The spinal accessory nerve is the main motor innervation to the trapezius muscle and also innervates the sternocleidomastoid muscle. In the context of a patient with a suspicion of injury to the spinal accessory nerve, a complete history should be performed including occupation, hobbies, history of trauma of the shoulder or neck, or previous cervical surgery.
The superficial location of this nerve in the posterior cervical triangle makes it extremely susceptible to injury. In fact, iatrogenic injury to the nerve after a surgical procedure is one of the most common causes of trapezius palsy. Other reported mechanisms of injury include compression by tumors or aneurysm formation at the base of the skull,13 direct trauma in the neck region,2,11,12,15,28 or a traction injury.1,14 However, most cases of isolated spinal accessory palsy are idiopathic and occur in the absence of a trauma.8,17,26 Spinal accessory neuritis is also uncommon and can be associated with autoimmune or infectious diseases. Neuritis after dengue infection rarely occurs. However, an increasing number of uncommon neurologic signs have been recently reported. This would include brachial neuritis and peripheral nerve palsy such as long thoracic nerve palsy, peripheral facial palsy, phrenic nerve palsy, or abducens nerve palsy.5,6,18,20,21,24,27
We present the case of spontaneous unilateral trapezius palsy caused by spinal accessory nerve palsy as a peripheral nervous system manifestation of dengue fever.
Case presentation
A 62-year-old Caucasian Spanish man with a medical history of hypertension and asthma presented to the emergency service with fever and fatigue associated with diarrhea episodes and retro-orbitary pain after traveling to Thailand. Routine hematology revealed thrombocytopenia. A blood film for malaria was negative. The diagnosis of suspected dengue fever was made and IgM anti-dengue virus was positive. Dengue infection was complicated with respiratory infection caused by Pseudomonas aeruginosa and the patient required 3 days of hospitalization in an intensive care unit.
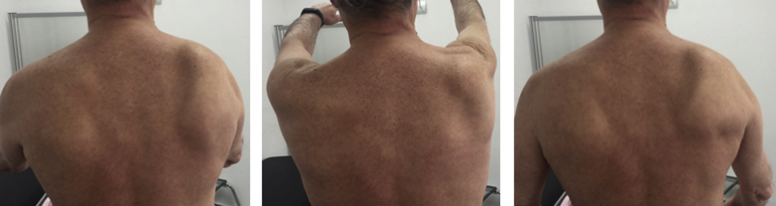
Few days after the resolution of the fever, the patient developed intense shoulder pain, weakness, and limited active range of motion of his right shoulder. Passive range of motion was preserved. He denied any specific traumatic event. Results of his complete blood count, serum biochemistry, erythrocyte sedimentation rate, and C-reactive protein were normal at this stage. X-rays of his right shoulder were unremarkable. Symptomatic treatment based on analgesia with nonsteroidal anti-inflammatory drugs was indicated, and 2 months after the onset of the symptoms, the patient continued with pain and physical examination revealed marked right trapezius atrophy, whereas wasting and weakness were not observed in the ipsilateral sternocleidomastoid muscle. Scapular winging was marked during abduction and forward elevation, whereas it was only slightly evident at rest (Figs. 1 and 2). Neurologic examination did not reveal any other nerve deficits. No brachial plexus neurologic signs were detected and rotator cuff seemed to be intact.
Figure 1.
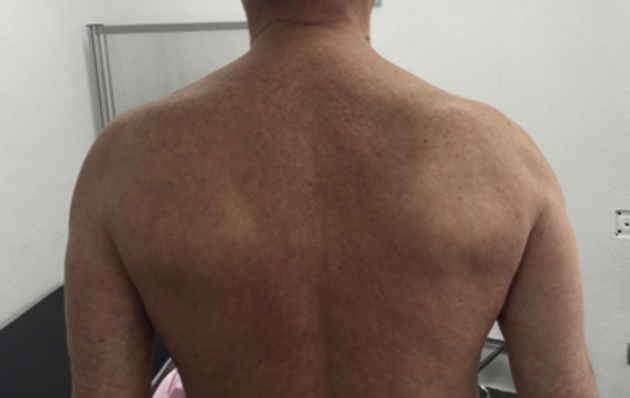
Scapular winging slightly evident at rest.
