Abstract
Background
Revision reverse total shoulder arthroplasty (RTSA) reliably improves shoulder pain and function in patients with failed shoulder arthroplasty, although it can lead to significant postoperative complications. The purpose of this study was to determine the effect of postoperative complications on shoulder pain and function after revision RTSA.
Methods
We evaluated 36 patients at an average of 4.3 years (range, 2-8.6 years) after revision of a shoulder arthroplasty to RTSA. Of these patients, 9 had a failed anatomic total shoulder arthroplasty, 23 had a failed hemiarthroplasty, and 4 had a failed RTSA. The American Shoulder and Elbow Surgeons (ASES) score and visual analog scale (VAS) pain score were evaluated postoperatively, and patients with and without postoperative complications were compared.
Results
The final ASES score and VAS pain score were 61 ± 23 and 2.4 ± 2.3, respectively. A major postoperative complication occurred in 7 patients (19%) (infection in 3, hematoma in 1, instability in 1, and acromial and/or scapular spine fracture in 2). Further surgical treatment was required in 5 patients (14%) (irrigation and débridement and component exchange for infection in 3, irrigation and débridement for hematoma in 1, and open reduction–internal fixation of scapular spine fracture in 1). On comparison of clinical outcomes between patients with and patients without complications, the ASES score and VAS pain score were significantly worse in patients with complications vs. those without them (ASES score, 43 ± 24 vs. 66 ± 21 [P = .04]; VAS pain score, 4.3 ± 2 vs. 2 ± 2.2 [P = .03]).
Conclusion
Revision RTSA resulted in postoperative pain and shoulder function comparable to primary RTSA reported in the literature, although postoperative complications led to clinically significant declines in function and increases in pain.
Keywords: Reverse total shoulder arthroplasty, revision, glenoid bone loss, bone grafting, shoulder arthroplasty, shoulder replacement
As the incidence of shoulder arthroplasty has increased,28 especially in younger patients,33,39 the burden of revision shoulder arthroplasty has increased.6,50 Historically, revision total shoulder arthroplasty (TSA) was associated with unacceptably high failure rates because of difficulty reconstructing glenoid bone defects,17,21,24 difficulty achieving stable fixation of the glenoid component,11 and a high prevalence of rotator cuff failure.37 The expectation after revision TSA in the past was the achievement of limited goals with modest levels of pain relief or functional improvement. Revision reverse total shoulder arthroplasty (RTSA) eliminates may problems associated with revision TSA. Glenoid bone grafting can be very successful in the setting of RTSA,8,25,50,56 baseplate loosening is uncommon,22,30,43 and RTSA does not rely on an intact rotator cuff.10,19 Unfortunately, revision RTSA comes at the cost of other unique postoperative complications including impingement resulting in instability and acromial or scapular spine fractures, which may affect final functional outcomes. Varying results of revision RTSA have been reported in several studies, with improvements in pain and function, although complication rates have reached as high as 50%4,7,18,22,23,27,31,35,36,48,50,53 (Table I).
Table I.
Results of revision to reverse total shoulder arthroplasty in prior series
| Authors | Year | Patients, n | Follow-up, mo | AFE, ° | VAS pain score | ASES score | Constant score | SST score | Cx, % |
|---|---|---|---|---|---|---|---|---|---|
| Alentorn-Geli et al1 | 2017 | 31 | 28 | 109 | 1 | NA | NA | NA | 21 |
| Black et al4 | 2015 | 16 | 59 | NA | 1.7 | 67 | NA | 5.3 | 56 |
| Black et al5 | 2014 | 36 | 55 | NA | 1.4 | 70 | NA | 5.9 | 28 |
| Boileau et al7 | 2013 | 37 | 34 | 111 | NA | NA | 47 | NA | 30 |
| Castagna et al12 | 2013 | 36 | 32 | 120 | NA | NA | 48 | NA | 0 |
| Chacon et al13 | 2009 | 25 | 30 | 82 | NA | 70 | NA | 4.5 | 16 |
| Cox et al15 | 2019 | 73 | 68 | 75 | NA | 51 | NA | 3.5 | 19 |
| Flury et al18 | 2011 | 21 | 46 | 97 | 3 | NA | 56 | NA | 43 |
| Hernandez et al20 | 2017 | 70 | 36 | 112 | NA | 68 | NA | 7 | NA |
| Holcomb et al22 | 2009 | 14 | 33 | 118 | NA | 70 | NA | 4.5 | 21 |
| Holschen et al23 | 2017 | ||||||||
| GHOA cohort | 23 | 24 | 126 | 1.5 | 59 | 67 | NA | 9 | |
| Fracture cohort | 21 | 24 | 115 | 2.2 | 71 | 73 | NA | 24 | |
| Kany et al26 | 2015 | 29 | 28 | 124 | 2 | NA | 60 | 8 | 0 |
| Kelly et al27 | 2012 | 28 | 34 | 106 | NA | 72 | 49 | NA | 50 |
| Melis et al31 | 2012 | 37 | 47 | 121 | NA | NA | 55 | NA | 30 |
| Merolla et al32 | 2018 | 157 | 49 | NA | 2.2 | 60 | NA | 6 | NA |
| Ortmaier et al35 | 2013 | 50 | 51 | 98 | 1 | NA | 49 | 5.6 | 24 |
| Patel et al36 | 2012 | 28 | 41 | 108 | 2.6 | 66 | NA | 7.6 | 11 |
| Sheth et al40 | 2019 | 110 | 57 | NA | 2.9 | 63 | NA | NA | 20 |
| Shields and Wiater41 | 2019 | 35 | 50 | NA | 2.4 | 68 | NA | NA | 31 |
| Stephens et al43 | 2016 | 58 | 24 | 97 | 3.5 | 53 | NA | 4.4 | 18 |
| Stephens et al45 | 2015 | 16 | 36 | 100 | 2.6 | 67 | NA | 5.3 | 31 |
| Valenti et al48 | 2014 | 30 | 36 | 108 | NA | NA | 52 | NA | 27 |
| Wagner et al50 | 2015 | 143 | 37 | NA | NA | 66 | NA | 6 | 18 |
| Wagner et al52 | 2017 | 39 | 36 | 121 | NA | 68 | NA | 6.8 | 15 |
| Wagner et al51 | 2017 | 38 | 44 | 108 | NA | 61 | NA | 5 | 26 |
| Walker et al53 | 2012 | 22 | 40 | 130 | NA | 68 | NA | 5 | 23 |
| Weighted mean or total | NA | 1223 | NA | 106 ± 15 | 3 ± 2 | 63 ± 6 | 54 ± 8 | 5.7 ± 1.1 | 22 |
AFE, active forward elevation; VAS, visual analog scale; ASES, American Shoulder and Elbow Surgeons; SST, Simple Shoulder Test score; Cx, complications; NA, not available; GHOA, glenohumeral osteoarthritis.
