 2 (1.4) | 20 (14.1) |
| Constant | 4 (2.1) | 1 (0.5) | 1 (0.5) | 10 (5.3) | 4 (2.8) | 3 (2.1) |
| PROMIS UE | 2 (1.1) | 3 (1.6) | 1 (0.5) | 5 (2.6) | 12 (8.5) | 24 (16.9) |
VAS, visual analog scale; qDASH, Quick Disabilities of the Arm, Shoulder, and Hand; ASES, American Shoulder and Elbow Surgeons score; SANE, Single Assessment Numeric Evaluation; SF12 PCS, Short Form 12 Physical Component Score; VR12 PCS, Veterans Rand 12 Physical Component Score; PROMIS, Patient-Reported Outcome Measurement Information System; UE, upper extremity.
Bold test represents significant floor or ceiling effect (≥15%); italics indicate the absence (0.0%) of minimum and maximum score achievement, resulting in relative floor and ceiling calculations based on relative minimum and maximum values from the cohort.
ESs calculated using Cohen's d revealed large ESs for VAS function, qDASH, ASES, SANE, Constant, and PROMIS UE at 6 and 12 months. VAS strength demonstrated a large ES at 6 months (d = −0.96) and a medium ES at 12 months (d = −0.75), whereas VAS pain demonstrated medium ESs at both 6 (d = −0.76) and 12 months (d = −0.74) (Table VII). The SF12 PCS and VR12 PCS instruments demonstrated the smallest ESs at both 6 months (−0.51, −0.63) and 12 months (−0.27, −0.36), respectively.
Table VII.
Effect size of PROMs by cohort
| Instrument | Cohen's d (6 mo) |
Cohen's d (12 mo) |
||
|---|---|---|---|---|
| Effect size (95% CI) | Magnitude | Effect size (95% CI) | Magnitude | |
| VAS function | −1.09 (−1.37, −0.81) | Large | −0.88 (−1.38, −0.37) | Large |
| VAS strength | −0.96 (−1.23, −0.68) | Large | −0.75 (−1.25, −0.25) | Medium |
| VAS pain | −0.76 (−1.03, −0.49) | Medium | −0.74 (−1.24, −0.24) | Medium |
| qDASH | 1.58 (−0.40, 3.56) | Large | 0.82 (0.26, 1.38) | Large |
| ASES | −1.03 (−1.30, −0.76) | Large | −1.20 (−1.72, −0.68) | Large |
| SANE | −1.05 (−1.38, −0.73) | Large | −0.96 (−1.48, −0.45) | Large |
| SF12 PCS | −0.51 (−0.78, −0.25) | Medium | −0.27 (−0.76, 0.21) | Small |
| VR12 PCS | −0.63 (−0.89, −0.36) | Medium | −0.36 (−0.85, 0.13) | Small |
| Constant | −0.98 (−1.26, −0.71) | Large | −1.13 (−1.65, −0.61) | Large |
| PROMIS UE | −0.82 (−1.09, −0.55) | Large | −0.95 (−1.46, −0.45) | Large |
PROM, patient-reported outcome measure; CI, confidence interval; VAS, visual analog scale; qDASH, Quick Disabilities of the Arm, Shoulder, and Hand; ASES, American Shoulder and Elbow Surgeons score; SANE, Single Assessment Numeric Evaluation; SF12 PCS, Short Form 12 Physical Component Score; VR12 PCS, Veterans Rand 12 Physical Component Score; PROMIS, Patient-Reported Outcome Measurement Information System; UE, upper extremity.
Effect sizes at 6 and 12 months displayed as means (95% CIs).
Discussion
In this study, we administered VAS questions for function, strength, and pain alongside traditional disease-specific PROMs to patients undergoing arthroscopic treatment of rotator cuff injury. We found that preoperatively, VAS questions demonstrate moderate correlations with traditional PROMs. Postoperatively, the performance of VAS improves to good/excellent correlations. In addition, we demonstrated the efficiency of applying VAS measures and confirmed that they do not have a significant floor or ceiling effects in the postoperative setting. Given the preoperative VAS floor effects and the moderate correlation to legacies, our study findings confirm that that legacy instruments such as the ASES are more appropriate for establishing preoperative functional baselines, whereas VAS measures may represent an efficient proxy in the postoperative setting. Overall, the psychometric and practical properties of VAS instruments suggest that they may greatly simplify the tracking of postoperative outcomes in an efficient, domain-specific manner that may be easily adopted across outcome groups and modes of administration (ie, paper, electronic registries, SMS). Utilization of VAS measures in the postoperative setting may also allow for outcomes to be tracked at more time points postoperatively without significantly increasing question burden.