Figure 2.
Scapular winging at different angles of arm abduction.
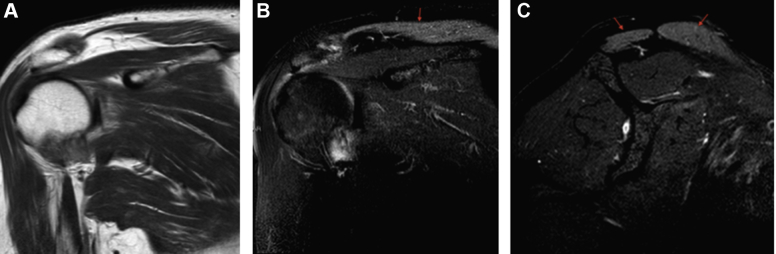
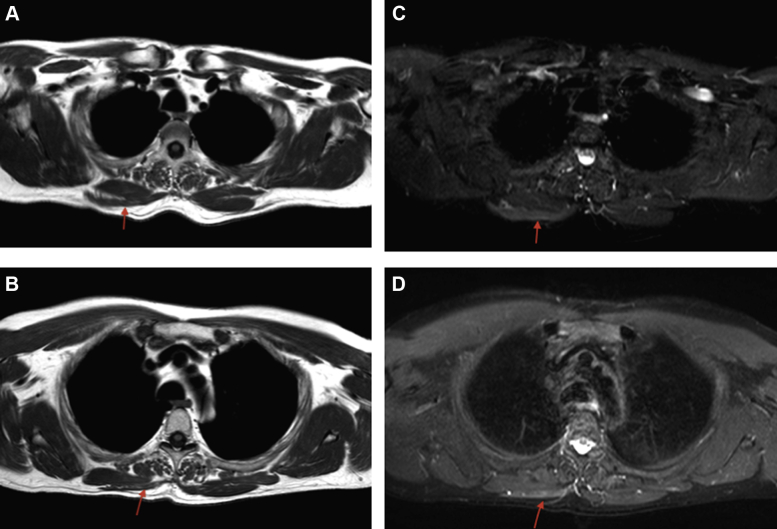
The magnetic resonance imaging (MRI) study of the shoulder was performed, 3 months from the onset of the symptoms, and revealed marked edema in the muscle bulk of the right trapezius (Fig. 3). Fatty infiltration was not observed in any muscle. A thoracic wall MRI revealed mild atrophy of the right trapezius muscle (Fig. 4). Atrophy was not observed in the ipsilateral sternocleidomastoid muscle.
Figure 3.
Coronal T1WI (A), coronal T2WI (B), and sagital T2FS (C) views of magnetic resonance imaging of the shoulder reveal marked edema in the muscle bulk of the right trapezius ( ). T1WI, T1 weighted image; T2WI, T2 weighted image; T2FS, fat-saturated T2 weighted image.
). T1WI, T1 weighted image; T2WI, T2 weighted image; T2FS, fat-saturated T2 weighted image.
Figure 4.
Transverse views of magnetic resonance T1WI (A, B) and STIR WI (C, D) imaging of the chest reveal marked edema and wasting in the muscle bulk of the right trapezius ( ). T1WI, T1 weighted image; STIR WI, short time inversion recovery weighted image.
). T1WI, T1 weighted image; STIR WI, short time inversion recovery weighted image.
Needle electromyography (EMG) of the right trapezius muscle, performed also 3 months after the onset of the symptoms, revealed signs of atrophy and subacute denervation, with motor unit potentials showing polyphasia and instability (increased jiggle). EMG of his right sternocleidomastoid muscle showed no findings of axonal injury, and his serratus anterior, levator scapulae, and rhomboid muscles were also normal. Clinical, radiographic, and EMG findings correlated with a diagnosis of right spinal accessory nerve palsy not affecting the sternocleidomastoid muscle.