Data are presented as mean values unless otherwise indicated.
Saltzman et al38 previously reported on the complication rates of primary vs. revision RTSA and TSA and determined that overall, minor, surgical, intraoperative, perioperative, and postoperative complications were greater after revision RTSA than after primary RTSA. Very few studies have examined the influence of postoperative complications in revision RTSA on functional outcomes. Holcomb et al22 reported the results of revision RTSA owing to baseplate failure in RTSA and found no difference in outcomes comparing pre-failure vs. post-revision American Shoulder and Elbow Surgeons (ASES) scores, supporting that the outcomes of primary vs. revision RTSA show no difference. Shields and Wiater41 reported on 36 revision RTSAs and noted significantly worse Subjective Shoulder Values in the revision RTSA group when patients sustained either a postoperative complication or required revision surgery compared with patients who did not require revision surgery or sustain a postoperative complication.
The purpose of this study was to describe the short-term clinical and radiographic results of revision to RTSA and determine whether patients sustaining a postoperative complication had worse functional results than those without a complication. We hypothesized that revision to RTSA would result in significant improvements in range of motion and functional outcomes and that patients sustaining a postoperative complication would have significantly worse shoulder pain and function.
Materials and methods
This was a retrospective study. The operative log of the senior author (R.Z.T.) was reviewed. We queried the log for patients who underwent revision from a humeral hemiarthroplasty (HA), anatomic TSA, or RTSA to an RTSA from May 2008 to May 2015. Patient who underwent conversion from an HA, anatomic TSA, or RTSA to an antibiotic spacer, anatomic TSA, or HA were excluded. We also excluded patients who underwent primary RTSA and patients with <2 years' potential follow-up. RTSA was indicated for patients with persistent pain, limited function, and restriction of motion in those with a failed arthroplasty. In the setting of a failed HA, the indications for conversion to RTSA included a painful arthroplasty with or without rotator cuff insufficiency with or without glenoid or humeral bone deficiency. No HA was converted to an anatomic TSA during the study period. In the setting of a failed anatomic TSA, the indications for conversion to RTSA included a loosened glenoid implant not amendable to revision anatomic glenoid component placement or rotator cuff failure. In the setting of a failed RTSA, indications for conversion to another RTSA included instability or implant loosening amendable to revision with or without bone grafting. Eligible patients were then contacted and returned for evaluation including functional outcome questionnaires, physical examinations, and radiographs or provided functional outcome data by telephone.
Operative protocol
In all cases, a deltopectoral approach was used. Both the Trabecular Metal RTSA (Zimmer, Warsaw, IN, USA) and Aequalis Reversed Shoulder Arthroplasty (Wright Medical Group, Bloomington, MN, USA) systems were used. Although initial attempts were made to free the humeral stem using a burr and thin osteotomes, when this method was not successful an osteotomy or window of the humeral shaft was performed and was fixated with cerclage wires as previously described.7 Both long (>130 mm) and standard-length (130 mm) stemmed implants were used depending on proximal humeral bone loss and the requirement for a humeral osteotomy or window. In cases of severe glenoid erosion, allograft or iliac crest autograft reconstruction of the glenoid defect was performed. Glenoid grafting was performed in cases in which severe cavitary defect limited the ability to implant a baseplate in a stable fashion or severe erosion limited the baseplate to be positioned in a neutral position or with inferior tilt. We used 36-, 40-, and 42-mm glenospheres depending on the implant system, as well as the size of the patient. Standard or constrained polyethylene liners were used at the discretion of the surgeon on the basis of overall stability of the shoulder during trialing. The subscapularis was not repaired in any case. Proximal humeral allograft was used in some cases at the discretion of the surgeon for proximal humeral bone loss in the setting of tuberosity resorption using the step-cut technique described by Chacon et al.13
Clinical data collection
For each patient, the following data were collected based on the preoperative documentation: age, operative side, sex, duration of preoperative symptoms, active forward elevation (AFE) of the shoulder, and whether the patient had an active workers' compensation claim. In addition, for each patient, the following data were collected based on the intraoperative documentation: diagnosis, procedure, implant company, whether a glenoid bone graft was required and the source of this graft, whether an allograft was required for proximal humeral bone deficiency, whether a constrained polyethylene liner was used, glenosphere size, glenoid baseplate peg length, whether a humeral osteotomy was required, whether a humeral window was required, length of the humeral stem (standard or long stem), and whether any intraoperative complications or fractures were noted. For each patient, the following data were collected at final follow-up: whether any major postoperative complication occurred (fracture, instability, and infection or hematoma requiring surgery), whether the patient underwent revision, AFE, visual analog scale (VAS) pain score, ASES score, and length of follow-up.