With respect to psychometrics, the VAS function and VAS strength instruments demonstrated comparable performance with each other. Both instruments exhibited poor-to-excellent correlations preoperatively, which improved to good to excellent at 12 months. The VAS function and ASES shared large ESs at 6 and 12 months, whereas the VAS strength demonstrated large and medium ESs at 6 and 12 months, respectively. These data suggest that the performance and discriminative ability of the VAS function and VAS strength measures are comparable with that of ASES in an arthroscopic RCR population, with the VAS function instrument slightly outperforming the VAS strength with respect to ESs. However, the psychometrics of both VAS function (r = 0.26-0.84) and VAS strength (r = 0.23-0.84) were limited preoperatively, suggesting limited applicability in the estimation of functional baselines preoperatively. With respect to outcome scores, all legacy function PROMs and VAS instruments demonstrated significant P values when comparing preoperative to postoperative PRO values (P < .001), suggesting that each may capture postoperative improvements in clinical status. Future research should consider examining VAS measures in the context of clinically significant outcome achievement (ie, minimally clinically important difference, substantial clinical benefit, patient acceptable symptomatic state), using either anchor-based or distribution-based methods to determine if significant differences in outcome discrimination exist.
The VAS instruments used in this study were devoid of significant floor and ceiling effects; however, the VAS function, strength, and pain measures demonstrated a trend toward an absolute floor effect in the preoperative setting (11.1%-13.2%). ASES demonstrated no significant preoperative floor or ceiling effects (<1.1%), but did trend toward both absolute (11.3%) and relative (14.8%) ceiling effects at 12 months. Differences in instrument design between the ASES and VAS instruments likely explain the difference in floor effects between the 2 instruments. After a full-thickness rotator cuff tear, patients may be particularly prone to perceiving their functional status as a new “baseline” deserving a score of 0% of normal. Specifically, it may be particularly difficult to gauge the level of functional deficit in terms of percentages of normal, with the deficit representing a new functional baseline that patients may rate as 0%. In comparison, the ASES instrument quantifies functional deficits based on activity-based questions with multilevel responses (ie, “Is it difficult for you to manage toileting,” “Is it difficult for you to put on a coat”). When considered in the context of comparable psychometrics and increased completion time, relative to VAS instruments, ASES may be most fit to establish preoperative baselines, whereas VAS instruments may more efficiently and accurately track postoperative improvement in function, strength, and pain.
The VAS instruments examined in our study displayed specific advantages relative to legacy PROMs, the first of which is optimized time-to-completion. Each measure consists of single question, with function, strength, and pain being assessed in an average of 1.36 minutes. This is an important advantage when compared with other function PROMs, representing nearly a 5-fold decrease from ASES. SANE was the only instrument that averaged less time to complete (0.75 ± 0.60); however, SANE is limited to 1 domain, whereas the VAS triad employed in this study assesses function in addition to pain and strength in an additional 37 seconds, on average. When administered together, the VAS measures used in this study may represent a particularly efficient methodology to examine the patient-reported outcomes instrument after RCR in a multidomain manner. As more institutions and orthopedic groups continue to adopt methods to track outcomes in the context of value-based care,11,25 it becomes increasingly important to use an efficient, psychometrically appropriate set of PROMs in the pre- and postoperative assessment of outcomes.
Our study has certain limitations that are worth noting. First, we were unable to assess the effect of questionnaire fatigue on patient response speeds and response rates. On the basis of the electronic registry used in this study, participants answered standardized, predetermined sets of questionnaires in a nonrandomized fashion. That is, every person initiated the questionnaire set with the ASES and VAS questionnaires, ending with SF12 and VR12 instruments. Theoretically, this may have led to “hasty completion,” previously been linked to a predisposition toward floor effects on the PROMIS Depression CAT.13 In addition, generalizability of our study results is most applicable to other patient populations receiving isolated arthroscopic RCR. Third, during our study, we experienced certain loss to follow-up at 12-month time point. Nonetheless, the performance metrics reported in our study reached significance thresholds, and there were no significant differences in demographics between the groups following up and those lost to follow-up. Lastly, the current study represents in-process research with respect to demonstrating validity, repeatability, and reproducibility of the VAS questionnaires. As such, further research is necessary to assess instrument validity, particularly because loss to follow-up occurred at the 12-month time point.