A specific program of physiotherapy was followed focusing on strengthening the scapular muscles and preserving the range of motion of his shoulder. A total of 15 sessions during the first 2 months were performed, and after 2 months of rest, a new physiotherapy program was developed with a total of 10 sessions. At the last follow-up, 13 months after the onset, a remarkable improvement in the active abduction and elevation of his arm was observed. However, scapular winging was still present. At this stage, MRI showed the resolution of muscle edema with persistent muscle atrophy. He was able to return to manual activity for his job and daily life although a complete range of motion had not been reached yet.
Discussion
This healthy young man developed an acute febrile illness with clinical and serologic evidence for a diagnosis of dengue fever. His illness was complicated by right-sided spinal accessory nerve palsy.
Dengue fever is an arbovirus disease, the most common in humans. It is endemic in most tropical countries, and, over the past 2 decades, an increasing incidence of dengue has been observed in Latin America, Asia (including Thailand), and the Caribbean islands. The clinical presentation of dengue infection ranges from clinical febrile illness to severe manifestations like dengue hemorrhagic fever and dengue shock syndrome.
Recent studies reported that virological characteristics of dengue viruses have changed. The development of neurotrophic and immunological mechanisms has resulted in increasing rates of neurologic complications, which has been reported in 0.5%-21% of dengue cases.4,16,19,22,23,26 Approximately 95% of neurologic complications involve the central nervous system (encephalitis and encephalopathy), whereas peripheral nervous system manifestations represent only 5% of neurologic manifestations of dengue fever.5,6,18,20,21,24,27
The development of spontaneous unilateral trapezius palsy caused by spinal accessory neuritis as a peripheral nervous system manifestation of dengue fever is rare. Isolated dysfunction of the spinal accessory nerve is a well-described but uncommon nerve palsy. Most cases are idiopathic and right-sided. When infections have been implicated, they normally started during convalescence. Our patient developed palsy of his right spinal accessory nerve that began after acute dengue fever had resolved. We hypothesized that post-dengue infection would lead to autoimmune response against myelin sheath causing neuritis due to patchy demyelination of the nerve.
However, the history of concomitant respiratory infection caused by P. aeruginosa and the stay in an intensive care unit may be a possible confounder. The reported findings can correlate with intensive care unit–acquired neuropathy seen in critically ill patients,10 although it is most frequently seen in prolonged stays, not in a 3-day stay as in this case. When making the differential diagnosis in any patient who presents with acute shoulder pain, Parsonage-Tuner syndrome (PTS) should always be entertained. PTS, also referred to as idiopathic brachial plexopathy or neuralgic amyotrophy, is a disorder characterized by acute severe unilateral shoulder pain lasting for several days to weeks, which decreases spontaneously followed by progressive weakness, dysesthesias, and numbness.9 It most commonly affects the suprascapular nerve, the long thoracic nerve, and the axillary nerve, and less commonly the anterior interosseous, musculocutaneous, and spinal accessory nerve. The most common associated risk factor is a recent viral infection directly involving the brachial plexus, reported in 25%-55% of patients,9 specifically upper respiratory tract infection including influenza, parvovirus B19, cytomegalovirus, HIV, and typhoid fever.
Differentiating between a dengue-associated neuritis and PTS secondary to respiratory infection could be complicated. However, there are some points to take into account. First, bacterial pneumonia as a cause of PTS is a rare condition. In addition, the absence of sensory symptoms made PTS diagnosis less probably, given that sensory loss is classically involved in up to 66% of patients.7 Moreover, pain in PTS is almost always self-limiting, lasting 1-2 weeks, but on rare occasions persisting for longer periods of time,25 and it is not positional, is usually worse at night, and may be associated with awakenings from sleep.
Other possible differential diagnoses that could also be considered are shingles and cervical pathology, although pain secondary to a cervical radiculopathy may be exacerbated by extension of the neck.
Because the trapezius is a major scapular stabilizer, trapezius palsy may cause weakness in the abduction and anterior elevation of the arm and winging of the scapula. The usual initial presentation is severe neck and shoulder pain, which becomes evident after a few days. It is essential to recognize this condition in the early stage to maintain glenohumeral and scapulothoracic motion. However, trapezius paralysis frequently is misdiagnosed on initial presentation, despite consultation with orthopedic surgeon, physiatrists, neurologist, and neurosurgeons.