Radiographic data collection
Preoperative and most recent final follow-up radiographs, including anteroposterior, Grashey anteroposterior, scapular-Y lateral, and axillary lateral views, were independently evaluated by an attending surgeon (P.N.C.) who had fellowship training in shoulder and elbow surgery but did not perform the index procedures. Preoperative humeral bone deficiency was classified using the PHAROS (Proximal Humeral Arthroplasty Revision Osseous Insufficiency) classification system.14 Postoperative radiographs were evaluated for incorporation or resorption of the bone grafts, migration or subsidence of the baseplate, humeral stem loosening, scapular spine or acromial fracture, proximal humeral osteolysis, and scapular notching. Scapular notching was graded using the Nerot-Sirveaux system.42
Statistical analysis
Descriptive statistics were calculated for preoperative, intraoperative, and postoperative variables. Preoperative AFE and postoperative AFE were compared using paired Student t tests. Postoperative outcome scores were compared between patients with and patients without complications using Student t tests. A power analysis was not performed because the procedure is an uncommon procedure that was examined with a retrospective design. All available subjects were included.
Results
In total, 56 patients met the inclusion and exclusion criteria, of whom 8 died, leaving a total of 48 patients available for review. Of these 48 patients, 12 were lost to follow-up, resulting in a total of 36 patients being reviewed and a 75% rate of follow-up. The length of follow-up was 4.3 years (range, 2-8.6 years). The right side was affected in 66% of patients. Female patients comprised 86% of the cohort, and the mean age (±standard deviation) was 69 ± 11.3 years. One patient had a prior workers' compensation claim. Preoperatively, patients showed significant restriction of motion, with AFE of only 55° ± 46.9°. Of the patients, 9 had a failed anatomic TSA (rotator cuff tear in 6, loosened glenoid component in 2, and periprosthetic fracture in 1), 23 had a failed HA (antibiotic spacer in 4 and rotator cuff failure or tuberosity nonunion and/or glenoid erosion in 19), and 4 had a failed RTSA (infection in 1, instability in 1, and loosened glenoid component in 2).
Preoperatively, 4 patients (11%) had no humeral bone loss. In contrast, 23 patients (64%) had type 1 epiphyseal humeral bone loss (15 with cortical thinning [type 1A], 3 with loss of the calcar [type 1AC], 4 with loss of the greater tuberosity [type 1AG], and 1 with loss of the greater tuberosity and calcar [type 1ACG]); 6 patients (17%) had type 2 metaphyseal bone loss (4 with cortical thinning [type 2A] and 2 with cortical loss [type 2B]); and 3 patients (8%) had type 3 diaphyseal bone loss (2 with cortical thinning [type 3A] and 1 with cortical loss [type 3B]).
Intraoperatively, a Trabecular Metal RTSA was used in 14 cases (39%) whereas an Aequalis Reversed Shoulder Arthroplasty was used in 22 cases (61%). A constrained polyethylene liner was used in 5 patients (14%). The humeral stem was not revised in 5 cases, whereas a long-stem implant (>130 mm) was used in 7 cases and a standard stem (130 mm) was used in 24 cases. A 36-mm glenosphere was used in all cases. In 8 cases, a 25-mm extended-length baseplate peg was used, whereas the remaining baseplates had a 15-mm-long central post. An episiotomy was required in 3 patients to remove the stem, and a humeral window was required in 2. Four patients underwent glenoid bone grafting with femoral head allograft for a large structural defect, and one underwent iliac crest autograft augmentation for a large glenoid defect. Five patients underwent proximal humeral allografting for large proximal humeral bone defects. Two intraoperative complications occurred; both were greater tuberosity fractures repaired with cerclage suture at the time of revision.
On analysis of final radiographs obtained at an average of 2.6 ± 2 years postoperatively, 31 of 36 patients (86%) had well-fixed components without osteolysis or loosening, 1 patient had a displaced scapular fracture that precluded full determination of whether the glenoid component was well fixed, 1 patient had a loose humeral component, and 3 patients had proximal humeral osteolysis but without loosening. By use of the Nerot-Sirveaux system, 20 patients (56%) had no notching, 9 (26%) had grade 1 notching, 1 (3%) had grade 2 notching, 1 (3%) had grade 3 notching, and 4 (11%) had grade 4 notching; in 1 patient, notching could not be evaluated because of a displaced scapular fracture. Of the 10 patients with humeral or glenoid grafts, 9 showed healing whereas 1 could not be evaluated because of a displaced scapular fracture.