Conclusion
Single-question VAS assessments for function, strength, and pain are an efficient means for assessing outcome in RCR surgery and may be particularly useful in the postoperative setting. VAS instruments collectively trended toward floor effects preoperatively, suggesting that legacy instruments may more appropriately establish preoperative baselines. However, in the postoperative setting, VAS instruments demonstrate good-to-excellent correlation, minimized time-to-completion, and no significant floor or ceiling effects.
Disclaimer
Brian Forsythe reports personal fees from Elsevier, Arthrex, Jace Medical, and Stryker; and grants from Smith and Nephew and Ossur, outside the submitted work.
Brian J. Cole reports other from Aesculap/B.Braun, American Journal of Orthopedics, American Journal of Sports Medicine, Arthroscopy Association of North America, Athletico, Cartilage, Elsevier Publishing, International Cartilage Repair Society, Journal of Shoulder and Elbow Surgery, Journal of the American Academy of Orthopaedic Surgeons, JRF Ortho, National Institutes of Health (NIAMS & NICHD), Operative Techniques in Sports Medicine, Ossio, and Smith & Nephew; grants, personal fees, nonfinancial support, and other from Arthrex, Inc.; and personal fees and other from Regentis and Zimmer, outside the submitted work.
Nikhil N. Verma reports personal fees and nonfinancial support from Arthrex, Inc.; nonfinancial support and other from Arthroscopy and Vindico Medical-Orthopedics Hyperguide; and personal fees from DJ Orthopaedics and Orthospace, outside the submitted work. In addition, Nikhil N. Verma has a patent Smith & Nephew—Instrumentation with royalties paid to Smith & Nephew; is a board or committee member in American Orthopaedic Society for Sports Medicine, American Shoulder and Elbow Surgeons, and Arthroscopy Association of North America; has stock or stock options in Cymedica and Omeros; is in editorial or governing board of Journal of Knee Surgery and SLACK Incorporated; and is a paid consultant at Minivasive.
The other authors, their immediate families, and any research foundations with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article27, 35.
Footnotes
Institutional review board approval was received from Rush University Medical Center (ORA-19022301).
References
- 1.Anagnostis C., Mayer T.G., Gatchel R.J., Proctor T.J. The million visual analog scale: its utility for predicting tertiary rehabilitation outcomes. Spine (Phila Pa 1976) 2003;28:1051–1060. doi: 10.1097/01.BRS.0000061989.94487.9B. [DOI] [PubMed] [Google Scholar]
- 2.Anthony C.A., Glass N., Hancock K., Bollier M., Hettrich C.M., Wolf B.R. Preoperative performance of the Patient-Reported Outcomes Measurement Information System in patients with rotator cuff pathology. Arthroscopy. 2017;33:1770–1774.e1. doi: 10.1016/j.arthro.2017.04.018. [DOI] [PubMed] [Google Scholar]
- 3.Anthony C.A., Glass N.A., Hancock K., Bollier M., Wolf B.R., Hettrich C.M. Performance of PROMIS instruments in patients with shoulder instability. Am J Sports Med. 2017;45:449–453. doi: 10.1177/0363546516668304. [DOI] [PubMed] [Google Scholar]
- 4.Ayers D.C., Bozic K.J. The importance of outcome measurement in orthopaedics. Clin Orthop Relat Res. 2013;471:3409–3411. doi: 10.1007/s11999-013-3224-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ayers D.C., Zheng H., Franklin P.D. Integrating patient-reported outcomes into orthopaedic clinical practice: proof of concept from FORCE-TJR. Clin Orthop Relat Res. 2013;471:3419–3425. doi: 10.1007/s11999-013-3143-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baumhauer J.F., Bozic K.J. Value-based healthcare: patient-reported outcomes in clinical decision making. Clin Orthop Relat Res. 2016;474:1375–1378. doi: 10.1007/s11999-016-4813-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beach W.R. Editorial Commentary: Patient Reported Outcomes Measurement Information System (PROMIS) may be our promise for the future. Arthroscopy. 2017;33:1775–1776. doi: 10.1016/j.arthro.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 8.Cancienne J., Kunze K.N., Beck E.C., Chahla J., Suppauksorn S., Nho S.J. Influence of cigarette smoking at the time of surgery on postoperative outcomes in patients with femoroacetabular impingement: a matched-pair cohort analysis. Am J Sports Med. 2019;47:1138–1144. doi: 10.1177/0363546519832545. [DOI] [PubMed] [Google Scholar]