Based on the hypothesis that dengue-associated neuritis is related to immune reactions against the myelin sheath, dengue-associated nerve palsy is mostly managed with conservative treatment including a specific program of physical therapy and specific treatment with steroids27 or intravenous immunoglobulins,3,20 although the mechanisms remain unclear.4
Conclusions
Trapezius paralysis frequently is misdiagnosed on initial presentation. A thorough examination of the patient from the back is always recommended to avoid underdiagnosing scapulothoracic pathology. The main cause of trapezius palsy is injury to its major nerve supply, the spinal accessory nerve. In the absence of previous surgery or trauma, a meticulous history is necessary. Peripheral nervous system manifestations represent only 5% of neurologic manifestations of dengue fever and could be attributed to immune reactions. Although conservative management is recommended, it is essential to achieve an early diagnosis in order to develop a specific physiotherapy program to maintain proper shoulder motion and function.
Disclaimer
The authors, their immediate families, and any research foundations with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article.
Footnotes
Written informed consent was obtained from the patient for publication of his case report and any accompanying images.
References
- 1.Baker S.K. Isolated spinal accessory mononeuropathy associated with neurogenic muscle hypertrophy: restricted neuralgic amyotrophy or stretch-palsy? A case report. Arch Phys Med Rehabil. 2008;89:559–563. doi: 10.1016/j.apmr.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 2.Bird S. Accessory nerve injury. Aust Fam Physician. 2006;35:535–536. [PubMed] [Google Scholar]
- 3.Biswas N.M., Pal S. Oculomotor nerve palsy in dengue encephalitis-a rare presentation. Indian J Med Res. 2014;140:793–794. [PMC free article] [PubMed] [Google Scholar]
- 4.Carod-Artal F.J., Wichmann O., Farrar J., Gascón J. Neurological complications of dengue virus infection. Lancet Neurol. 2013;12:906–919. doi: 10.1016/S1474-4422(13)70150-9. [DOI] [PubMed] [Google Scholar]
- 5.Chappuis F., Justafré J.C., Duchunstang L., Loutan L., Taylor W.R. Dengue fever and long thoracic nerve palsy in a traveler returning from Thailand. J Travel Med. 2004;11:112–114. doi: 10.2310/7060.2004.16983. [DOI] [PubMed] [Google Scholar]
- 6.Chien J., Ong A., Low S.Y. An unusual complication of dengue fever. Singapore Med J. 2008;49:e340–e342. [PubMed] [Google Scholar]
- 7.Cwik V.A., Wilbourn A.J., Rorick M. Acute brachial neuropathy: detailed EMG findings in a large series. Muscle Nerve. 1990;13:859. [Google Scholar]
- 8.Eisen A., Bertrand G. Isolated accessory nerve palsy of spontaneous origin. Arch Neurol. 1972;27:496–502. doi: 10.1001/archneur.1972.00490180032008. [DOI] [PubMed] [Google Scholar]
- 9.Feinberg J.H., Radecki J. Parsonage-turner syndrome. HSS J. 2010;6:199–205. doi: 10.1007/s11420-010-9176-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedrich O., Reid M.B., Van den Berghe G., Vanhorebeek I., Hermans G., Rich M.M. The sick and the weak: neuropathies/myopathies in the critically ill. Physiol Rev. 2015;95:1025–1109. doi: 10.1152/physrev.00028.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gordon S.L., Graham W.P., III, Black J.T., Miller S.H. Accessory nerve function after surgical procedures in the posterior triangle. Arch Surg. 1977;112:264–268. doi: 10.1001/archsurg.1977.01370030036005. [DOI] [PubMed] [Google Scholar]
- 12.Hirasawa Y., Sakakida K. Sports and peripheral nerve injury. Am J Sports Med. 1983;11:420–426. doi: 10.1177/036354658301100607. [DOI] [PubMed] [Google Scholar]