Final postoperative AFE was 117° ± 43°, which improved by 62° ± 51° from preoperatively (P < .0001). The final ASES score and VAS pain score were 61 ± 23 and 2.4 ± 2.3, respectively. A major postoperative complication occurred in 7 patients (19%) (infection in 3, hematoma in 1, instability in 1, and acromial and/or scapular spine fracture in 2). Further surgical treatment was required in 5 patients (14%) (3 underwent irrigation and débridement and component exchange for infection, 1 underwent irrigation and débridement for hematoma, and 1 underwent open reduction–internal fixation of a scapular spine fracture that went on to achieve union although the final ASES and VAS pain scores were poor, at 25 and 5, respectively). The humeral stem and the baseplates were maintained in all patients until final follow-up. On comparison of clinical outcomes between patients with and patients without complications, the ASES score and VAS pain score at final follow-up were significantly worse in patients with complications vs. those without them (ASES score, P = .04; VAS pain score, P = .03) (Table II). The ASES score and VAS pain score were also worse in those patients requiring revision surgery vs. those not requiring revision, although the differences did not achieve statistical significance (ASES score, P = .22; VAS pain score, P = .21).
Table II.
Clinical results of patients with complications or need for reoperation vs. patients without complications or need for reoperation
| Patients, n | Final ASES score | Final VAS pain score | |
|---|---|---|---|
| Complications | |||
| Yes | 7 | 43 ± 24 | 4.3 ± 2 |
| No | 29 | 66 ± 21 | 2 ± 2.2 |
| P value | .04 | .03 | |
| Reoperations | |||
| Yes | 5 | 45 ± 28 | 3.8 ± 1.9 |
| No | 31 | 64 ± 22 | 2.2 ± 2.2 |
| P value | .22 | .21 |
ASES, American Shoulder and Elbow Surgeons; VAS, visual analog scale.
The Student t test was used to compare outcome scores; P < .05 was considered statistically significant.
Discussion
Revision RTSA has been shown to reliably improve pain and function in the treatment of failed anatomic and reverse shoulder arthroplasty. Despite high rates of clinical success, complication rates are high, ranging up to 50%. Our data are comparable to those of other studies reporting clinical results, with an ASES score averaging 61 and VAS pain score averaging 2.3 with final average AFE of 117°. Of the patients, 19% sustained a postoperative complication and 14% required further surgery. We have also shown a 90% rate of radiographic healing of bulk humeral and glenoid allograft reconstructions, with only 1 baseplate and 1 humeral implant showing loosening. On the basis of our data, avoidance of hematoma, fracture, infection, and instability after revision RTSA is critical to success as these complications will result in a clinically significant decline in shoulder function.
Multiple prior studies have examined the results of RTSA as revision from a prior HA, TSA, or RTSA.4,5,13,23,26,27,29,31,34, 35, 36,44,48, 49, 50,53,54 Nearly all prior reports have suggested that revision RTSA does decrease patient pain and improve patient-reported function.4,5,13,22,23,26,27,29,31,34, 35, 36,43,44,48, 49, 50,54 However, numerous reports have also noted that final postoperative outcomes after revision RTSA are inferior to those after primary RTSA.2,5,9,54 In addition, several reports have noted concerning complication rates as high as 50%-69%.4,18,23,27,39,54 Within these reports, predictors of complications and inferior outcomes included a high body mass index,38 medical comorbidities,57 glenoid bone grafting,50 and proximal humeral bone loss.44 Overall, our results are comparable to the findings in the prior literature regarding shoulder function, pain, and complication rates. Unlike prior studies in which patients were not stratified by complications, our study has shown that revision RTSA can lead to similar outcomes to primary RTSA as long as complications are avoided. Only Shields and Wiater41 determined that the development of a postoperative complication negatively influenced outcomes. Our data support the finding that if a major postoperative complication occurs (Fig. 1), pain and functional outcome will decline, and the inferior results are clinically significant as the differences exceed the minimal clinically important differences previously reported for the ASES score and VAS pain score.47
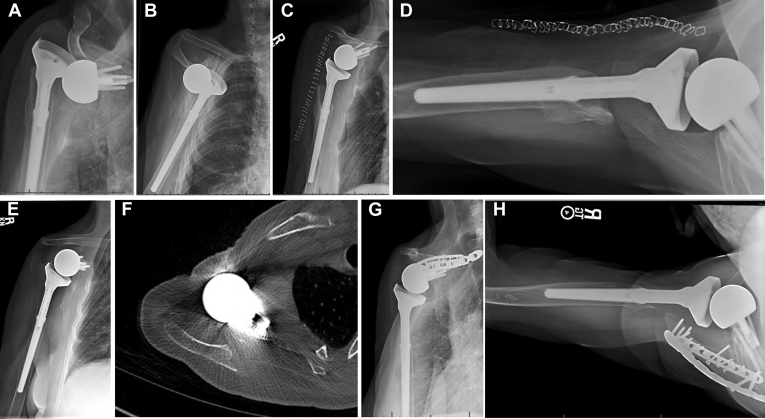
Figure 1.
Anteroposterior (A) and axillary lateral (B) radiographs showing a 70-year-old female patient who underwent revision of a recurrently unstable reverse total shoulder arthroplasty with cerclage of the glenosphere to the humerus and 15 mm of lengthening on the humeral side. Immediately postoperatively, anteroposterior (C) and axillary lateral (D) radiographs showed a good outcome. However, a displaced scapular spine fracture occurred at 1.5 years postoperatively; an anteroposterior radiograph (E) and axillary computed tomography slice (F) demonstrate displacement. This progressed to a nonunion, which was painful and limited elevation; thus, the patient underwent open reduction–internal reduction (G, H). Her function remained limited, with final active forward elevation of 30°, and a final American Shoulder and Elbow Surgeons score of 25.