- 9.Carr A.J. Evidence-based orthopaedic surgery: what type of research will best improve clinical practice? J Bone Joint Surg Br. 2005;87:1593–1594. doi: 10.1302/0301-620X.87B12.17085. [DOI] [PubMed] [Google Scholar]
- 10.Downie W.W., Leatham P.A., Rhind V.M., Pickup M.E., Wright V. The visual analogue scale in the assessment of grip strength. Ann Rheum Dis. 1978;37:382–384. doi: 10.1136/ard.37.4.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franklin P.D., Lewallen D., Bozic K., Hallstrom B., Jiranek W., Ayers D.C. Implementation of patient-reported outcome measures in U.S. Total joint replacement registries: rationale, status, and plans. J Bone Joint Surg Am. 2014;96(Suppl 1):104–109. doi: 10.2106/JBJS.N.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fung C.H., Hays R.D. Prospects and challenges in using patient-reported outcomes in clinical practice. Qual Life Res. 2008;17:1297–1302. doi: 10.1007/s11136-008-9379-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guattery J.M., Dardas A.Z., Kelly M., Chamberlain A., McAndrew C., Calfee R.P. Floor effect of PROMIS depression CAT associated with hasty completion in orthopaedic surgery patients. Clin Orthop Relat Res. 2018;476:696–703. doi: 10.1007/s11999.0000000000000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hancock K.J., Glass N., Anthony C.A., Hettrich C.M., Albright J., Amendola A. Performance of PROMIS for healthy patients undergoing meniscal surgery. J Bone Joint Surg Am. 2017;99:954–958. doi: 10.2106/JBJS.16.00848. [DOI] [PubMed] [Google Scholar]
- 15.Karaaslan F., Karaoglu S., Yurdakul E. Reducing intra-articular hemarthrosis after arthroscopic anterior cruciate ligament reconstruction by the administration of intravenous tranexamic acid: a prospective, randomized controlled trial. Am J Sports Med. 2015;43:2720–2726. doi: 10.1177/0363546515599629. [DOI] [PubMed] [Google Scholar]
- 16.Klimek L., Bergmann K.C., Biedermann T., Bousquet J., Hellings P., Jung K. Visual analogue scales (VAS): measuring instruments for the documentation of symptoms and therapy monitoring in cases of allergic rhinitis in everyday health care: Position Paper of the German Society of Allergology (AeDA) and the German Society of Allergy and Clinical Immunology (DGAKI), ENT Section, in collaboration with the working group on Clinical Immunology, Allergology and Environmental Medicine of the German Society of Otorhinolaryngology, Head and Neck Surgery (DGHNOKHC) Allergo J Int. 2017;26:16–24. doi: 10.1007/s40629-016-0006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krishnamoorthy V.P., Kunze K.N., Beck E.C., Cancienne J.M., O'Keefe L.S., Ayeni O.R. Radiographic prevalence of symphysis pubis abnormalities and clinical outcomes in patients with femoroacetabular impingement syndrome. Am J Sports Med. 2019;47:1467–1472. doi: 10.1177/0363546519837203. [DOI] [PubMed] [Google Scholar]
- 18.Limaye V., Frankham A., Disney A., Pile K. Evaluation of hand function in patients undergoing long term haemodialysis. Ann Rheum Dis. 2001;60:278–280. doi: 10.1136/ard.60.3.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Magasi S., Ryan G., Revicki D., Lenderking W., Hays R.D., Brod M. Content validity of patient-reported outcome measures: perspectives from a PROMIS meeting. Qual Life Res. 2012;21:739–746. doi: 10.1007/s11136-011-9990-8. [DOI] [PubMed] [Google Scholar]
- 20.Makhni E.C., Hamamoto J.T., Higgins J.D., Patterson T., Griffin J.W., Romeo A.A. How comprehensive and efficient are patient-reported outcomes for rotator cuff tears? Orthop J Sports Med. 2017;5 doi: 10.1177/2325967117693223. 2325967117693223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Makhni E.C., Padaki A.S., Petridis P.D., Steinhaus M.E., Ahmad C.S., Cole B.J. High variability in outcome reporting patterns in high-impact ACL literature. J Bone Joint Surg Am. 2015;97:1529–1542. doi: 10.2106/JBJS.O.00155. [DOI] [PubMed] [Google Scholar]