- 13.Kubota M., Ushikubo O., Miyata A., Yamaura A. Schwannoma of the spinal accessory nerve. J Clin Neuroscience. 1998;5:436–437. doi: 10.1016/s0967-5868(98)90281-8. [DOI] [PubMed] [Google Scholar]
- 14.Logigian E.L., McInnes J.M., Berger A.R., Busis N.A., Lehrich J.R., Shahani B.T. Stretch-induced spinal accessory nerve palsy. Muscle Nerve. 1988;11:146–150. doi: 10.1002/mus.880110210. [DOI] [PubMed] [Google Scholar]
- 15.Lu L., Haman S.P., Ebraheim N.A. Vulnerability of the spinal accessory nerve in the posterior triangle of the neck: a cadaveric study. Orthopedics. 2002;25:71–74. doi: 10.3928/0147-7447-20020101-20. [DOI] [PubMed] [Google Scholar]
- 16.Mamdouh K.H., Mroog K.M., Hani N.H., Nabil E.M. Atypical dengue meningitis in Makkah, Saudi Arabia with slow resolving, prominent migraine like headache, phobia, and arrhythmia. J Glob Infect Dis. 2013;5:183–186. doi: 10.4103/0974-777X.122021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mariani P., Santoriello P., Maresca G. Spontaneous accessory nerve palsy. J Shoulder Elbow Surg. 1998;7:545–546. doi: 10.1016/s1058-2746(98)90211-7. [DOI] [PubMed] [Google Scholar]
- 18.Mishra A., Shukla S., Aggarwal S., Chaudhary B. Lateral rectus palsy in a case of dengue fever. Med J Armed Forces India. 2013;1:10–12. doi: 10.1016/j.mjafi.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murthy J.M. Neurological complications of dengue infection. Neurol India. 2010;58:581–584. doi: 10.4103/0028-3886.68654. [DOI] [PubMed] [Google Scholar]
- 20.Patey O., Ollivaud J., Breuil J., Lafaix C. Unusual neurological manifestations occuring during dengue fever infection. Am J Trop Med Hyg. 1993;48:793–802. doi: 10.4269/ajtmh.1993.48.793. [DOI] [PubMed] [Google Scholar]
- 21.Peter S., Malhotra N., Peter P., Sood R. Isolated bell's palsy—an unusual presentation of dengue infection. Asian Pac J Trop Med. 2013;6:82–84. doi: 10.1016/S1995-7645(12)60207-7. [DOI] [PubMed] [Google Scholar]
- 22.Sahu R., Verma R., Jain A., Garg R.K., Singh M.K., Malhotra H.S. Neurologic complications in dengue virus infection: a prospective cohort study. Neurology. 2014;83:1601–1609. doi: 10.1212/WNL.0000000000000935. [DOI] [PubMed] [Google Scholar]
- 23.Saini L., Chakrabarty B., Pastel H., Israni A., Kumar A., Gulati S. Dengue fever triggering hemiconvulsion hemiplegia epilepsy in a child. Neurol India. 2017;65:636–638. doi: 10.4103/neuroindia.NI_1367_15. [DOI] [PubMed] [Google Scholar]
- 24.Shivanthan M.C., Ratnayake E.C., Wijesiriwardena B.C., Somaratna K.C., Gamagedara L.K. Paralytic squint due to abducens nerve palsy: a rare consequence of dengue fever. BMC Infect Dis. 2012;12:156. doi: 10.1186/1471-2334-12-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsairis P., Dyck P.J., Milder D.W. Natural history of brachial plexus neuropathy: report on 99 patients. Arch Neurol. 1972;27:109–117. doi: 10.1001/archneur.1972.00490140013004. [DOI] [PubMed] [Google Scholar]
- 26.Van Tuijl J.H., Schmid A., Van Kranen-Mastenbroek V., Faber C.G., Vles J.S.H. Isolated spinal accessory neuropathy in an adolescent: a case study. Eur J Paed Neurol. 2006;10:83–85. doi: 10.1016/j.ejpn.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 27.Verma R., Sharma P., Garg R.K., Atam V., Singh M.K., Mehrotra H.S. Neurological complications of dengue fever: experience from a tertiary center of North India. Ann Indian Acad Neurol. 2011;14:272–278. doi: 10.4103/0972-2327.91946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wright T.A. Accessory spinal nerve injury. Clin Orthop. 1975;108:15–18. doi: 10.1097/00003086-197505000-00004. [DOI] [PubMed] [Google Scholar]