Glenoid and humeral bone graft is common with revision RTSA, although the ideal glenoid or humeral grafting technique remains unclear. Various authors have reported on structural glenoid bone grafting,16,46,55 bulk proximal humeral allografting,13 femoral neck allografting for glenoid defects,3 and a BIO-RSA technique for glenoid defects.6 Wagner et al50 examined a series of 143 revision RTSAs with a minimum follow-up period of 2 years, of which 41 received glenoid bone grafts. Glenoid bone grafting was associated with decreased prosthesis survival at 2 and 5 years' follow-up. Within the series, allograft vs. autograft and corticocancellous vs. structural bone grafting did not influence survival, although the study may have been underpowered for these comparisons.46,50 Bulk humeral allografting for proximal humeral defects was evaluated by Chacon et al,13 who reported 84% metaphyseal healing and 76% diaphyseal healing with ASES scores averaging 69 at an average of 30 months. Implant survival in our series was not affected by the presence or absence of glenoid or humeral bone grafting using allograft (Fig. 2) as all implants were in place at final follow-up and all bone grafts that could be evaluated by postoperative imaging were healed.
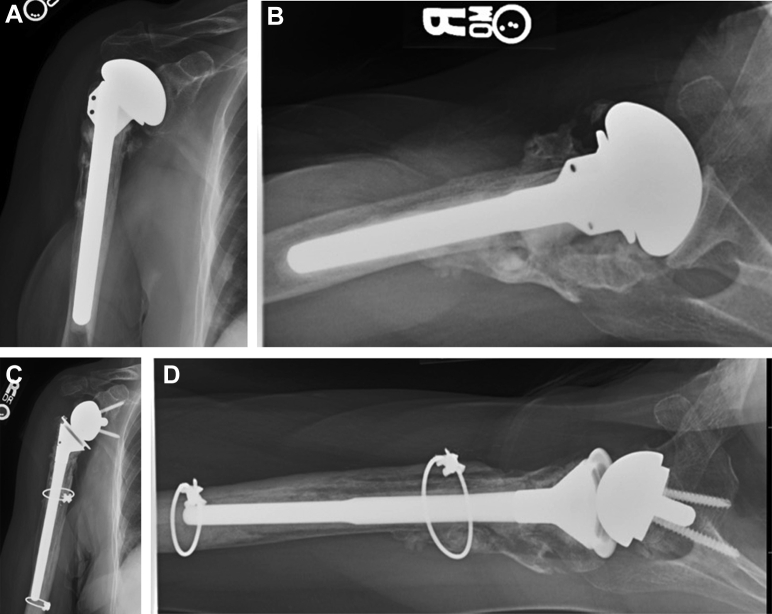
Figure 2.
Anteroposterior (A) and axillary lateral (B) radiographs showing an 87-year-old female patient who underwent revision of a painful cemented hemiarthroplasty performed for a proximal humeral fracture with malunion of the greater tuberosity with a proximal humeral allograft and femoral strut allograft. (C, D) Stable implants and graft incorporation were observed at 4 years postoperatively. The patient did not have any complications and was satisfied with her final active forward elevation.
The strengths of this study include a high rate of follow-up; the inclusion of validated, patient-related outcome scores; the comparison of outcomes in patients with and without postoperative complications; and the inclusion of radiographic outcomes. Our study also has several limitations: This was a single-center, retrospective review of a small sample with short-term follow-up and no control group. Given the relative rarity of revision RTSA compared with primary RTSA, larger sample sizes might be difficult to obtain, and nonsignificant differences in analyses could be due to a type II error.
Conclusion
Revision RTSA resulted in postoperative pain and shoulder function comparable to primary RTSA reported in the literature, although postoperative complications led to clinically significant declines in function and increases in pain.
Disclaimer
Robert Z. Tashjian is a paid consultant for Zimmer/Biomet, Wright Medical, and Mitek; owns stock in Conextions, Intrafuse, Genesis, and Kator; receives intellectual property royalties from Wright Medical, Shoulder Innovations, and Zimmer/Biomet; receives publishing royalties from the Journal of Bone and Joint Surgery and Springer; and serves on the editorial boards of the Journal of the American Academy of Orthopaedic Surgeons and Shoulder & Elbow.
Peter N. Chalmers is a paid consultant for Mitek and Arthrex; serves on the editorial board of the Journal of Shoulder and Elbow Surgery; is a paid speaker for DePuy; and receives intellectual property royalties from DePuy.
The other authors, their immediate families, and any research foundations with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article.
Footnotes
Institutional Review Board approval was obtained from the University of Utah School of Medicine prior to study initiation (no. 101394).