- 22.Makhni E.C., Swart E., Steinhaus M.E., Mather R.C., III, Levine W.N., Bach B.R., Jr. Cost-effectiveness of reverse total shoulder arthroplasty versus arthroscopic rotator cuff repair for symptomatic large and massive rotator cuff tears. Arthroscopy. 2016;32:1771–1780. doi: 10.1016/j.arthro.2016.01.063. [DOI] [PubMed] [Google Scholar]
- 23.Middel B., van Sonderen E. Statistical significant change versus relevant or important change in (quasi) experimental design: some conceptual and methodological problems in estimating magnitude of intervention-related change in health services research. Int J Integr Care. 2002;2:e15. doi: 10.5334/ijic.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nwachukwu B.U., Chang B., Adjei J., Schairer W.W., Ranawat A.S., Kelly B.T. Time required to achieve minimal clinically important difference and substantial clinical benefit after arthroscopic treatment of femoroacetabular impingement. Am J Sports Med. 2018;46:2601–2606. doi: 10.1177/0363546518786480. [DOI] [PubMed] [Google Scholar]
- 25.Nwachukwu B.U., Chang B., Voleti P.B., Berkanish P., Cohn M.R., Altchek D.W. Preoperative Short Form Health Survey score is predictive of return to play and minimal clinically important difference at a minimum 2-year follow-up after anterior cruciate ligament reconstruction. Am J Sports Med. 2017;45:2784–2790. doi: 10.1177/0363546517714472. [DOI] [PubMed] [Google Scholar]
- 26.Nwachukwu B.U., Hamid K.S., Bozic K.J. Measuring value in orthopaedic surgery. JBJS Rev. 2013;1 doi: 10.2106/JBJS.RVW.M.00067. 01874474-201311000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Oak S.R., Strnad G.J., Bena J., Farrow L.D., Parker R.D., Jones M.H. Responsiveness comparison of the EQ-5D, PROMIS Global Health, and VR-12 Questionnaires in knee arthroscopy. Orthop J Sports Med. 2016;4 doi: 10.1177/2325967116674714. 2325967116674714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oka K., Tanaka H., Okada K., Sahara W., Myoui A., Yamada T. Three-dimensional corrective osteotomy for malunited fractures of the upper extremity using patient-matched instruments: a prospective, multicenter, open-label, single-arm trial. J Bone Joint Surg Am. 2019;101:710–721. doi: 10.2106/JBJS.18.00765. [DOI] [PubMed] [Google Scholar]
- 29.Seker V., Hackett L., Lam P.H., Murrell G.A.C. Evaluating the outcomes of rotator cuff repairs with polytetrafluoroethylene patches for massive and irreparable rotator cuff tears with a minimum 2-year follow-up. Am J Sports Med. 2018;46:3155–3164. doi: 10.1177/0363546518801014. [DOI] [PubMed] [Google Scholar]
- 30.Selim A.J., Rogers W., Qian S.X., Brazier J., Kazis L.E. A preference-based measure of health: the VR-6D derived from the veterans RAND 12-Item Health Survey. Qual Life Res. 2011;20:1337–1347. doi: 10.1007/s11136-011-9866-y. [DOI] [PubMed] [Google Scholar]
- 31.Stone A.V., Jacobs C.A., Luo T.D., Meadows M.C., Nho S.J., Stubbs A.J. High degree of variability in reporting of clinical and patient-reported outcomes after hip arthroscopy. Am J Sports Med. 2018;46:3040–3046. doi: 10.1177/0363546517724743. [DOI] [PubMed] [Google Scholar]
- 32.Terwee C.B., Bot S.D., de Boer M.R., van der Windt D.A., Knol D.L., Dekker J. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol. 2007;60:34–42. doi: 10.1016/j.jclinepi.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 33.Waterman B.R., Newgren J., Gowd A.K., Cabarcas B.C., Bach B.R., Cole B.J. Randomized prospective trial of arthroscopic rotator cuff with or without acromioplasty: no difference in patient-reported outcomes at long-term follow-up. Orthop J Sports Med. 2018;6 doi: 10.1177/2325967118S00080. 2325967118S00080. [DOI] [PubMed] [Google Scholar]
- 34.Windle G., Bennett K.M., Noyes J. A methodological review of resilience measurement scales. Health Qual Life Outcomes. 2011;9:8. doi: 10.1186/1477-7525-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yim J.H., Seon J.K., Song E.K., Choi J.I., Kim M.C., Lee K.B. A comparative study of meniscectomy and nonoperative treatment for degenerative horizontal tears of the medial meniscus. Am J Sports Med. 2013;41:1565–1570. doi: 10.1177/0363546513488518. [DOI] [PubMed] [Google Scholar]