References
- 1.Alentorn-Geli E., Clark N.J., Assenmacher A.T., Samuelsen B.T., Sanchez-Sotelo J., Cofield R.H. What are the complications, survival, and outcomes after revision to reverse shoulder arthroplasty in patients older than 80 years? Clin Orthop Relat Res. 2017;475:2744–2751. doi: 10.1007/s11999-017-5406-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Austin L., Zmistowski B., Chang E.S., Williams G.R., Jr. Is reverse shoulder arthroplasty a reasonable alternative for revision arthroplasty? Clin Orthop Relat Res. 2011;469:2531–2537. doi: 10.1007/s11999-010-1685-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bateman E., Donald S.M. Reconstruction of massive uncontained glenoid defects using a combined autograft-allograft construct with reverse shoulder arthroplasty: preliminary results. J Shoulder Elbow Surg. 2012;21:925–934. doi: 10.1016/j.jse.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 4.Black E.M., Roberts S.M., Siegel E., Yannopoulos P., Higgins L.D., Warner J.J. Failure after reverse total shoulder arthroplasty: what is the success of component revision? J Shoulder Elbow Surg. 2015;24:1908–1914. doi: 10.1016/j.jse.2015.05.029. [DOI] [PubMed] [Google Scholar]
- 5.Black E.M., Roberts S.M., Siegel E., Yannopoulos P., Higgins L.D., Warner J.J. Reverse shoulder arthroplasty as salvage for failed prior arthroplasty in patients 65 years of age or younger. J Shoulder Elbow Surg. 2014;23:1036–1042. doi: 10.1016/j.jse.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 6.Boileau P. Complications and revision of reverse total shoulder arthroplasty. Orthop Traumatol Surg Res. 2016;102:S33–S43. doi: 10.1016/j.otsr.2015.06.031. [DOI] [PubMed] [Google Scholar]
- 7.Boileau P., Melis B., Duperron D., Moineau G., Rumian A.P., Han Y. Revision surgery of reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22:1359–1370. doi: 10.1016/j.jse.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Boileau P., Moineau G., Roussanne Y., O'Shea K. Bony increased-offset reversed shoulder arthroplasty: minimizing scapular impingement while maximizing glenoid fixation. Clin Orthop Relat Res. 2011;469:2558–2567. doi: 10.1007/s11999-011-1775-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boileau P., Watkinson D., Hatzidakis A.M., Hovorka I. Neer Award 2005: the Grammont reverse shoulder prosthesis: results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J Shoulder Elbow Surg. 2006;15:527–540. doi: 10.1016/j.jse.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Boileau P., Watkinson D.J., Hatzidakis A.M., Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg. 2005;14:147S–161S. doi: 10.1016/j.jse.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 11.Bonnevialle N., Melis B., Neyton L., Favard L., Mole D., Walch G. Aseptic glenoid loosening or failure in total shoulder arthroplasty: revision with glenoid reimplantation. J Shoulder Elbow Surg. 2013;22:745–751. doi: 10.1016/j.jse.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 12.Castagna A., Delcogliano M., de Caro F., Ziveri G., Borroni M., Gumina S. Conversion of shoulder arthroplasty to reverse implants: clinical and radiological results using a modular system. Int Orthop. 2013;37:1297–1305. doi: 10.1007/s00264-013-1907-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chacon A., Virani N., Shannon R., Levy J.C., Pupello D., Frankle M. Revision arthroplasty with use of a reverse shoulder prosthesis-allograft composite. J Bone Joint Surg Am. 2009;91:119–127. doi: 10.2106/JBJS.H.00094. [DOI] [PubMed] [Google Scholar]
- 14.Chalmers P.N., Romeo A.A., Nicholson G.P., Boileau P., Keener J.D., Gregory J.M. Humeral bone loss in revision total shoulder arthroplasty: the Proximal Humeral Arthroplasty Revision Osseous Insufficiency (PHAROS) classification system. Clin Orthop Relat Res. 2019;477:432–441. doi: 10.1097/CORR.0000000000000590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cox J.L., McLendon P.B., Christmas K.N., Simon P., Mighell M.A., Frankle M.A. Clinical outcomes following reverse shoulder arthroplasty-allograft composite for revision of failed arthroplasty associated with proximal humeral bone deficiency: 2- to 15-year follow-up. J Shoulder Elbow Surg. 2019;28:900–907. doi: 10.1016/j.jse.2018.10.023. [DOI] [PubMed] [Google Scholar]
- 16.De Biase C.F., Ziveri G., De Caro F., Roberts N., Delcogliano M. Reverse shoulder arthroplasty using a “L” shaped allograft for glenoid reconstruction in a patient with massive glenoid bone loss: case report. Eur Rev Med Pharmacol Sci. 2014;18(Suppl):44–49. [PubMed] [Google Scholar]
- 17.Elhassan B., Ozbaydar M., Higgins L.D., Warner J.J. Glenoid reconstruction in revision shoulder arthroplasty. Clin Orthop Relat Res. 2008;466:599–607. doi: 10.1007/s11999-007-0108-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flury M.P., Frey P., Goldhahn J., Schwyzer H.K., Simmen B.R. Reverse shoulder arthroplasty as a salvage procedure for failed conventional shoulder replacement due to cuff failure—midterm results. Int Orthop. 2011;35:53–60. doi: 10.1007/s00264-010-0990-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerber C., Pennington S.D., Nyffeler R.W. Reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2009;17:284–295. doi: 10.5435/00124635-200905000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Hernandez N.M., Chalmers B.P., Wagner E.R., Sperling J.W., Cofield R.H., Sanchez-Sotelo J. Revision to reverse total shoulder arthroplasty restores stability for patients with unstable shoulder prostheses. Clin Orthop Relat Res. 2017;475:2716–2722. doi: 10.1007/s11999-017-5429-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hill J.M., Norris T.R. Long-term results of total shoulder arthroplasty following bone-grafting of the glenoid. J Bone Joint Surg Am. 2001;83:877–883. [PubMed] [Google Scholar]
- 22.Holcomb J.O., Cuff D., Petersen S.A., Pupello D.R., Frankle M.A. Revision reverse shoulder arthroplasty for glenoid baseplate failure after primary reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18:717–723. doi: 10.1016/j.jse.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 23.Holschen M., Franetzki B., Witt K.A., Liem D., Steinbeck J. Conversions from anatomic shoulder replacements to reverse total shoulder arthroplasty: do the indications for initial surgery influence the clinical outcome after revision surgery? Arch Orthop Trauma Surg. 2017;137:167–172. doi: 10.1007/s00402-016-2595-5. [DOI] [PubMed] [Google Scholar]
- 24.Iannotti J.P., Frangiamore S.J. Fate of large structural allograft for treatment of severe uncontained glenoid bone deficiency. J Shoulder Elbow Surg. 2012;21:765–771. doi: 10.1016/j.jse.2011.08.069. [DOI] [PubMed] [Google Scholar]
- 25.Jones R.B., Wright T.W., Roche C.P. Bone grafting the glenoid versus use of augmented glenoid baseplates with reverse shoulder arthroplasty. Bull Hosp Jt Dis (2013) 2015;73(Suppl 1):S129–S135. [PubMed] [Google Scholar]
- 26.Kany J., Amouyel T., Flamand O., Katz D., Valenti P. A convertible shoulder system: is it useful in total shoulder arthroplasty revisions? Int Orthop. 2015;39:299–304. doi: 10.1007/s00264-014-2563-z. [DOI] [PubMed] [Google Scholar]
- 27.Kelly J.D., II, Zhao J.X., Hobgood E.R., Norris T.R. Clinical results of revision shoulder arthroplasty using the reverse prosthesis. J Shoulder Elbow Surg. 2012;21:1516–1525. doi: 10.1016/j.jse.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 28.Kim S.H., Wise B.L., Zhang Y., Szabo R.M. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93:2249–2254. doi: 10.2106/JBJS.J.01994. [DOI] [PubMed] [Google Scholar]
- 29.Matsoukis J., Billuart F., Houssam K., Dujardin F., Walch G. Conversion of total shoulder arthroplasty to reverse shoulder arthroplasty made possible by custom humeral adapter. Orthop Traumatol Surg Res. 2015;101:759–761. doi: 10.1016/j.otsr.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 30.McFarland E.G., Huri G., Hyun Y.S., Petersen S.A., Srikumaran U. Reverse total shoulder arthroplasty without bone-grafting for severe glenoid bone loss in patients with osteoarthritis and intact rotator cuff. J Bone Joint Surg Am. 2016;98:1801–1807. doi: 10.2106/JBJS.15.01181. [DOI] [PubMed] [Google Scholar]
- 31.Melis B., Bonnevialle N., Neyton L., Levigne C., Favard L., Walch G. Glenoid loosening and failure in anatomical total shoulder arthroplasty: is revision with a reverse shoulder arthroplasty a reliable option? J Shoulder Elbow Surg. 2012;21:342–349. doi: 10.1016/j.jse.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 32.Merolla G., Wagner E., Sperling J.W., Paladini P., Fabbri E., Porcellini G. Revision of failed shoulder hemiarthroplasty to reverse total arthroplasty: analysis of 157 revision implants. J Shoulder Elbow Surg. 2018;27:75–81. doi: 10.1016/j.jse.2017.06.038. [DOI] [PubMed] [Google Scholar]
- 33.Muh S.J., Streit J.J., Wanner J.P., Lenarz C.J., Shishani Y., Rowland D.Y. Early follow-up of reverse total shoulder arthroplasty in patients sixty years of age or younger. J Bone Joint Surg Am. 2013;95:1877–1883. doi: 10.2106/JBJS.L.10005. [DOI] [PubMed] [Google Scholar]
- 34.Neyton L., Boileau P., Nove-Josserand L., Edwards T.B., Walch G. Glenoid bone grafting with a reverse design prosthesis. J Shoulder Elbow Surg. 2007;16:S71–S78. doi: 10.1016/j.jse.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 35.Ortmaier R., Resch H., Matis N., Blocher M., Auffarth A., Mayer M. Reverse shoulder arthroplasty in revision of failed shoulder arthroplasty-outcome and follow-up. Int Orthop. 2013;37:67–75. doi: 10.1007/s00264-012-1742-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patel D.N., Young B., Onyekwelu I., Zuckerman J.D., Kwon Y.W. Reverse total shoulder arthroplasty for failed shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21:1478–1483. doi: 10.1016/j.jse.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 37.Raiss P., Schmitt M., Bruckner T., Kasten P., Pape G., Loew M. Results of cemented total shoulder replacement with a minimum follow-up of ten years. J Bone Joint Surg Am. 2012;94:e171. doi: 10.2106/JBJS.K.00580. [DOI] [PubMed] [Google Scholar]
- 38.Saltzman B.M., Chalmers P.N., Gupta A.K., Romeo A.A., Nicholson G.P. Complication rates comparing primary with revision reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23:1647–1654. doi: 10.1016/j.jse.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 39.Sershon R.A., Van Thiel G.S., Lin E.C., McGill K.C., Cole B.J., Verma N.N. Clinical outcomes of reverse total shoulder arthroplasty in patients aged younger than 60 years. J Shoulder Elbow Surg. 2014;23:395–400. doi: 10.1016/j.jse.2013.07.047. [DOI] [PubMed] [Google Scholar]
- 40.Sheth M.M., Sholder D., Getz C.L., Williams G.R., Namdari S. Revision of failed hemiarthroplasty and anatomic total shoulder arthroplasty to reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2019;28:1074–1081. doi: 10.1016/j.jse.2018.10.026. [DOI] [PubMed] [Google Scholar]
- 41.Shields E., Wiater J.M. Patient outcomes after revision of anatomic total shoulder arthroplasty to reverse shoulder arthroplasty for rotator cuff failure or component loosening: a matched cohort study. J Am Acad Orthop Surg. 2019;27:e193–e198. doi: 10.5435/JAAOS-D-17-00350. [DOI] [PubMed] [Google Scholar]
- 42.Sirveaux F., Favard L., Oudet D., Huquet D., Walch G., Mole D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J Bone Joint Surg Br. 2004;86:388–395. doi: 10.1302/0301-620X.86B3.14024. [DOI] [PubMed] [Google Scholar]
- 43.Stephens B.C., Simon P., Clark R.E., Christmas K.N., Stone G.P., Lorenzetti A.J. Revision for a failed reverse: a 12-year review of a lateralized implant. J Shoulder Elbow Surg. 2016;25:e115–e124. doi: 10.1016/j.jse.2015.09.027. [DOI] [PubMed] [Google Scholar]
- 44.Stephens S.P., Paisley K.C., Giveans M.R., Wirth M.A. The effect of proximal humeral bone loss on revision reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24:1519–1526. doi: 10.1016/j.jse.2015.02.020. [DOI] [PubMed] [Google Scholar]
- 45.Stephens S.P., Paisley K.C., Jeng J., Dutta A.K., Wirth M.A. Shoulder arthroplasty in the presence of posterior glenoid bone loss. J Bone Joint Surg Am. 2015;97:251–259. doi: 10.2106/JBJS.N.00566. [DOI] [PubMed] [Google Scholar]
- 46.Tashjian R. No bone? No problem! Is bone-grafting at the time of revision to a reverse shoulder arthroplasty a reasonable option? Commentary on an article by Eric Wagner, MD, et al.: “Glenoid bone-grafting in revision to a reverse total shoulder arthroplasty”. J Bone Joint Surg Am. 2015;97:e68. doi: 10.2106/JBJS.O.00850. [DOI] [PubMed] [Google Scholar]
- 47.Tashjian R.Z., Hung M., Keener J.D., Bowen R.C., McAllister J., Chen W. Determining the minimal clinically important difference for the American Shoulder and Elbow Surgeons score, Simple Shoulder Test, and visual analog scale (VAS) measuring pain after shoulder arthroplasty. J Shoulder Elbow Surg. 2017;26:144–148. doi: 10.1016/j.jse.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 48.Valenti P., Kilinc A.S., Sauzieres P., Katz D. Results of 30 reverse shoulder prostheses for revision of failed hemi- or total shoulder arthroplasty. Eur J Orthop Surg Traumatol. 2014;24:1375–1382. doi: 10.1007/s00590-013-1332-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.von Engelhardt L.V., Manzke M., Filler T.J., Jerosch J. Short-term results of the reverse Total Evolutive Shoulder System (TESS) in cuff tear arthropathy and revision arthroplasty cases. Arch Orthop Trauma Surg. 2015;135:897–904. doi: 10.1007/s00402-015-2218-6. [DOI] [PubMed] [Google Scholar]
- 50.Wagner E., Houdek M.T., Griffith T., Elhassan B.T., Sanchez-Sotelo J., Sperling J.W. Glenoid bone-grafting in revision to a reverse total shoulder arthroplasty. J Bone Joint Surg Am. 2015;97:1653–1660. doi: 10.2106/JBJS.N.00732. [DOI] [PubMed] [Google Scholar]
- 51.Wagner E.R., Houdek M.T., Hernandez N.M., Cofield R.H., Sanchez-Sotelo J., Sperling J.W. Cement-within-cement technique in revision reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2017;26:1448–1453. doi: 10.1016/j.jse.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 52.Wagner E.R., Statz J.M., Houdek M.T., Cofield R.H., Sanchez-Sotelo J., Sperling J.W. Use of a shorter humeral stem in revision reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2017;26:1454–1461. doi: 10.1016/j.jse.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 53.Walker M., Willis M.P., Brooks J.P., Pupello D., Mulieri P.J., Frankle M.A. The use of the reverse shoulder arthroplasty for treatment of failed total shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21:514–522. doi: 10.1016/j.jse.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 54.Wall B., Nove-Josserand L., O'Connor D.P., Edwards T.B., Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am. 2007;89:1476–1485. doi: 10.2106/JBJS.F.00666. [DOI] [PubMed] [Google Scholar]
- 55.Werner B.C., Burrus M.T., Begho I., Gwathmey F.W., Brockmeier S.F. Early revision within 1 year after shoulder arthroplasty: patient factors and etiology. J Shoulder Elbow Surg. 2015;24:e323–e330. doi: 10.1016/j.jse.2015.05.035. [DOI] [PubMed] [Google Scholar]
- 56.Werner B.S., Bohm D., Abdelkawi A., Gohlke F. Glenoid bone grafting in reverse shoulder arthroplasty for long-standing anterior shoulder dislocation. J Shoulder Elbow Surg. 2014;23:1655–1661. doi: 10.1016/j.jse.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 57.Xu S., Baker D.K., Woods J.C., Brabston E.W., III, Ponce B.A. Risk factors for early readmission after anatomical or reverse total shoulder arthroplasty. Am J Orthop (Belle Mead NJ) 2016;45:E386–E392. [PubMed] [Google Scholar]